Prevention of Root Caries Using Oxalic Acid
Abstract
:1. Introduction
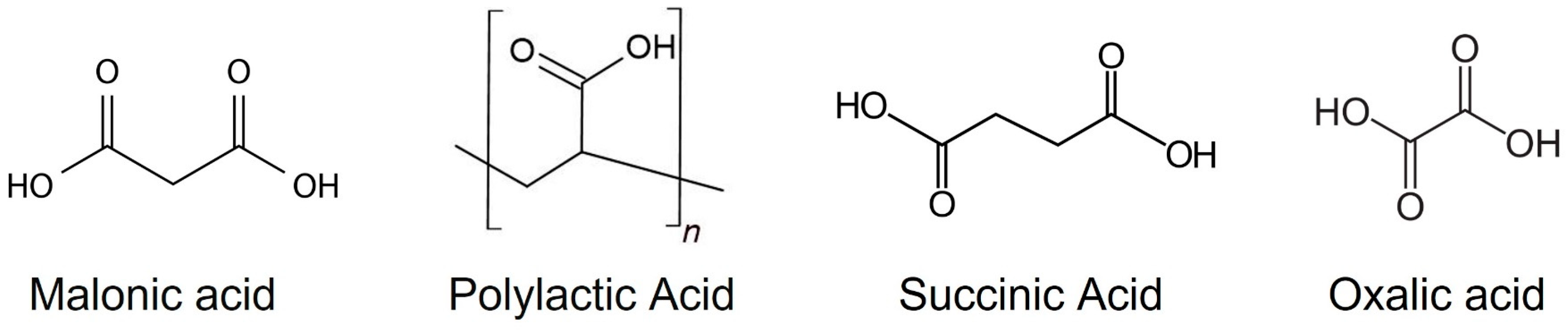
2. Materials and Method
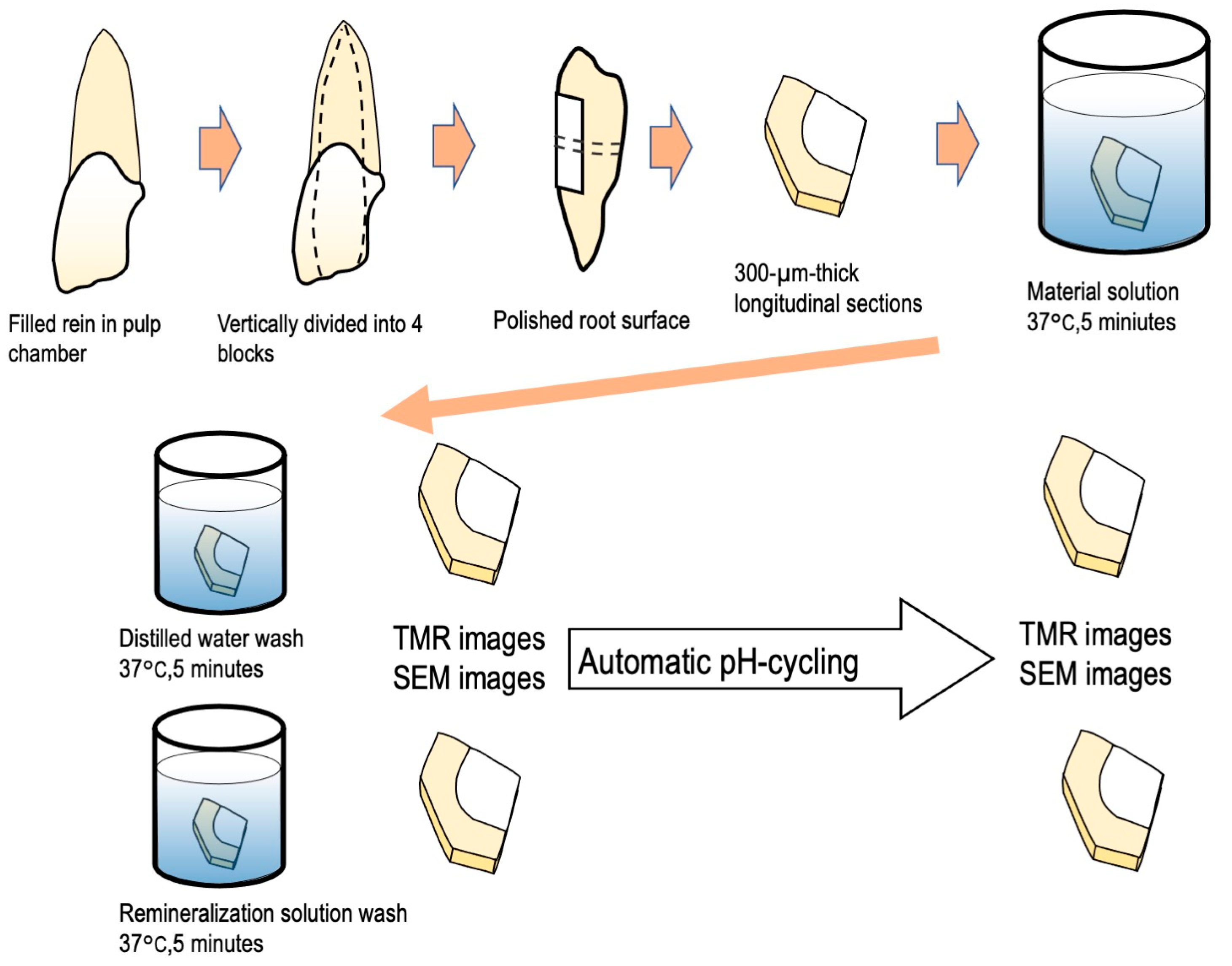
2.1. Specimen Preparation
2.2. Automatic pH Cycling System
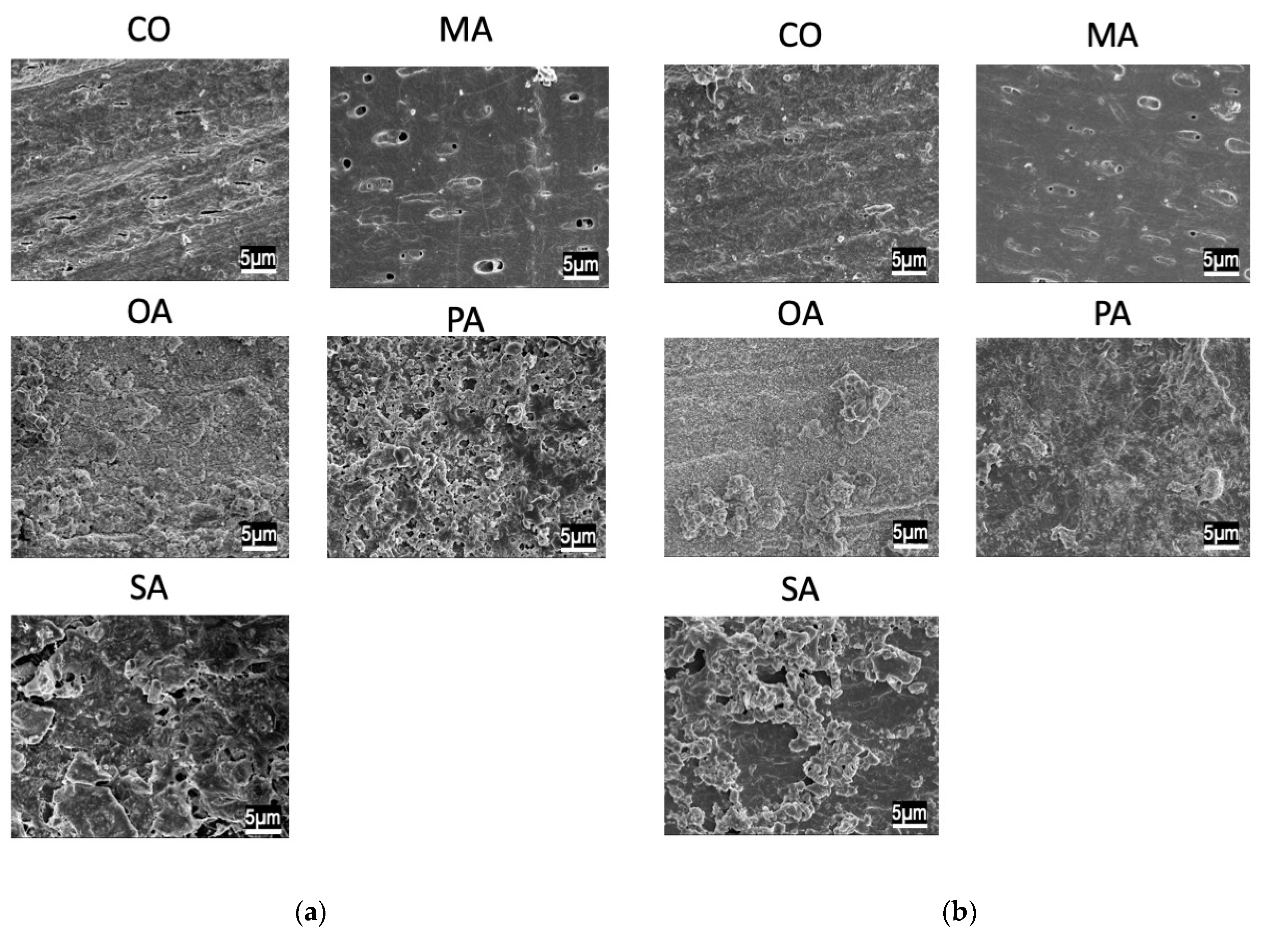
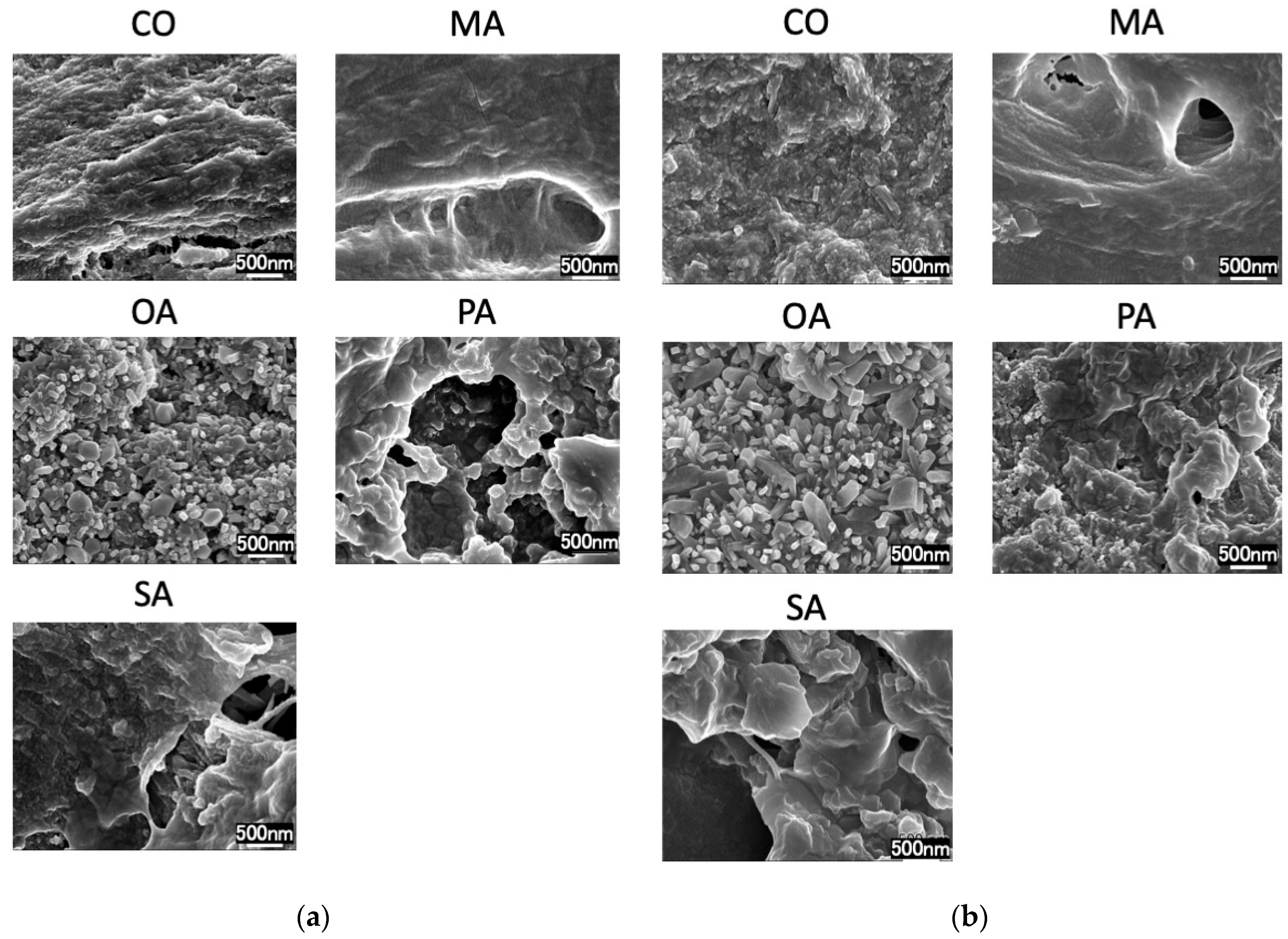
2.3. Scanning Electron Microscopy (SEM) Observation
2.4. Mineral Loss Analysis
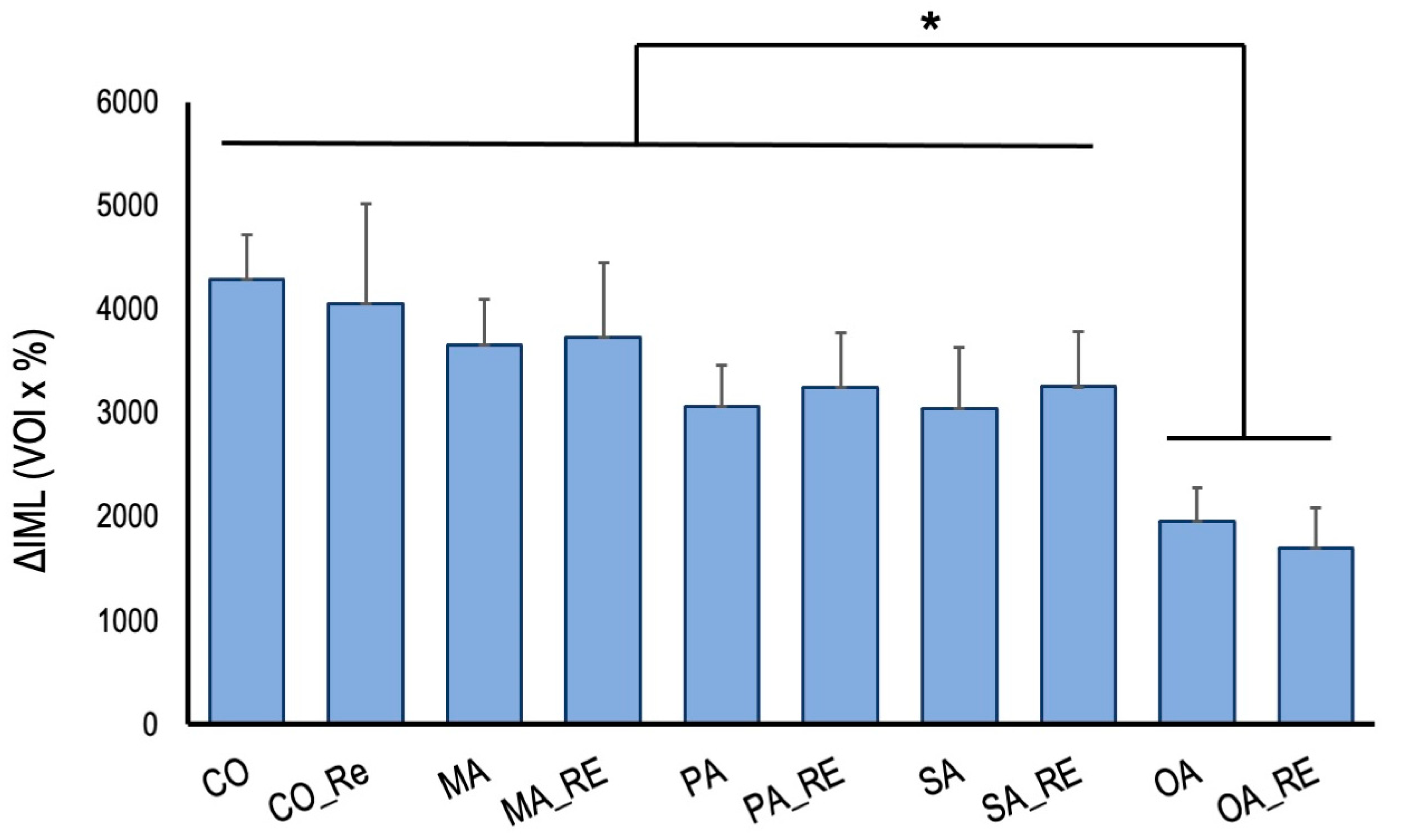
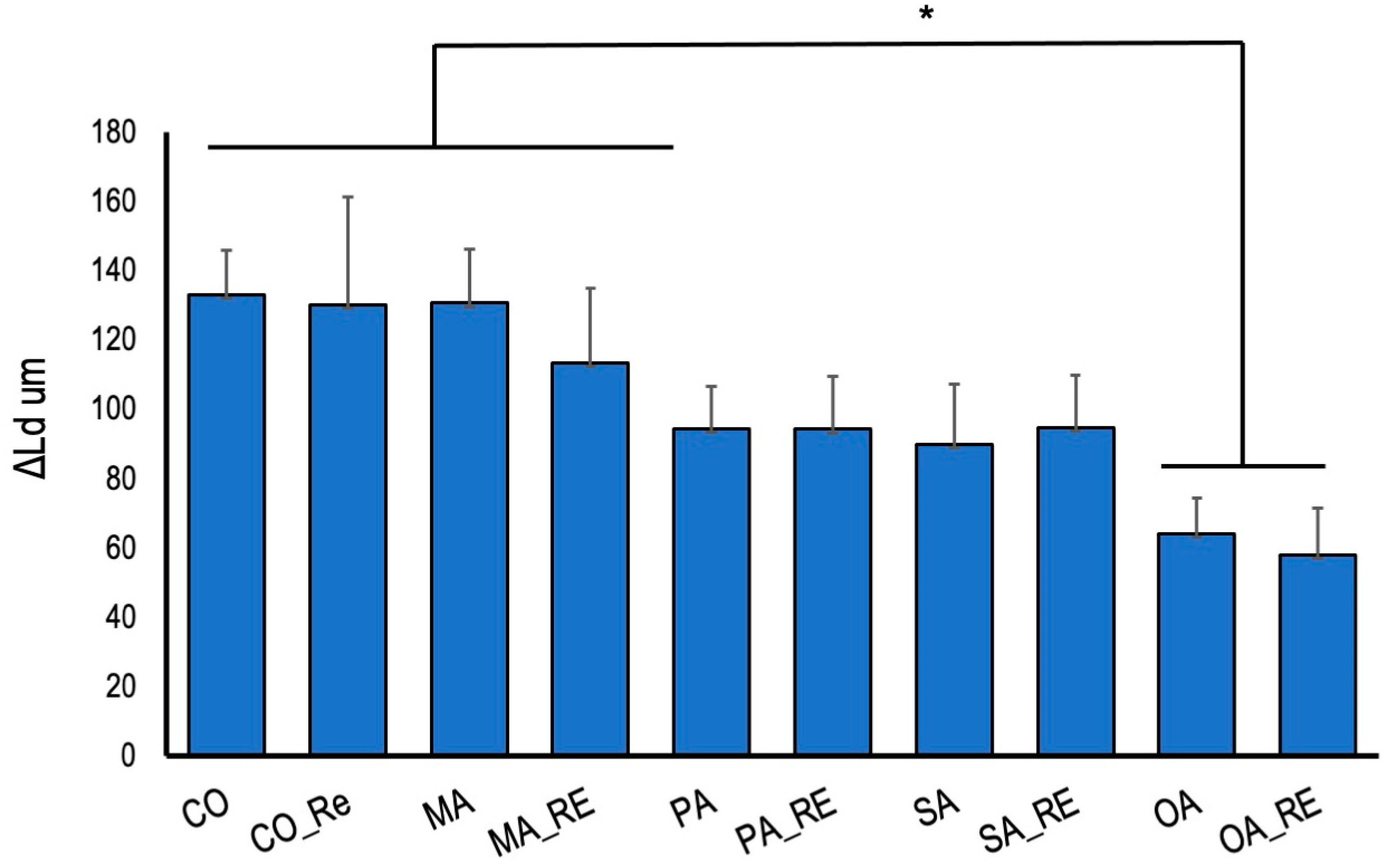
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Abyu, G.Y.; Alsharif, U.; et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Kuriyama, S.; Nakaya, N.; Ohmori-Matsuda, K.; Shimazu, T.; Kikuchi, N.; Kakizaki, M.; Sone, T.; Sato, F.; Nagai, M.; Sugawara, Y.; et al. The Ohsaki Cohort 2006 Study: Design of study and profile of participants at baseline. J. Epidemiol. 2010, 20, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Hahnel, S.; Behr, M.; Handel, G.; Bürgers, R. Saliva substitutes for the treatment of radiation-induced xerostomia—A review. Support. Care Cancer 2009, 17, 1331–1343. [Google Scholar] [CrossRef]
- Zandim-Barcelos, D.L.; Kielbassa, A.M.; Sampaio, J.E.; Tschoppe, P. Saliva substitutes in combination with high-fluoride gel on dentin remineralization. Clin. Oral. Investig. 2015, 19, 289–297. [Google Scholar] [CrossRef]
- Su, N.; Marek, C.L.; Ching, V.; Grushka, M. Caries prevention for patients with dry mouth. J. Can. Dent. Assoc. 2011, 77, b85. [Google Scholar]
- Slayton, R.L.; Urquhart, O.; Araujo, M.W.B.; Fontana, M.; Guzmán-Armstrong, S.; Nascimento, M.M.; Nový, B.B.; Tinanoff, N.; Weyant, R.J.; Wolff, M.S.; et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J. Am. Dent. Assoc. 2018, 149, 837–849.e819. [Google Scholar] [CrossRef]
- Mitchell, C.; Gross, A.J.; Milgrom, P.; Mancl, L.; Prince, D.B. Silver diamine fluoride treatment of active root caries lesions in older adults: A case series. J. Dent. 2021, 105, 103561. [Google Scholar] [CrossRef]
- Zandim, D.L.; Tschoppe, P.; Sampaio, J.E.C.; Kielbassa, A.M. Effect of saliva substitutes in combination with fluorides on remineralization of subsurface dentin lesions. Support. Care Cancer 2011, 19, 1143–1149. [Google Scholar] [CrossRef]
- Sleibi, A.; Tappuni, A.R.; Baysan, A. Reversal of Root Caries with Casein Phosphopeptide-Amorphous Calcium Phosphate and Fluoride Varnish in Xerostomia. Caries Res. 2021, 55, 475–484. [Google Scholar] [CrossRef]
- Cai, J.; Burrow, M.F.; Manton, D.J.; Hardiman, R.; Palamara, J.E.A. Remineralising effects of fluoride varnishes containing calcium phosphate on artificial root caries lesions with adjunctive application of proanthocyanidin. Dent. Mater. 2021, 37, 143–157. [Google Scholar] [CrossRef]
- McComas, W.H., Jr.; Rieman, W., III. The Solubility of Calcium Oxalate Monohydrate in Pure Water and Various Neutral Salt Solutions at 25°. J. Am. Chem. Soc. 1942, 64, 2946–2947. [Google Scholar] [CrossRef]
- Saori, M.; Saori, T.; Yasuhiro, M.; Naoki, H.; Hidehiko, S.; Masamitsu, K. Caries Prevention after Surface Reaction-Type Prereacted Glass Ionomer Filler-Containing Coating Resin Removal from Root Surfaces. J. Nanosci. Nano-Technol. 2016, 16, 12996–13000. [Google Scholar] [CrossRef]
- Lynch, M.C.; Perfekt, R.; McGuire, J.A.; Milleman, J.; Gallob, J.; Amini, P.; Milleman, K. Potassium oxalate mouthrinse reduces dentinal hypersensitivity: A randomized controlled clinical study. J. Am. Dent. Assoc. 2018, 149, 608–618. [Google Scholar] [CrossRef]
- Matsuda, Y.; Altankhishig, B.; Okuyama, K.; Yamamoto, H.; Naito, K.; Hayashi, M.; Sano, H.; Sidhu, S.K.; Saito, T. Inhibition of Demineralization of Dentin by Fluoride-Containing Hydrogel Desensitizers: An In Vitro Study. J. Funct. Biomater. 2022, 13, 246. [Google Scholar] [CrossRef]
- Oaki, Y.; Kotachi, A.; Miura, T.; Imai, H. Bridged nanocrystals in biominerals and their biomimetics: Classical yet modern crystal growth on the nanoscale. Adv. Funct. Mater. 2006, 16, 1633–1639. [Google Scholar] [CrossRef]
- Niu, L.N.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef]
- Matsuda, Y.; Komatsu, H.; Murata, Y.; Tanaka, T.; Sano, H. A Newly Designed Automatic pH-cycling System to Simulate Daily pH Fluctuations. Dent. Mater. J. 2006, 25, 280–285. [Google Scholar] [CrossRef]
- Herkströter, F.M.; Witjes, M.; Arends, J. Demineralization of human dentine compared with enamel in a pH-cycling apparatus with a constant composition during de- and remineralization periods. Caries Res. 1991, 25, 317–322. [Google Scholar] [CrossRef]
- Matsuda, Y.; Murata, Y.; Tanaka, T.; Komatsu, H.; Sano, H. Development of new software as a convenient analysis method for dental microradiography. Dent. Mater. J. 2007, 26, 414–421. [Google Scholar] [CrossRef]
- Matsuda, Y.; Komatsu, H.; Murata, Y.; Tanaka, T.; Sano, H. Effect of the Width of Grooves on Caries Progression Using an Automatic pH-cycling System. Jpn. J. Conserv. Dent. 2005, 48, 828–834. (In Japanese) [Google Scholar]
- Varoni, E.M.; Zuccheri, T.; Carletta, A.; Palazzo, B.; Cochis, A.; Colonna, M.; Rimondini, L. In vitro efficacy of a novel potassium oxalate hydrogel for dentin hypersensitivity. Eur. J. Oral Sci. 2017, 125, 151–159. [Google Scholar] [CrossRef]
- Zeola, L.F.; Soares, P.V.; Cunha-Cruz, J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019, 81, 1–6. [Google Scholar] [CrossRef]
- Shimazu, K.; Karibe, H.; Ogata, K. Effect of artificial saliva contamination on adhesion of dental restorative materials. Dent. Mater. J. 2014, 33, 545–550. [Google Scholar] [CrossRef]
- Göstemeyer, G.; Woike, H.; Paris, S.; Schwendicke, F.; Schlafer, S. Root Caries Preventive Effect of Varnishes Containing Fluoride or Fluoride + Chlorhexidine/Cetylpyridinium Chloride In Vitro. Microorganisms 2021, 9, 737. [Google Scholar] [CrossRef]
- Suge, T.; Kawasaki, A.; Ishikawa, K.; Matsuo, T.; Ebisu, S. Comparison of the occluding ability of dentinal tubules with different morphology between calcium phosphate precipitation method and potassium oxalate treatment. Dent. Mater. J. 2005, 24, 522–529. [Google Scholar] [CrossRef]
- Werner, H.; Bapat, S.; Schobesberger, M.; Segets, D.; Schwaminger, S.P. Calcium Oxalate Crystallization: Influence of pH, Energy Input, and Supersaturation Ratio on the Synthesis of Artificial Kidney Stones. ACS Omega 2021, 6, 26566–26574. [Google Scholar] [CrossRef]
- Oshima, M.; Hamba, H.; Sadr, A.; Nikaido, T.; Tagami, J. Effect of polymer-based desensitizer with sodium fluoride on prevention of root dentin demineralization. Am. J. Dent. 2015, 28, 123–127. [Google Scholar]
- Shao, C.; Zhao, R.; Jiang, S.; Yao, S.; Wu, Z.; Jin, B.; Yang, Y.; Pan, H.; Tang, R. Citrate Improves Collagen Mineralization via Interface Wetting: A Physicochemical Understanding of Biomineralization Control. Adv. Mater. 2018, 30, 1704876. [Google Scholar] [CrossRef]
- He, H.; Shao, C.; Mu, Z.; Mao, C.; Sun, J.; Chen, C.; Tang, R.; Gu, X. Promotion effect of immobilized chondroitin sulfate on intrafibrillar mineralization of collagen. Carbohydr. Polym. 2020, 229, 115547. [Google Scholar] [CrossRef]
- Jin, W.; Jin, Y.; Duan, P.; Wu, H.; Zhang, L.; Du, Q.; Pan, H.; Tang, R.; Shao, C. Promotion of collagen mineralization and dentin repair by succinates. J. Mater. Chem. B 2022, 10, 5826–5834. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef]
- Liou, S.-C.; Chen, S.-Y.; Liu, D.-M. Manipulation of nanoneedle and nanosphere apatite/poly(acrylic acid) nanocomposites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 117–122. [Google Scholar] [CrossRef]
- Pashley, D.H. Dynamics of the Pulpo-Dentin Complex. Crit. Rev. Oral Biol. Med. 1996, 7, 104–133. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguma, H.; Matsuda, Y.; Yoshihara, K.; Okuyama, K.; Sakurai, M.; Saito, T.; Inoue, S.; Yoshida, Y. Prevention of Root Caries Using Oxalic Acid. Materials 2023, 16, 1454. https://doi.org/10.3390/ma16041454
Oguma H, Matsuda Y, Yoshihara K, Okuyama K, Sakurai M, Saito T, Inoue S, Yoshida Y. Prevention of Root Caries Using Oxalic Acid. Materials. 2023; 16(4):1454. https://doi.org/10.3390/ma16041454
Chicago/Turabian StyleOguma, Hidetoshi, Yasuhiro Matsuda, Kumiko Yoshihara, Katsushi Okuyama, Masahiko Sakurai, Takashi Saito, Satoshi Inoue, and Yasuhiro Yoshida. 2023. "Prevention of Root Caries Using Oxalic Acid" Materials 16, no. 4: 1454. https://doi.org/10.3390/ma16041454
APA StyleOguma, H., Matsuda, Y., Yoshihara, K., Okuyama, K., Sakurai, M., Saito, T., Inoue, S., & Yoshida, Y. (2023). Prevention of Root Caries Using Oxalic Acid. Materials, 16(4), 1454. https://doi.org/10.3390/ma16041454








