A Novel Sugar-Assisted Solvothermal Method for FeF2 Nanomaterial and Its Application in LIBs
Abstract
:1. Introduction
2. Experiment
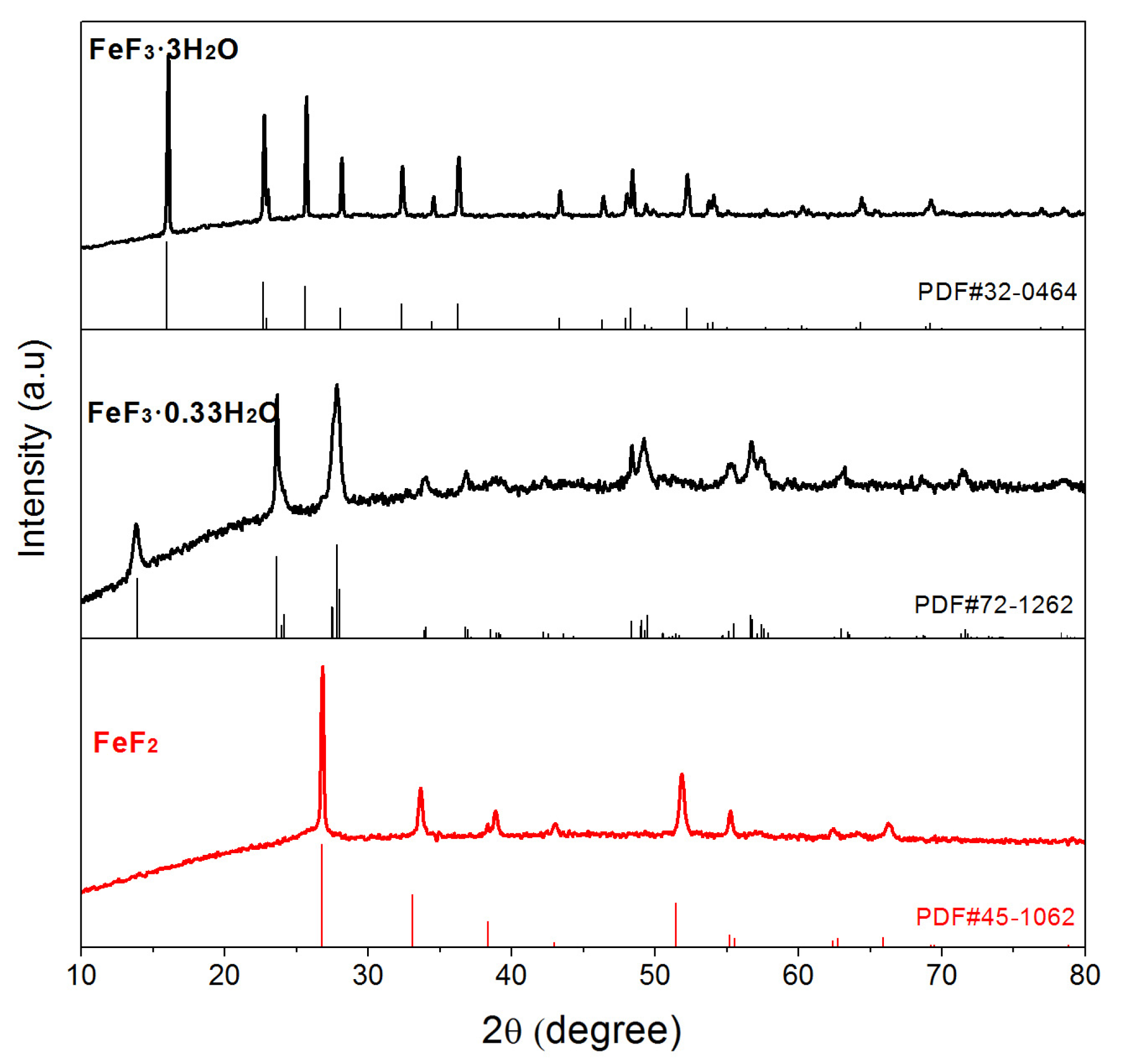
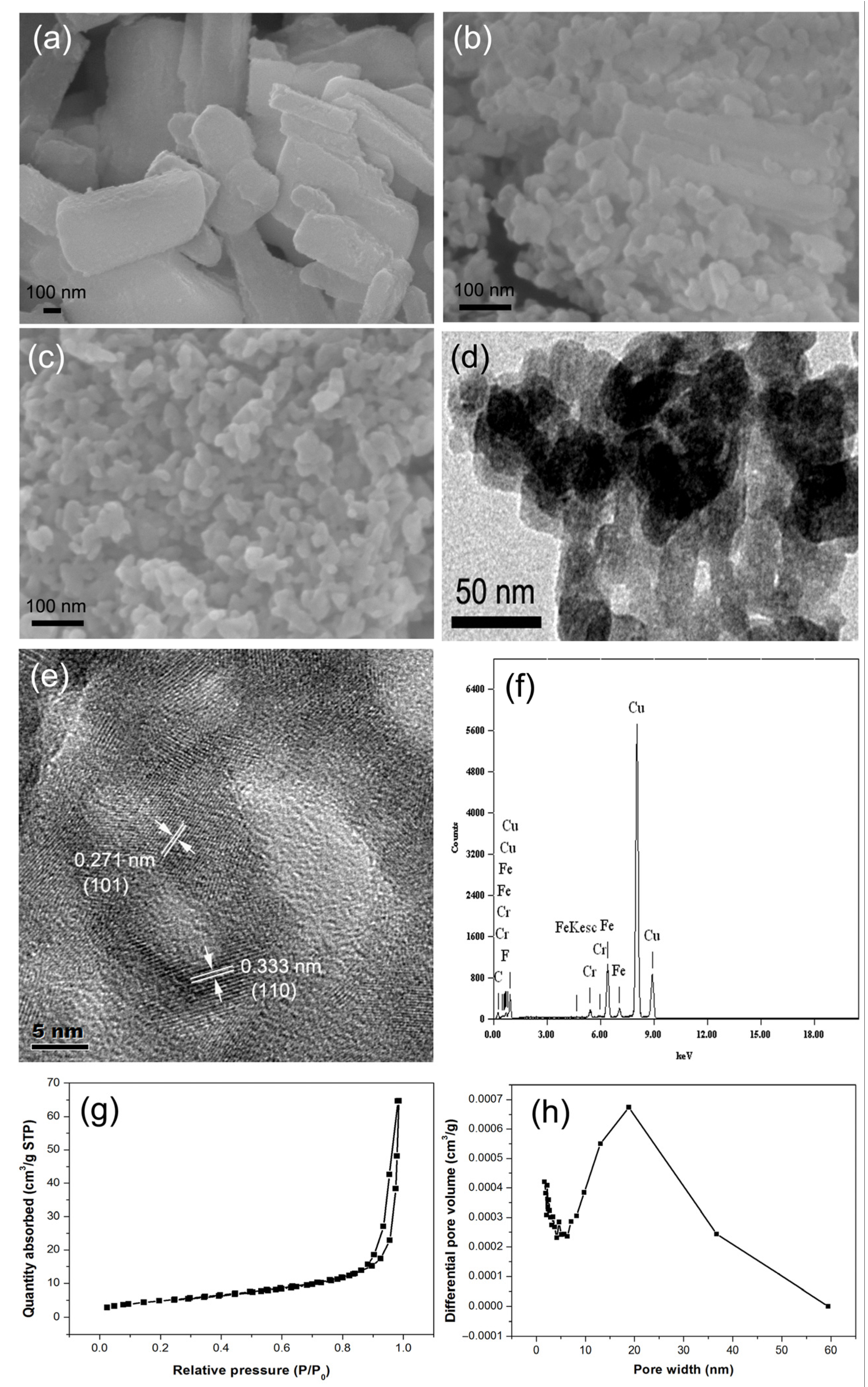
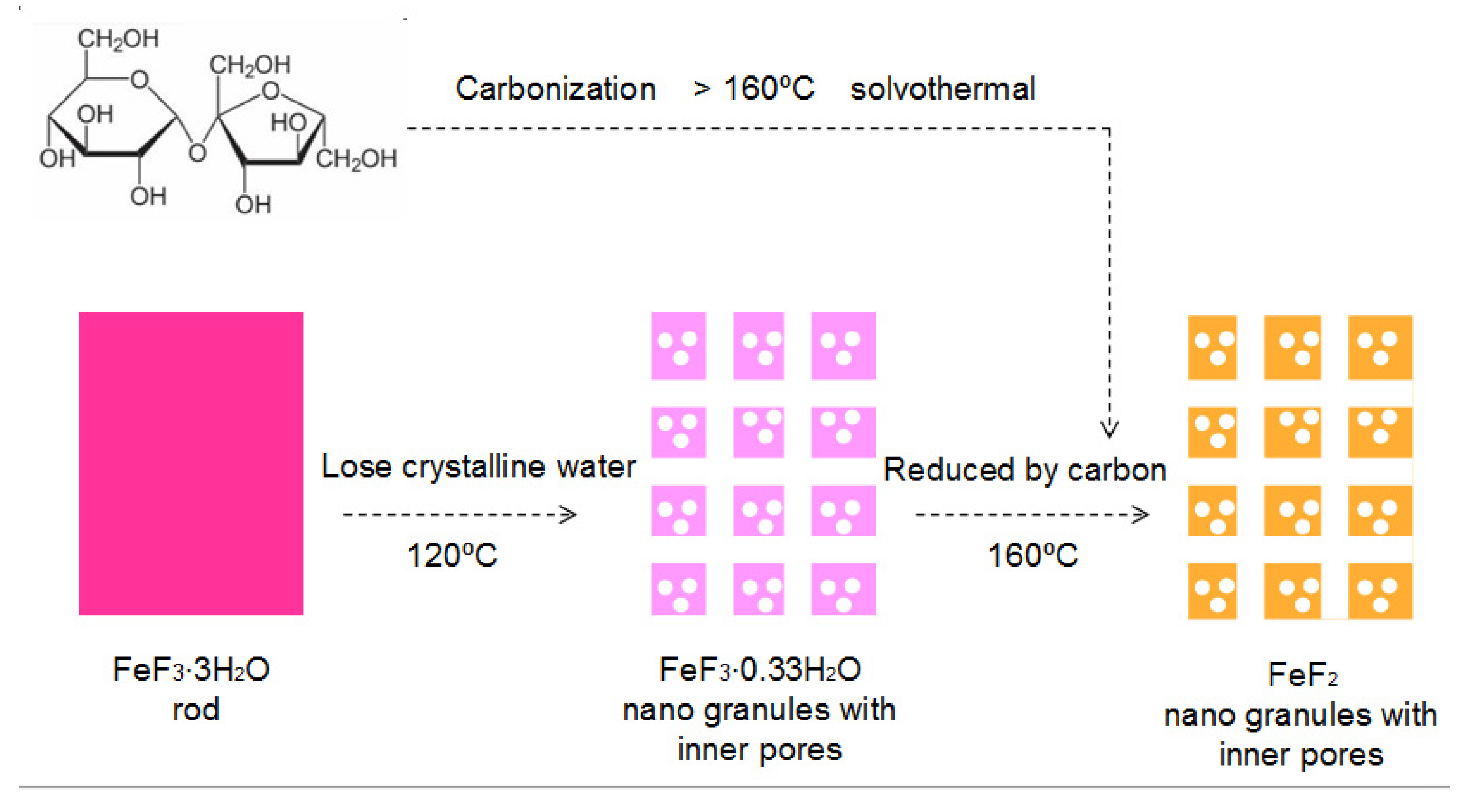
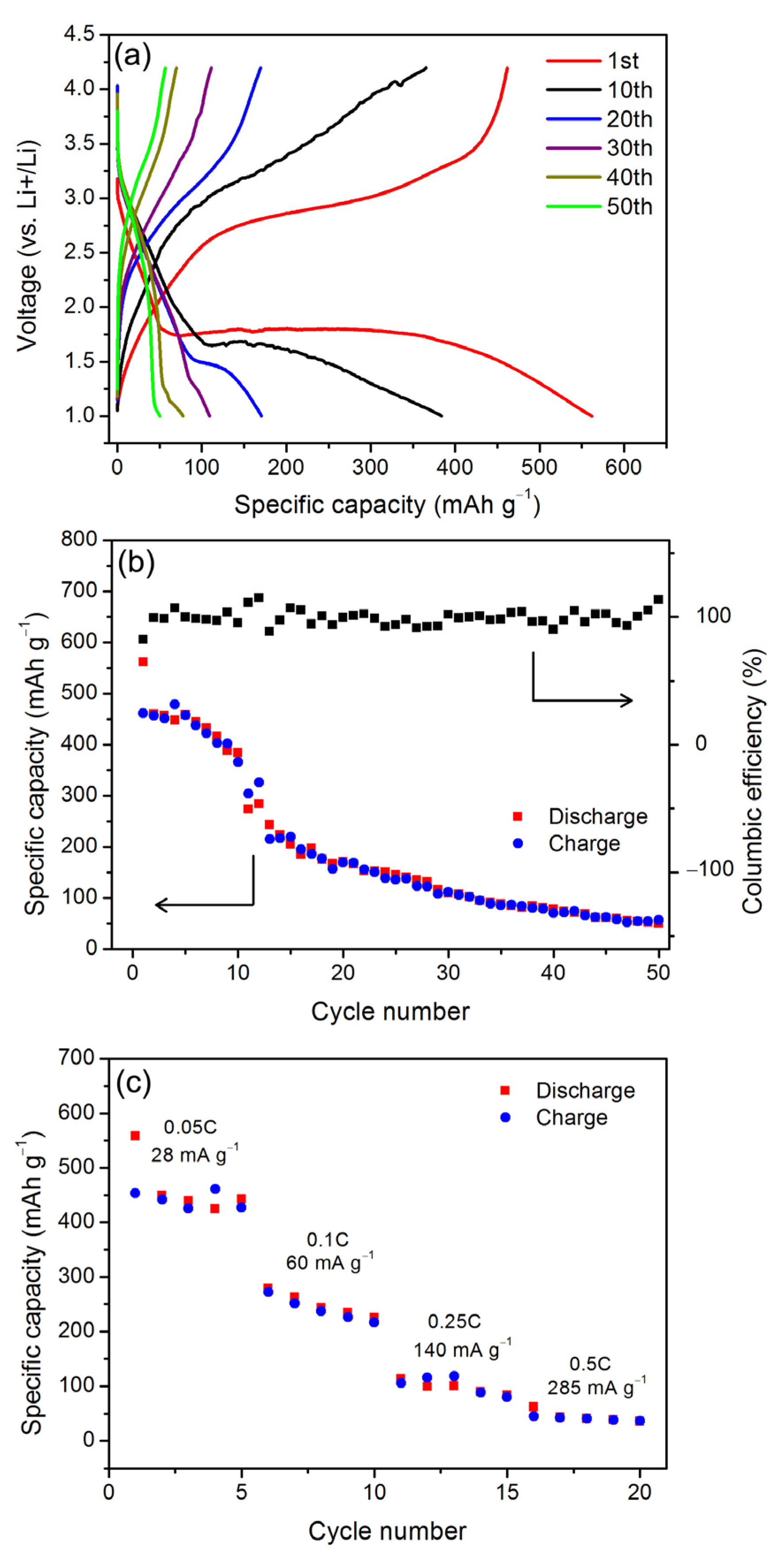
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Venkatesan, S.V.; Nandy, A.; Karan, K.; Larter, S.R.; Thangadurai, V. Recent Advances in the Unconventional Design of Electrochemical Energy Storage and Conversion Devices. Electrochem. Energy Rev. 2022, 5, 16. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, C.; Chen, K.; Wang, Y.; Liu, Q.; Liu, Y.; Ma, B.; Mi, L.; Mao, W. Perspectives on strategies and techniques for building robust thick electrodes for lithium-ion batteries. J. Power Sources 2022, 551, 232176. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wang, J.; Sheng, L.; Wang, L.; Xie, Y.; Hao, Y.; Dong, L.; He, X. Insight mechanism of nano iron difluoride cathode material for high-energy lithium-ion batteries: A review. J. Solid State Electrochem. 2022, 26, 2601–2626. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Su, Y.; Zheng, C.; Zhu, D.; Zhang, X.; Zhou, X.; Ouyang, R.; Huang, Q.; He, Y.; et al. Exploiting the Iron Difluoride Electrochemistry by Constructing Hierarchical Electron Pathways and Cathode Electrolyte Interface. Small 2022, 18, 2202006. [Google Scholar] [CrossRef]
- Jayasree, S.S.; Murali, A.S.; Nair, S.; Santhanagopalan, D. Recent progress on the low and high temperature performance of nanoscale engineered Li-ion battery cathode materials. Nanotechnology 2022, 33, 352001. [Google Scholar] [CrossRef]
- Ghosh, S.; Polaki, S.R.R.; Macrelli, A.; Casari, C.S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Nanoparticle-enhanced multifunctional nanocarbons-recent advances on electrochemical energy storage applications. J. Phys. D-Appl. Phys. 2022, 55, 413001. [Google Scholar] [CrossRef]
- Guntlin, C.P.; Kravchyk, K.V.; Erni, R.; Kovalenko, M.V. Transition metal trifluoroacetates (M = Fe, Co, Mn) as precursors for uniform colloidal metal difluoride and phosphide nanoparticles. Sci. Rep. 2019, 9, 6613. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Panneerselvam, A.; O’Regan, C.; Morris, M.A.; Holmes, J.D. Supercritical-fluid synthesis of FeF2 and CoF2 Li-ion conversion materials. J. Mater. Chem. A 2013, 1, 10667–10676. [Google Scholar] [CrossRef]
- Helen, M.; Fichtner, M.; Reddy, M.A. Electrochemical synthesis of carbon-metal fluoride nanocomposites as cathode materials for lithium batteries. Electrochem. Commun. 2020, 120, 106846. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, Z.; Wang, Z.; Liu, J.; Yan, H.; Peng, J.; Xu, L.; Guo, S.; Ju, S.; Chen, G. Synthesis of FeF2/carbon composite nanoparticle by one-pot solid state reaction as cathode material for lithium-ion battery. J. Mater. Res. Technol.-JmrT 2018, 7, 73–76. [Google Scholar] [CrossRef]
- Reddy, M.A.; Breitung, B.; Chakravadhanula, V.S.K.; Helen, M.; Witte, R.; Rongeat, C.; Kübel, C.; Hahn, H.; Fichtner, M. Facile synthesis of C-FeF2 nanocomposites from CFx: Influence of carbon precursor on reversible lithium storage. RSC Adv. 2018, 8, 36802–36811. [Google Scholar] [CrossRef]
- Reddy, M.A.; Breitung, B.; Wall, C.; Trivedi, S.; Chakravadhanula, V.S.K.; Helen, M.; Fichtner, M. Facile Synthesis of Carbon-Metal Fluoride Nanocomposites for Lithium-Ion Batteries. Energy Technol. 2016, 4, 201–211. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Zhang, X.; Song, H.; Chen, X. Carbon-Nanotube-Encapsulated FeF2 Nanorods for High-Performance Lithium-Ion Cathode Materials. ACS Appl. Mater. Interfaces 2014, 6, 21223–21229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Li, J.; Wen, L.; He, X. A one-pot approach towards FeF2-carbon core-shell composite and its application in lithium ion batteries. J. Alloy Compd. 2014, 606, 226–230. [Google Scholar] [CrossRef]
- Reddy, M.A.; Breitung, B.; Chakravadhanula, V.S.K.; Wall, C.; Engel, M.; Kübel, C.; Powell, A.K.; Hahn, H.; Fichtner, M. CFx Derived CarbonFeF2 Nanocomposites for Reversible Lithium Storage. Adv. Energy Mater. 2013, 3, 308–313. [Google Scholar] [CrossRef]
- Wygant, B.R.; Schorr, N.B.; Kolesnichenko, I.V.; Lambert, T.N. Nanoparticulate FeF2@C as a Li Battery Conversion Cathode. ACS Appl. Energy Mater. 2022, 5, 13346–13355. [Google Scholar] [CrossRef]
- Song, H.; Cui, H.; Wang, C. Extremely high-rate capacity and stable cycling of a highly ordered nanostructured carbon-FeF2 battery cathode. J. Mater. Chem. A 2015, 3, 22377–22384. [Google Scholar] [CrossRef]
- Gu, W.; Magasinski, A.; Zdyrko, B.; Yushin, G. Metal Fluorides Nanoconfined in Carbon Nanopores as Reversible High Capacity Cathodes for Li and Li-Ion Rechargeable Batteries: FeF2 as an Example (vol 5, 1401148, 2015). Adv. Energy Mater. 2015, 5, 1401148. [Google Scholar] [CrossRef]
- Pereira, N.; Badway, F.; Wartelsky, M.; Gunn, S.; Amatucci, G.G. Iron Oxyfluorides as High Capacity Cathode Materials for Lithium Batteries. J. Electrochem. Soc. 2009, 156, A407–A416. [Google Scholar] [CrossRef]
- Li, L.; Meng, F.; Jin, S. High-Capacity Lithium-Ion Battery Conversion Cathodes Based on Iron Fluoride Nanowires and Insights into the Conversion Mechanism. Nano Lett 2012, 12, 6030–6037. [Google Scholar] [CrossRef]
- Baruah, P.; Das, B.K.; Bora, M.; Saikia, B.K. Hydrothermally prepared sugar-derived carbon spheres for all-solid-state symmetric electrochemical capacitors. Mater. Today Commun. 2022, 33, 104219. [Google Scholar] [CrossRef]
- Balda, M.; Mackenzie, K.; Kopinke, F.-D.; Georgi, A. Uniform and dispersible carbonaceous microspheres as quasi-liquid sorbent. Chemosphere 2022, 307, 136079. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xiong, H.; Dong, H.; Liu, Y.; Lu, Y.; Liu, K.; Wang, J. Carbon thermal reduction of waste ternary cathode materials and wet magnetic separation based on Ni/MnO nanocomposite particles. Process Saf. Environ. Prot. 2022, 165, 278–285. [Google Scholar] [CrossRef]
- Li, Y.; Yao, S.; Zhang, C.; He, Y.; Wang, Y.; Liang, Y.; Shen, X.; Li, T.; Qin, S.; Wen, W. Molybdenum carbide nanocrystals modified carbon nanofibers as electrocatalyst for enhancing polysulfides redox reactions in lithium-sulfur batteries. Int. J. Energy Res. 2020, 44, 8388–8398. [Google Scholar] [CrossRef]
- Kumar, M.M.; Raj, C. Carbothermal-Reduction-Assisted Phosphidation of Cobalt Affords Mesoporous Nitrogen-Doped Carbon-Embedded CoP Nanoelectrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2019, 2, 643–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Li, J.; He, X.; Wen, L.; Gao, J.; Liu, H.; Zhang, Y.; Zhao, P. Electrochemical Performance of FeF3 center dot 0.33H(2)O/MWCNTs Composite Cathode Synthesized by Solvothermal Process. J. New Mater. Electrochem. Syst. 2015, 18, 103–109. [Google Scholar] [CrossRef]
- Xiao, A.W.; Lee, H.J.; Capone, I.; Robertson, A.; Wi, T.U.; Fawdon, J.; Wheeler, S.; Lee, H.W.; Grobert, N.; Pasta, M. Understanding the conversion mechanism and performance of monodisperse FeF2 nanocrystal cathodes. Nat. Mater. 2020, 19, 644–654. [Google Scholar] [CrossRef]
- Sina, M.; Thorpe, R.; Rangan, S.; Pereira, N.; Bartynski, R.A.; Amatucci, G.G.; Cosandey, F. Investigation of SEI Layer Formation in Conversion Iron Fluoride Cathodes by Combined STEM/EELS and XPS. J. Phys. Chem. C 2015, 119, 9762–9773. [Google Scholar] [CrossRef]
- Su, Y.; Chen, J.; Li, H.; Sun, H.; Yang, T.; Liu, Q.; Ichikawa, S.; Zhang, X.; Zhu, D.; Zhao, J.; et al. Enabling Long Cycle Life and High Rate Iron Difluoride Based Lithium Batteries by In Situ Cathode Surface Modification. Adv. Sci. 2022, 9, 2201419. [Google Scholar] [CrossRef]
| One-Step Method | ||||||
|---|---|---|---|---|---|---|
| Precursors | Synthesis Process | Final Product (FeF2) | ||||
| Fe(CO)5 + PVDF | Ar 500 °C | FeF2@C nanorod [14] | ||||
| Fe(CO)5 + CFx | Ar 250 °C | FeF2-C nanocomposite [15] | ||||
| Fe(ClO4)2·xH2O + CFx | Percolate in CAN, electrochemical | FeF2-C nanocomposite [9] | ||||
| Two-Step Method | ||||||
| Precursors | Synthesis Process | Intermediate Phase | Synthesis Process | Final Product (FeF2) | ||
| Fe + H2SiF6 | Liquid synthesis | FeSiF6·6H2O | Ar 250 °C | FeF2 nanoparticle (20 nm) [19] | ||
| Fe + H2SiF6 | Liquid synthesis | FeSiF6·6H2O solution, vacuum infiltration, porous carbon | Ar 250 °C | FeF2 in porous carbon [18] | ||
| HF + Fe(NO3)3·9H2O | Liquid synthesis | FeF3·3H2O | Ar 350 °C | FeF3 + FeF2 (few) [20] | ||
| FeCl3 solution + CMK-3 | hydrothermal | Fe2O3-CMK-3 + HF | seal | FeF3·3H2O-CMK-3 | N2 300 °C | FeF2-CMK-3 [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Q.; He, X.; Wang, L.; Wang, J.; Dong, L.; Xie, Y.; Hao, Y. A Novel Sugar-Assisted Solvothermal Method for FeF2 Nanomaterial and Its Application in LIBs. Materials 2023, 16, 1437. https://doi.org/10.3390/ma16041437
Zhang Y, Zhang Q, He X, Wang L, Wang J, Dong L, Xie Y, Hao Y. A Novel Sugar-Assisted Solvothermal Method for FeF2 Nanomaterial and Its Application in LIBs. Materials. 2023; 16(4):1437. https://doi.org/10.3390/ma16041437
Chicago/Turabian StyleZhang, Yanli, Qiang Zhang, Xiangming He, Li Wang, Jingxin Wang, Liangliang Dong, Yingpeng Xie, and Yongsheng Hao. 2023. "A Novel Sugar-Assisted Solvothermal Method for FeF2 Nanomaterial and Its Application in LIBs" Materials 16, no. 4: 1437. https://doi.org/10.3390/ma16041437
APA StyleZhang, Y., Zhang, Q., He, X., Wang, L., Wang, J., Dong, L., Xie, Y., & Hao, Y. (2023). A Novel Sugar-Assisted Solvothermal Method for FeF2 Nanomaterial and Its Application in LIBs. Materials, 16(4), 1437. https://doi.org/10.3390/ma16041437








