Solution Precursor Plasma Spraying of TiO2 Coatings Using a Catalyst-Free Precursor
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Preparation and Plasma Spraying
2.2. Characterization of Solution Precursor and Coatings
3. Results
3.1. Thermal and Crystallization Behaviors of the As-Dried Solution Precursor
3.2. Single-Pass Spray Deposits
3.3. Full-Pass Spray Coatings
3.3.1. Microstructure of Coatings
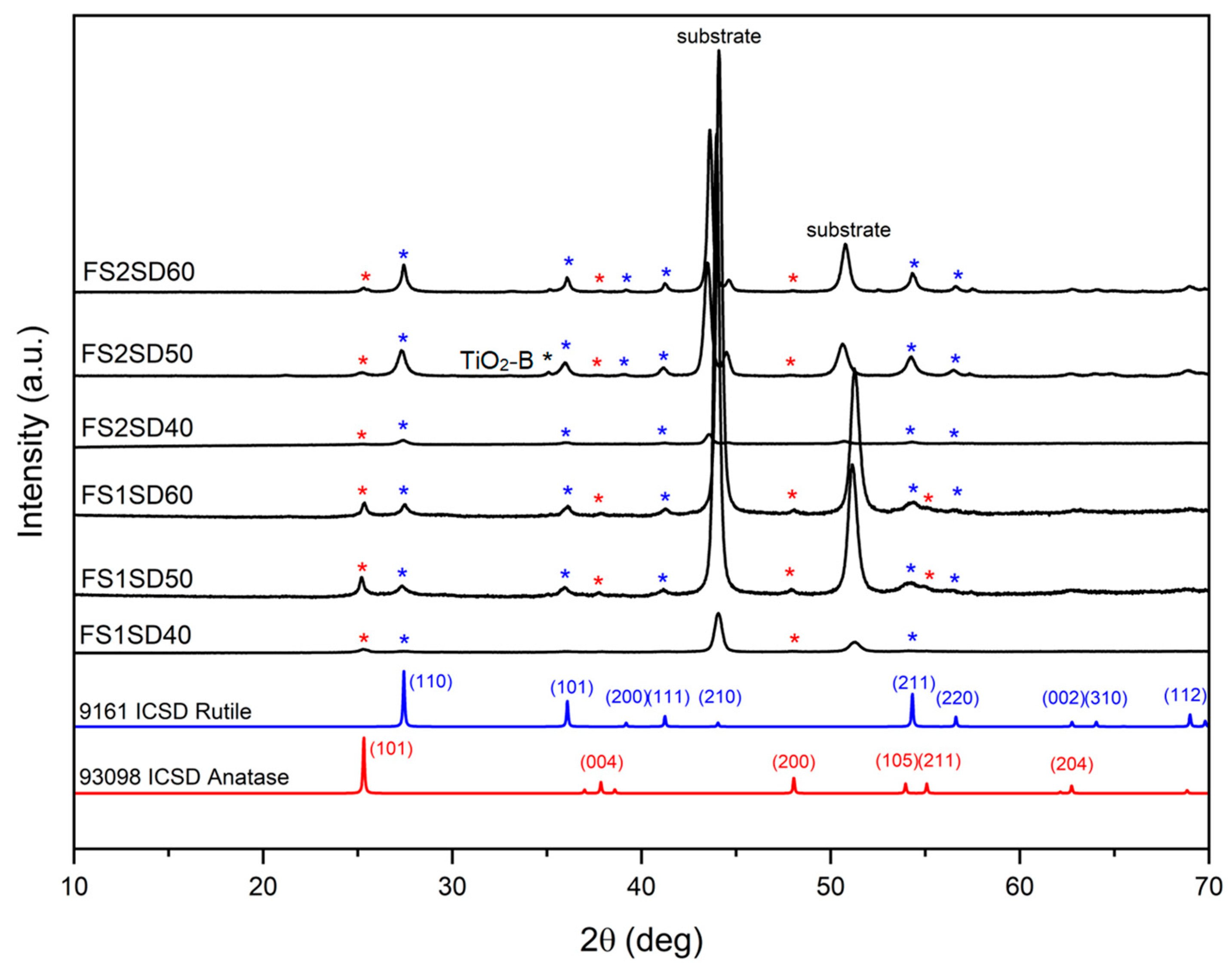
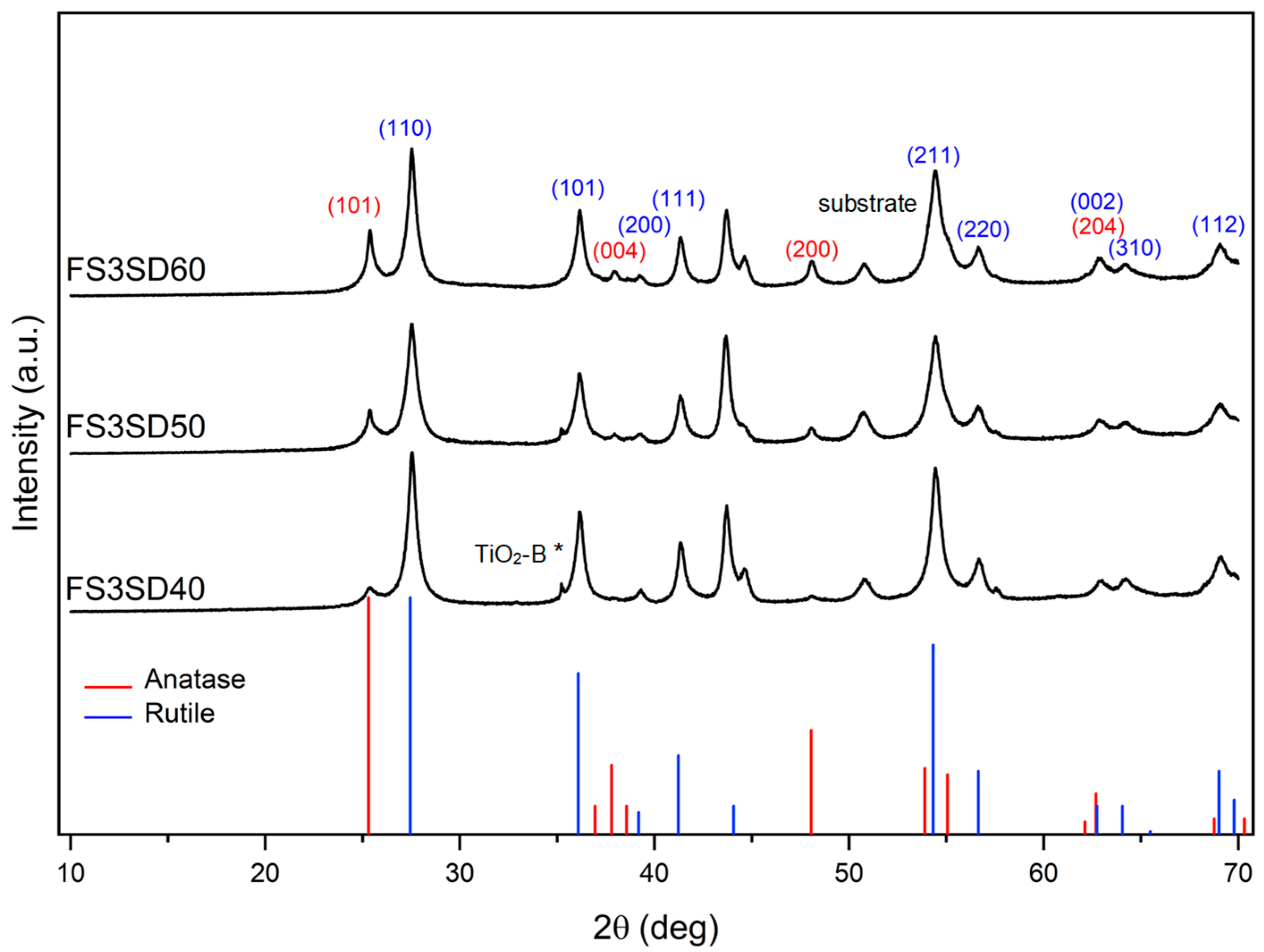
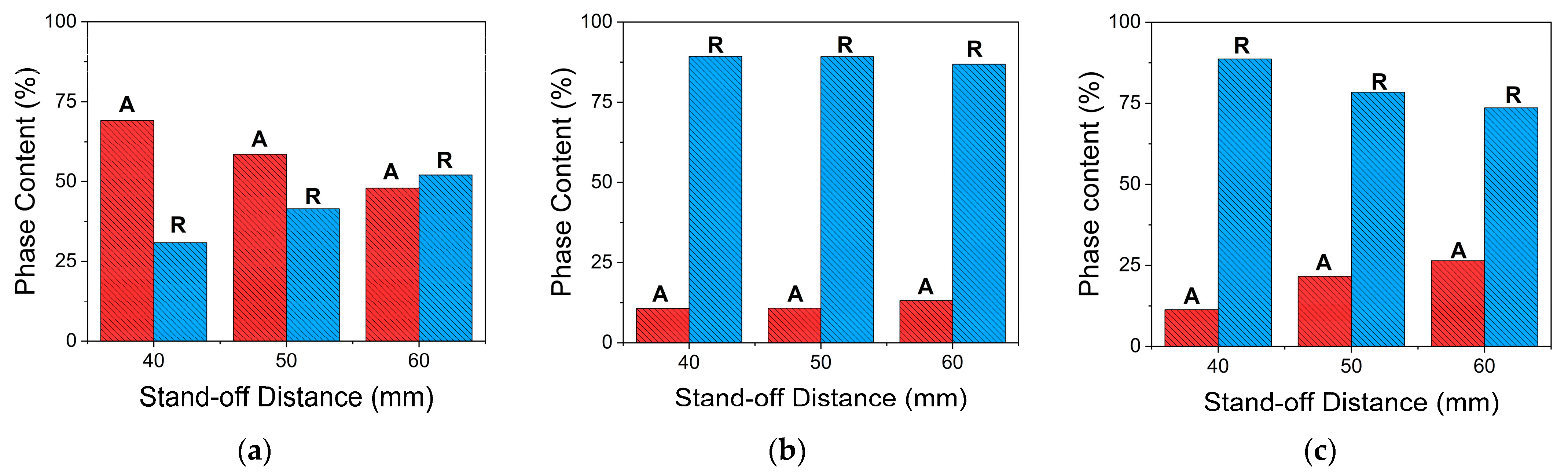
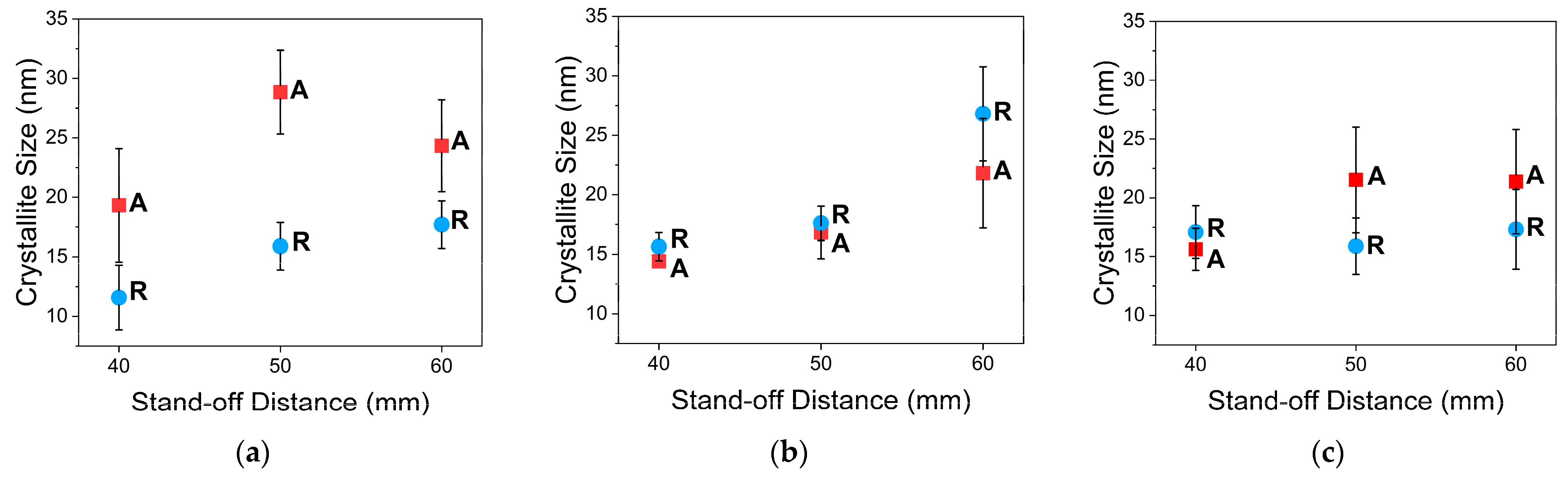
3.3.2. XRD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grela, M.A.; Brusa, M. Harnessing excess photon energy in photoinduced surface electron transfer between salicylate and illuminated titanium dioxide nanoparticles. J. Phys. Chem. B 1997, 101, 10986–10989. [Google Scholar] [CrossRef]
- Marien, C.B.D.; Marchal, C.; Koch, A.; Robert, D.; Drogui, P. Sol-gel synthesis of TiO2 nanoparticles: Effect of Pluronic P123 on particle’s morphology and photocatalytic degradation of paraquat. Environ. Sci. Pollut. Res. 2016, 24, 12582–12588. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property. Materials 2018, 11, 450. [Google Scholar] [CrossRef]
- Sahoo, M.; Yadav, A.K.; Ghosh, S.; Jha, S.N.; Bhattacharyya, D.; Mathews, T. Structural studies of spray pyrolysis synthesized oxygen deficient anatase TiO2 thin films by using X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 6198–6206. [Google Scholar] [CrossRef] [PubMed]
- Aloitabi, A.M.; Sathasivam, S.; Williamson, B.A.D.; Kafizas, A.; Sotelo-Vasquez, C.; Taylor, A.; Scanlon, D.O.; Parkin, I.P. Chemical Vapor Deposition of Photocatalytically Active Pure Brookite TiO2 Thin Films. Chem. Mater. 2018, 30, 1353–1361. [Google Scholar] [CrossRef]
- Yamada, M.; Isago, H.; Nakano, H.; Fukumoto, M. Cold Spraying of TiO2 Photocatalyst Coating With Nitrogen Process Gas. J. Therm. Spray Technol. 2010, 19, 1218–1223. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Robinson, B.W.; de Villiers Lovelock, H.L.; Wood, R.J.K. Structure-Property Relationships in Suspension HVOF Nano-TiO2 Coatings. Coatings 2019, 9, 504. [Google Scholar] [CrossRef]
- Forghani, S.M.; Ghazali, M.J.; Muchtar, A.; Daud, A.R.; Yusoff, N.H.N.; Azhari, C.H. Effects of plasma spray parameters on TiO2-coated mild steel using design of experiment (DoE) approach. Ceram. Int. 2013, 39, 3121–3127. [Google Scholar] [CrossRef]
- Zhai, M.; Liu, Y.; Huang, J.; Wang, Y.; Chen, K.; Fu, Y.; Li, H. Efficient suspension plasma spray fabrication of black titanium dioxide coatings with visible light absorption performances. Ceram. Int. 2018, 45, 930–935. [Google Scholar] [CrossRef]
- Du, L.; Coyle, T.W.; Chien, K.; Pershin, L.; Li, T.; Golozar, M. Titanium Dioxide Coating Prepared by Use of a Suspension-Solution Plasma-Spray Process. J. Therm. Spray Technol. 2015, 24, 915–924. [Google Scholar] [CrossRef]
- Aruna, S.T.; Vismaya, A.; Balaji, N. Photocatalytic behavior of titania coatings fabricated by suspension and solution precursor plasma spray processes. Mater. Manuf. Process. 2021, 36, 868–875. [Google Scholar] [CrossRef]
- Viswanathan, V.; Filmalter, R.; Patil, S.; Deshpande, S.; Seal, S. High-Temperature Oxidation Behavior of Solution Precursor Plasma Sprayed Nanoceria Coating on Martensitic Steels. J. Am. Ceram. Soc. 2007, 90, 870–877. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M.; Ma, X. Dense Alumina–Zirconia Coatings Using the Solution Precursor Plasma Spray Process. J. Am. Ceram. Soc. 2008, 91, 359–365. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, B.; Wang, J.; Luo, Y. Solution precursor plasma sprayed tungsten oxide particles and coatings. Mater. Manuf. Process. 2017, 33, 1107–1114. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Liao, H.; Li, C.-J.; Debliquy, M. Room-temperature nitrogen-dioxide sensors based on ZnO1−x coatings deposited by solution precursor plasma spray. Sens. Actuators B Chem. 2016, 242, 102–111. [Google Scholar] [CrossRef]
- Yu, Z.; Moussa, H.; Liu, M.; Chouchene, B.; Schneider, R.; Wang, W.; Moliere, M.; Liao, H. Tunable morphologies of ZnO films via the solution precursor plasma spray process for improved photocatalytic degradation performance. Appl. Surf. Sci. 2018, 455, 970–979. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M.; Ma, X. Dense TiO2 Coating Using the Solution Precursor Plasma Spray Process. J. Am. Ceram. Soc. 2008, 91, 865–872. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M. Porous TiO2 coating using the solution precursor plasma spray process. Surf. Coat. Technol. 2008, 202, 6113–6119. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; van Grieken, R.; Chien, K.; Pershin, L.; Coyle, T.; Mostaghimi, J. Effect of Liquid Feed-Stock Composition on the Morphology of Titanium Dioxide Films Deposited by Thermal Plasma Spray. J. Nanosci. Nanotechnol. 2015, 15, 6651–6662. [Google Scholar] [CrossRef]
- Fröberg, L. Thermal Analysis: TGA/DTA Lecture; Process Chemistry Centre, Abo Akademi University: Turku, Finland.
- Hosseini, S.N.; Chen, X.; Baesjou, P.J.; Imhof, A.; van Blaaderen, A. Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly. Appl. Sci. 2022, 12, 1614. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Metallurgy Series; Addison-Wesley Publishing: Boston, MA, USA, 1956. [Google Scholar]
- Baszczuk, A.; Jasiorski, M.; Winnicki, M. Low-Temperature Transformation of Amorphous Sol–Gel TiO2 Powder to Anatase During Cold Spray Deposition. J. Therm. Spray Technol. 2018, 27, 1551–1562. [Google Scholar] [CrossRef]
- Ni, X.; Liao, A. Effects of Cooling Rate and Solution Concentration on Solution Crystallization of L-Glutamic Acid in an Oscillatory Baffled Crystallizer. Cryst. Growth Des. 2008, 8, 2875–2881. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Z.-J.; Zhang, S.-L.; Luo, X.-T.; Li, C.-J. Effect of Powder Particle Size and Spray Parameters on the Ni/Al Reaction During Plasma Spraying of Ni-Al Composite Powders. J. Therm. Spray Technol. 2021, 30, 181–195. [Google Scholar] [CrossRef]
- Pateyron, B.; Calve, N.; Pawlowski, L. Influence of water and ethanol on transport properties of the jets used in suspension plasma spraying. Surf. Coat. Technol. 2013, 220, 257–260. [Google Scholar] [CrossRef]
- Xiong, H.; Sun, W. Investigation of Droplet Atomization and Evaporation in Solution Precursor Plasma Spray Coating. Coatings 2017, 7, 207. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M. Effect of solution concentration on splat formation and coating microstructure using the solution precursor plasma spray process. Surf. Coat. Technol. 2008, 202, 2132–2138. [Google Scholar] [CrossRef]
- Jayanthi, G.V.; Zhang, S.C.; Messing, G.L. Modeling of Solid Particle Formation During Solution Aerosol Thermolysis: The Evaporation Stage. Aerosol Sci. Technol. 1993, 19, 478–490. [Google Scholar] [CrossRef]
- Durante, O.; Di Giorgio, C.; Granata, V.; Neilson, J.; Fittipaldi, R.; Vecchione, A.; Carapella, G.; Chiadini, F.; DeSalvo, R.; Dinelli, F.; et al. Emergence and Evolution of Crystallization in TiO2 Thin Films: A Structural and Morphological Study. Nanomaterials 2021, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, M.; Streitenberger, P. Crystallization of amorphous superlattices in the limit of ultrathin films with oxide interfaces. Phys. Rev. B 2000, 62, 8391–8396. [Google Scholar] [CrossRef]
- Nichtová, L.; Kužel, R.; Matěj, Z.; Šícha, J.; Musil, J. Time and thickness dependence of crystallization of amorphous magnetron deposited TiO2 thin films. In Proceedings of the Eleventh European Powder Diffraction Conference, Warsaw, Poland, 19–22 September 2008; Oldenbourg Wissenschaftsverlag: München, Germany, 2009; Volume 30, pp. 235–240. [Google Scholar]
- Tobon Valencia, V.; Pawlowski, L.; Lecomte-Nana, G.; Constantinescu, C.; Pateyron, B. Preliminary analysis of physical and chemical phenomena occurring in droplet at solution precursor plasma spraying of zirconia coatings. Surf. Coat. Technol. 2020, 397, 126059. [Google Scholar] [CrossRef]
- Carpio, P.; Pawlowski, L.; Pateyron, B. Numerical investigation of influence of precursors on transport properties of the jets used in solution precursor plasma spraying. Surf. Coat. Technol. 2018, 371, 131–135. [Google Scholar] [CrossRef]
- Simfroso, K.T.; Liboon, A.Q., Jr.; Candidato, R.T., Jr. A Numerical Investigation of the Thermal Transport Properties of Argon + Hydrogen Plasma Working Gases in the Presence of Various TiO2 Precursor Solutions. Phys. Chem. Res. 2023, 11, 897–912. [Google Scholar]
- Chen, R.Q.; Lu, Q.Q.; Cheng, Q.D.; Ao, L.B.; Zhang, C.Y.; Hou, H.; Liu, Y.M.; Li, D.W.; Yin, D.C. An ignored variable: Solution preparation temperature in protein crystallization. Sci. Rep. 2015, 5, 7797. [Google Scholar] [CrossRef]
- Jang, J.; Takana, H.; Ando, Y.; Solonenko, O.P.; Nishiyama, H. Preparation of Carbon-doped TiO2 Nanopowder Synthesized by Droplet Injection of Solution Precursor in a DC-RF Hybrid Plasma Flow System. J. Therm. Spray Technol. 2013, 22, 974–982. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Li, M.; Feng, Z.; Li, C. UV Raman Spectroscopic Study on TiO2. II. Effect of Nanoparticle Size on the Outer/Inner Phase Transformations. J. Phys. Chem. C 2009, 113, 1698–1704. [Google Scholar] [CrossRef]
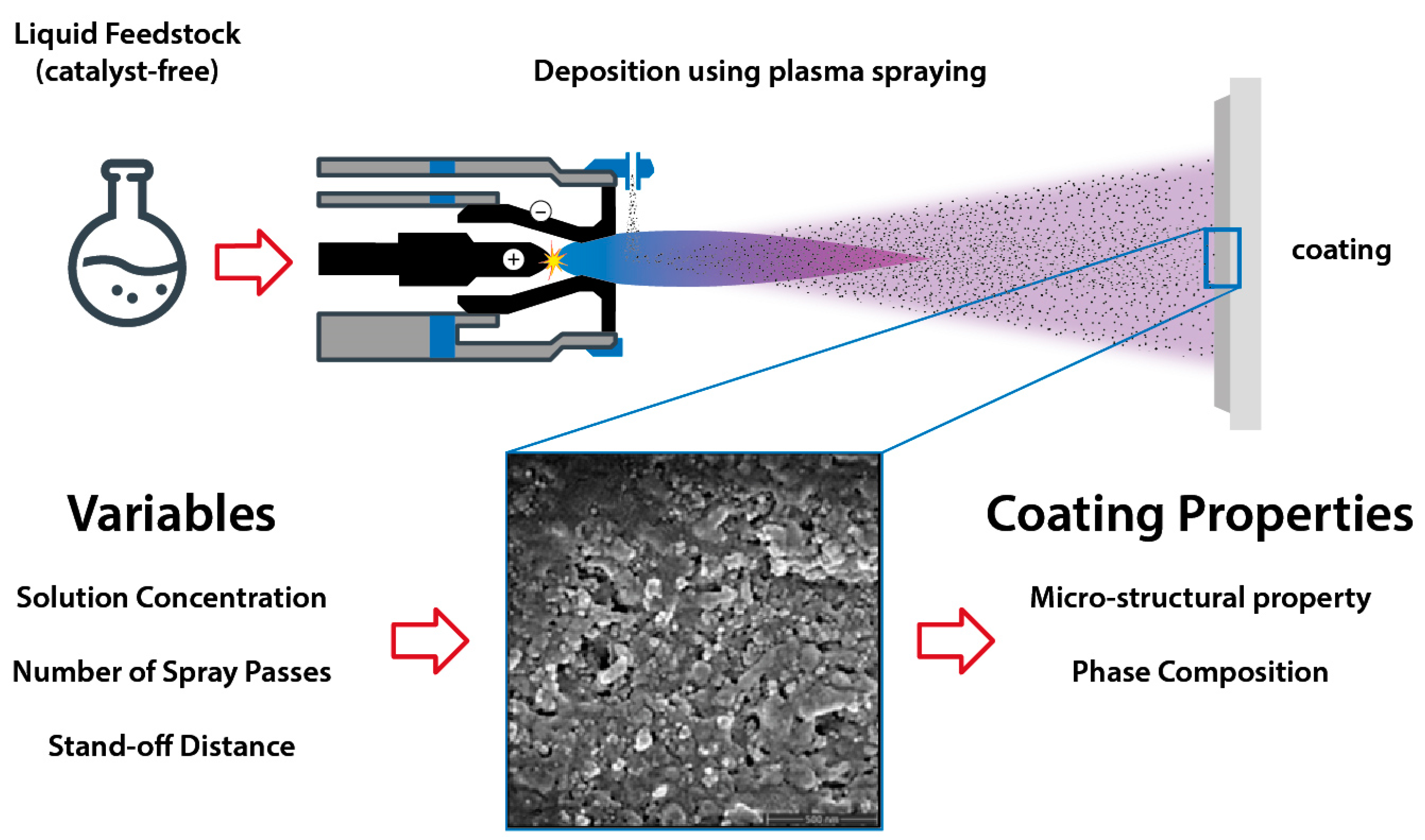
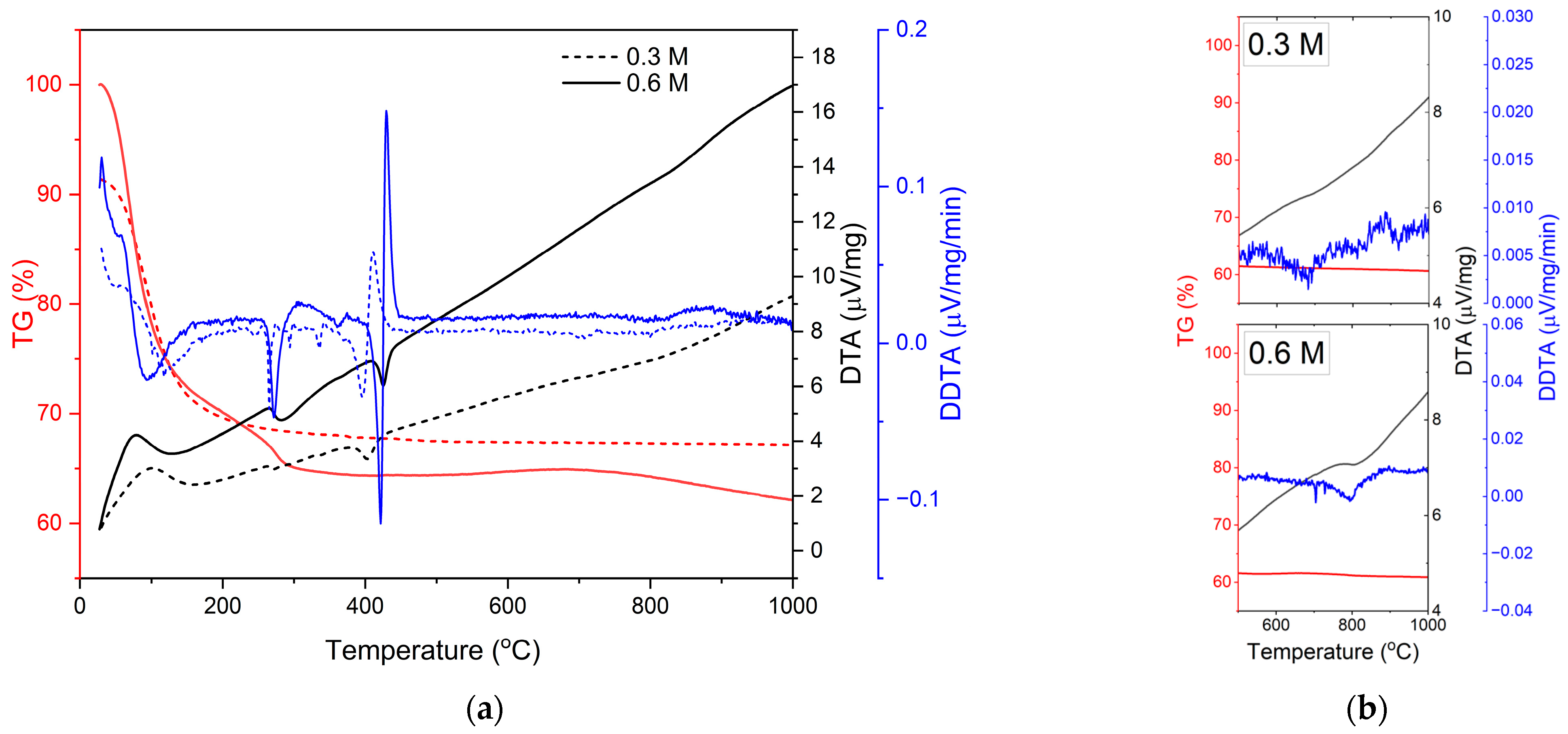

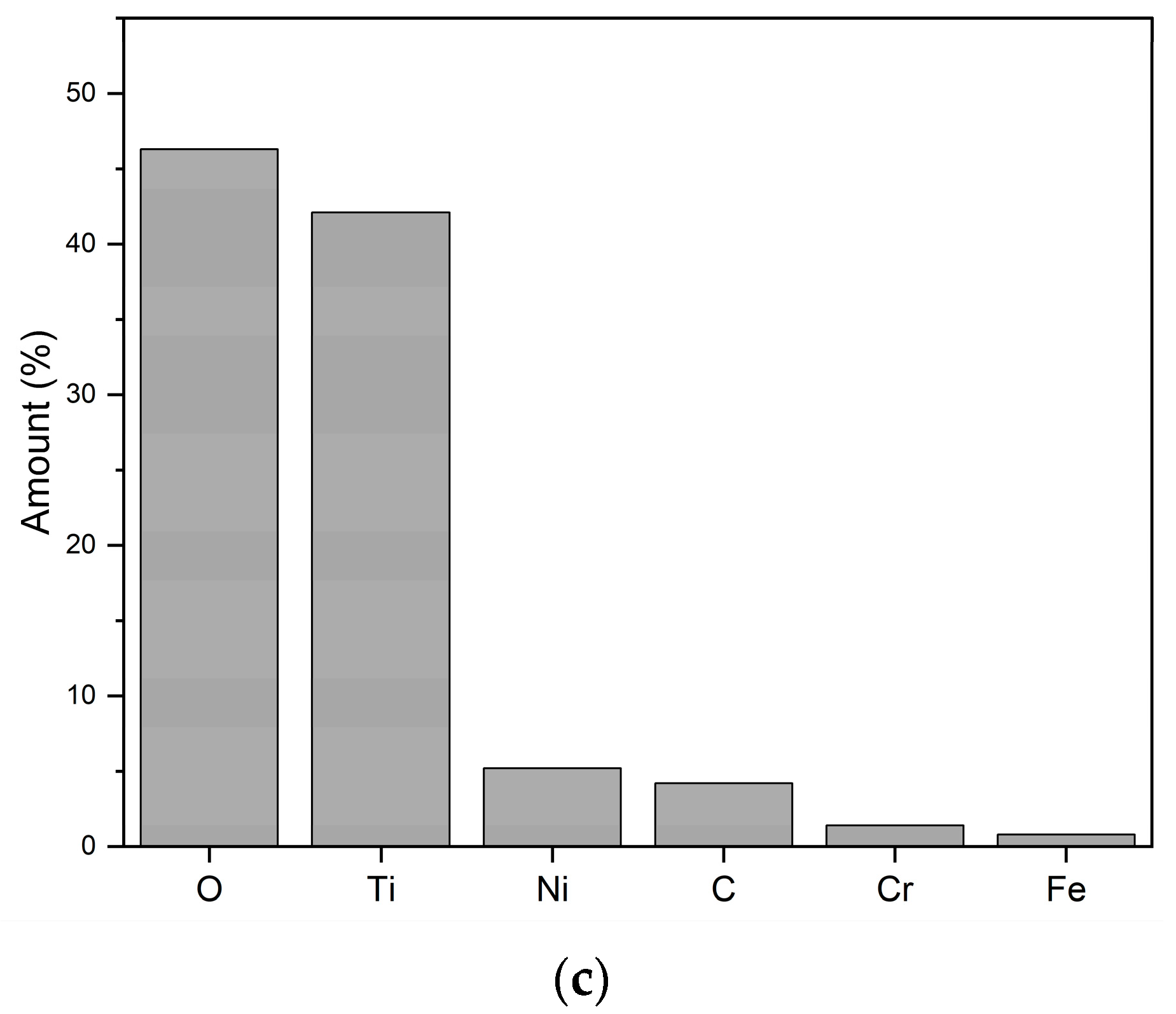

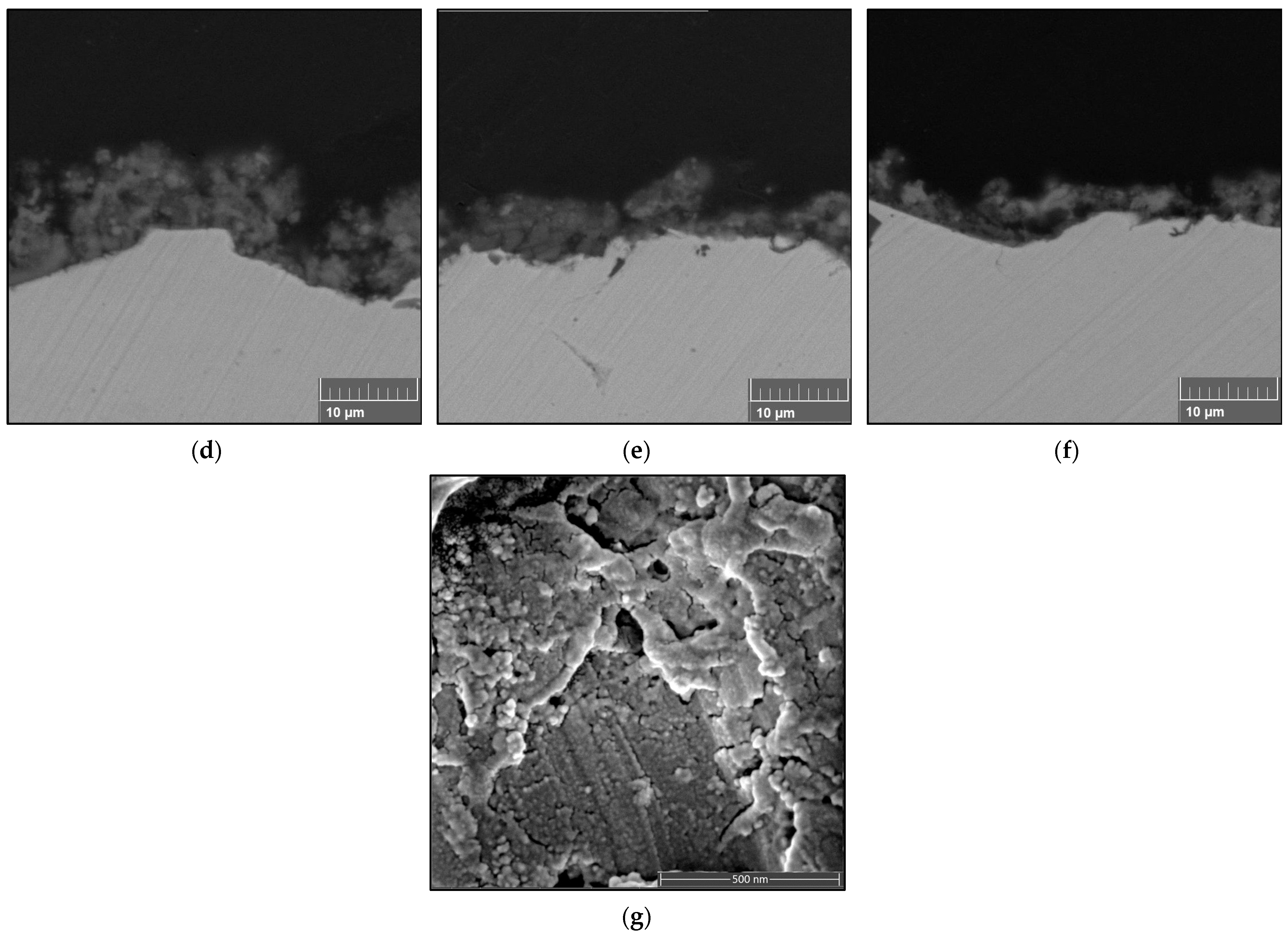


| Sample | Concentration | No. of Passes | Stand-Off Distance | Sample Label |
|---|---|---|---|---|
| 0.3 M | 10 | 40 mm | FS1SD40 | |
| FS1 | 50 mm | FS1SD50 | ||
| 60 mm | FS1SD60 | |||
| 0.6 M | 10 | 40 mm | FS2SD40 | |
| FS2 | 50 mm | FS2SD50 | ||
| 60 mm | FS2SD60 | |||
| 0.6 M | 30 | 40 mm | FS3SD40 | |
| FS3 | 50 mm | FS3SD50 | ||
| 60 mm | FS3SD60 |
| Parameters | Value |
|---|---|
| Plasma power | 28 kW |
| Ar flow rate | 45 slpm |
| H2 flow rate | 5 slpm |
| Torch velocity | 500 mm/s |
| Feedstock feed rate | ~35 g/min |
| Stand-off distance | 40, 50, 60 mm |
| Substrate | Stainless steel |
| Number of spray passes | 10, 30 |
| Nozzle diameter | 0.2 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simfroso, K.; Cabo, S.R.; Unabia, R.; Britos, A.; Sokołowski, P.; Candidato, R., Jr. Solution Precursor Plasma Spraying of TiO2 Coatings Using a Catalyst-Free Precursor. Materials 2023, 16, 1515. https://doi.org/10.3390/ma16041515
Simfroso K, Cabo SR, Unabia R, Britos A, Sokołowski P, Candidato R Jr. Solution Precursor Plasma Spraying of TiO2 Coatings Using a Catalyst-Free Precursor. Materials. 2023; 16(4):1515. https://doi.org/10.3390/ma16041515
Chicago/Turabian StyleSimfroso, Key, Shena Ramyr Cabo, Romnick Unabia, Angelito Britos, Paweł Sokołowski, and Rolando Candidato, Jr. 2023. "Solution Precursor Plasma Spraying of TiO2 Coatings Using a Catalyst-Free Precursor" Materials 16, no. 4: 1515. https://doi.org/10.3390/ma16041515
APA StyleSimfroso, K., Cabo, S. R., Unabia, R., Britos, A., Sokołowski, P., & Candidato, R., Jr. (2023). Solution Precursor Plasma Spraying of TiO2 Coatings Using a Catalyst-Free Precursor. Materials, 16(4), 1515. https://doi.org/10.3390/ma16041515








