Efficiency of Diclofenac Removal Using Activated Sludge in a Dynamic System (SBR Reactor) with Variable Parameters of pH, Concentration, and Sludge Oxygenation
Abstract
:1. Introduction
2. Materials and Methods
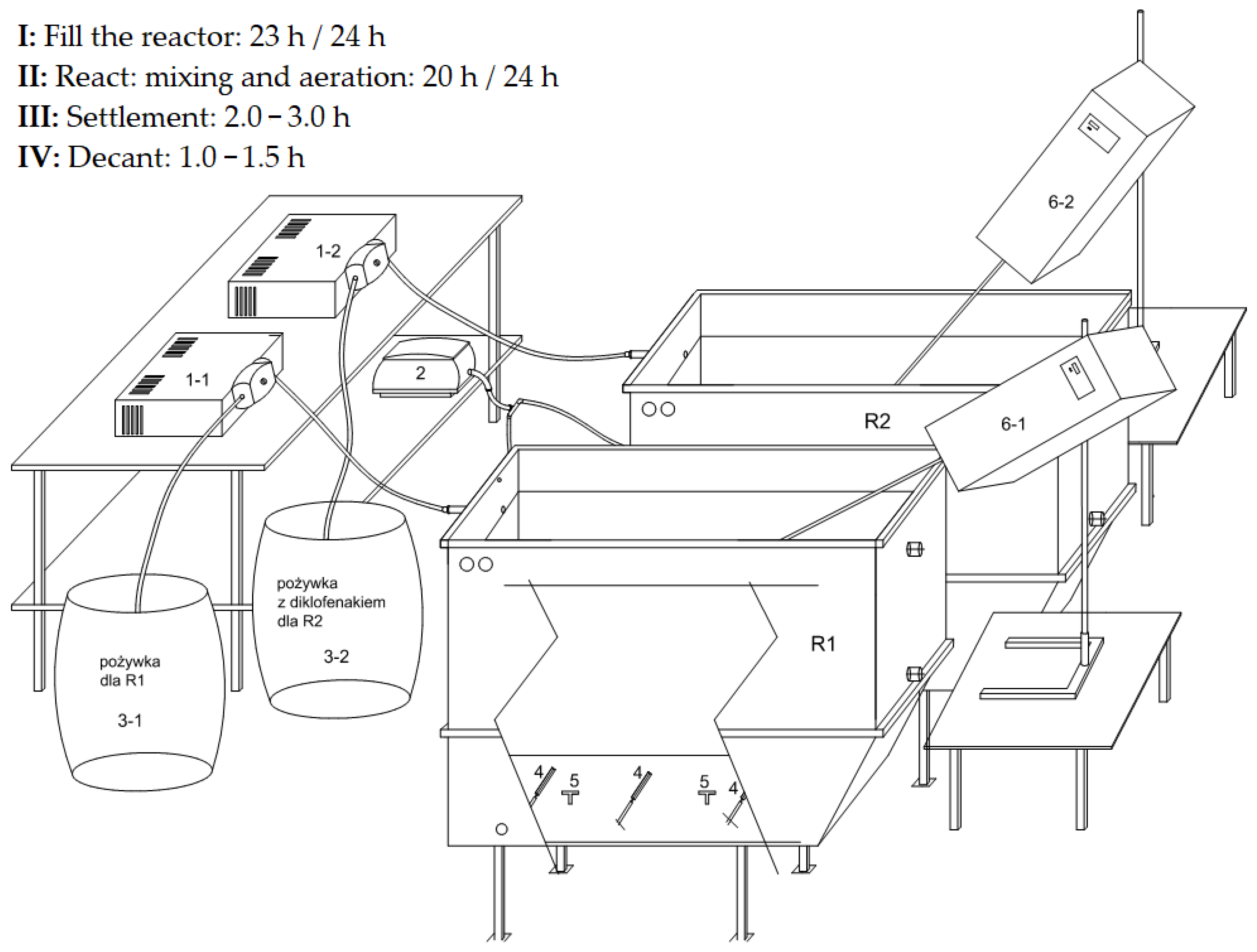
2.1. Construction of the Test Stand
2.2. Operation of the Test Stand and Equipment
2.3. Cycles of the Dynamic Test
2.4. The Composition of the Used Medium
2.5. LC-MS/MS Analysis
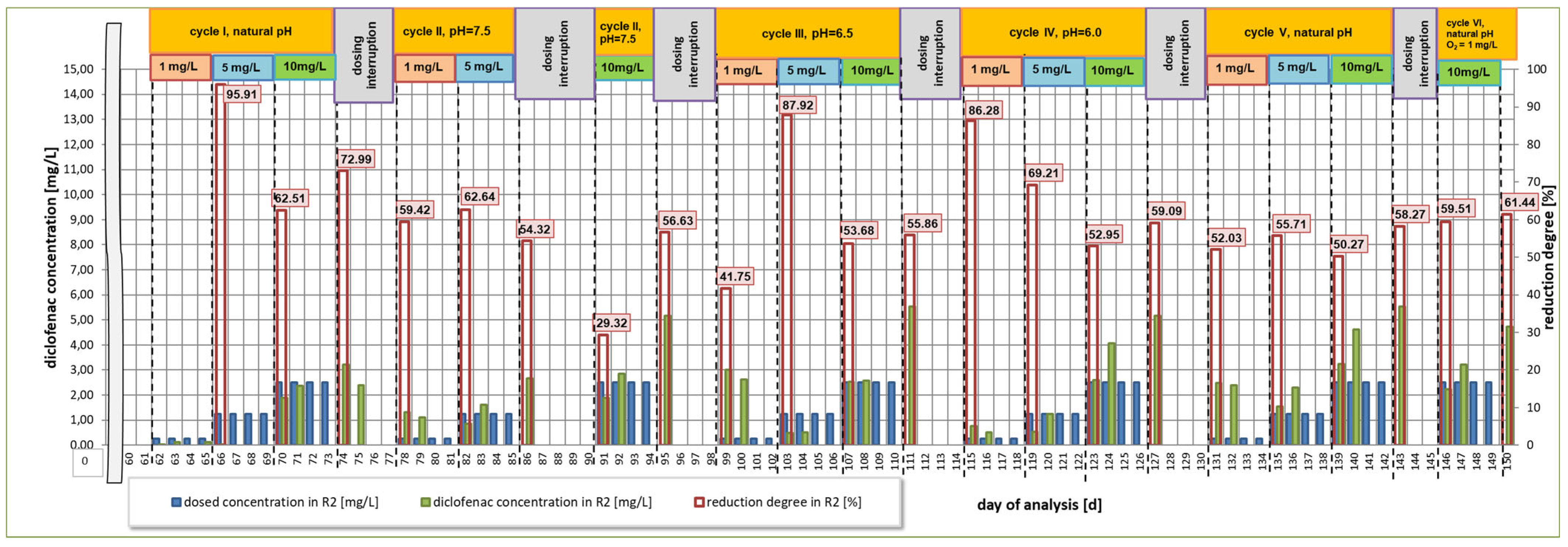
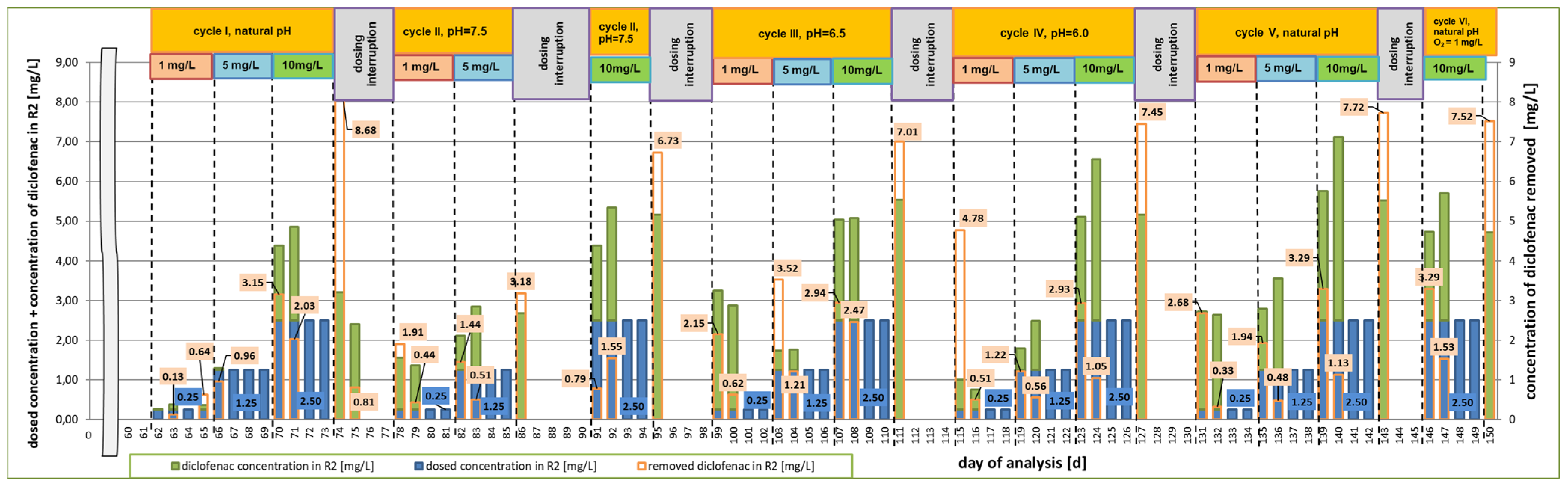
3. Results
3.1. First Research Cycle
3.2. Second Research Cycle
3.3. Third Research Cycle
3.4. Fourth Research Cycle
3.5. Fifth Research Cycle
3.6. Statistical Analysis
- Comparison of the test results of selected parameters, determined throughout the period of diclofenac dosing (i.e., from 62 to 150 days) in the R2 reactor, with the results determined for these parameters in the R1 reactor;
- Comparison of the test results of selected parameters for each research cycle, including the dosing of three concentrations of diclofenac to the R2 reactor (i.e., cycles I–V) in relation to individual research series carried out in parallel in the R1 reactor;
- Comparison of the test results for each of the selected parameters analyzed in the first cycle (adopted as a model) in relation to the remaining second to fifth research cycles, in the R2 reactor, during the dosing of diclofenac.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identifed by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Lapworth, D.J.; Civil, W.; Williams, P. Tracking changes in the occurrence and source of pharmaceuticals within the River Tames, UK; from source to sea. Environ. Pollut. 2019, 249, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kołecka, K.; Gajewska, M.; Caban, M. From the pills to environment—Prediction and tracking of non-steroidal an-ti-inflammatory drug concentrations in wastewater. Sci. Total Environ. 2022, 825, 153611. [Google Scholar] [CrossRef] [PubMed]
- Diclofenac. Drug Usage Statistics ClinCalc. Available online: https://clincalc.com/DrugStats/Drugs/Diclofenac (accessed on 5 December 2022).
- Polish Register of Medicinal Products. Available online: https://rejestrymedyczne.ezdrowie.gov.pl/rpl/search/public (accessed on 5 December 2022).
- Available online: https://www.industryarc.com/Research/Diclofenac-Market-Research-502786 (accessed on 15 January 2023).
- Available online: https://www.ncbi.nlm.nih.gov/books/NBK557879/ (accessed on 15 January 2023).
- Mohamed, S.E.; Hussein, D.S.; Al-khattaf, F.S.; El-Naggar, R.A.R.; Almaary, K.A. Diclofenac removal from the wastewater using activated sludge and analysis of multidrug resistant bacteria from the sludge. Environ. Res. 2022, 208, 112723. [Google Scholar]
- Styszko, K.; Durak, J.; Kończak, B.; Głodniok, M.; Borgulat, A. The impact of sewage sludge processing on the safety of its use. Sci. Rep. 2022, 12, 12227. [Google Scholar] [CrossRef]
- Linson Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Envi-ronmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Matejczyk, M. Toksyczność Wybranych Farmaceutyków i ich Mieszanin w Ściekach w Stosunku do Escherichia coli; Oficyna Wydawnicza Politechniki Białostockiej: Bialystok, Poland, 2022; ISBN 978-83-67185-25-7. [Google Scholar]
- Al-Kindi, G.Y.; al Ani, F.H.; Al-Bidri, N.K.; Alhaidri, H.A. Diclofenac Removal from Wastewater by Activated Carbon. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Baghdad, Iraq; Istanbul, Turkey, 2021; Volume 779. [Google Scholar]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic effects of NSAIDs in non-target species: A review from the perspective of the aquatic environment. Environ. Pollut. 2021, 273, 115891. [Google Scholar] [CrossRef]
- Harshkova, D.; Aksmann, A. Zanieczyszczenie środowiska niesteroidowymi lekami przeciwzapalnymi na przykładzie diklofenaku—Przyczyny, skutki, bioindykacja. Kosmos 2019, 68, 185–194. [Google Scholar] [CrossRef]
- European Commission Brussels. 26.10.2022 COM(2022) 540 Final 2022/0344 (COD) Proposal for a Directive of The European Parliament and of The Council amending Directive 2000/60/EC Establishing a Framework for Community Action in the Field of Water Policy, Directive 2006/118/EC on the Protection of Groundwater against Pollution and Deterioration and Directive 2008/105/EC on Environmental Quality Standards in the Field of Water Policy. Available online: https://environment.ec.europa.eu (accessed on 15 January 2023).
- European Commission Brussels. 26.10.2022 COM(2022) 541 Final 2022/0345 (COD) Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast). Available online: https://environment.ec.europa.eu (accessed on 15 January 2023).
- Decree No. 28 of 2004 (XII. 25.) KvVM of the Ministry of Environmental Protection and Water Management Concerning Emission Standards of Water-Pollutant Substances and Laying Down Rules of Application. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC124621/ (accessed on 15 January 2023).
- Bungau, S.; Suciu, R.; Bumbu, A.; Cioca, G.; Tit, D.M. Study on hospital waste management in medical rehabilitation clinical hospital, Baile Felix. J. Environ. Prot. Ecol. 2015, 16, 980–987. [Google Scholar]
- Rodríguez-Serin, H.; Gamez-Jara, A.; De La Cruz-Noriega, M.; Rojas-Flores, S.; Rodriguez-Yupanqui, M.; Cardenas, M.G.; Cruz-Monzon, J. Literature Review: Evaluation of Drug Removal Techniques in Municipal and Hospital Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 13105. [Google Scholar] [CrossRef]
- Tiwari, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Vaudreuil, M.A.; Sauvé, S.; Buelna, G.; Dubé, R. Fate of Pharmaceuticals in a Submerged Membrane Bioreactor Treating Hospital Wastewater. Front. Water 2021, 3, 730479. [Google Scholar] [CrossRef]
- Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-N/part-439 (accessed on 15 January 2023).
- Leverett, D.; Merrington, G.; Crane, M.; Ryan, J.; Wilson, I. Environmental quality standards for diclofenac derived under the European Water Framework Directive: 1. Aquatic organisms. ESEU 2021, 33, 133. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council, Official Journal of the European Union, L 78/40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495 (accessed on 15 January 2023).
- Gomez Cortes, L.; Marinov, D.; Sanseverino, I.; Navarro Cuenca, A.; Niegowska, M.; Porcel Rodriguez, E.; Lettieri, T. Selection of substances for the 3rd Watch List under the Water Framework Directive; EUR 30297 EN; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-19426-2. [Google Scholar] [CrossRef]
- Communication From the Commission To The European Parliament, The Council and The European Economic and Social Committee. European Union Strategic Approach to Pharmaceuticals in the Environment, European Commission, Brussels, 11.3.2019, COM 2019 128 Final. Available online: https://eur-lex.europa.eu (accessed on 15 January 2023).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy Official Journal of the European Union, L 435/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013L0039&from=PL (accessed on 15 January 2023).
- Directive (EU) 2020/2184 of the European Parliament and of the Council, Official Journal of the European Union, EN, L 435/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 15 January 2023).
- COMMISSION IMPLEMENTING DECISION (EU) 2022/679 of 19 January 2022 Establishing a Watch List of Substances and Compounds of Concern for Water Intended for Human Consumption as Provided for in Directive (EU) 2020/2184 of the European Parliament and of the Council, Official Journal of the European Union, EN, L 124/41. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022D0679 (accessed on 15 January 2023).
- Kawase, A.; Hashimoto, R.; Shibata, M.; Shimada, H.; Iwaki, M. Involvement of Reactive Metabolites of Diclofenac in Cytotoxicity in Sandwich-Cultured Rat Hepatocytes. Int. J. Toxicol. 2017, 36, 260–267. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Klopčič, I.; Markovič, T.; Mlinarič-Raščan, I.; Dolenc, M.S. Endocrine disrupting activities and immunomodulatory effects in lymphoblastoid cell lines of diclofenac, 4-hydroxydiclofenac and paracetamol. Toxicol. Lett. 2018, 294, 95–104. [Google Scholar] [CrossRef]
- Memmert, U.; Peither, A.; Burri, R.; Weber, K.; Schmidt, T.; Sumpter, J.P.; Hartmann, A. Diclofenac: New data on chronic toxicity and bioconcentration in fish. Environ. Toxicol. Chem. 2013, 32, 442–452. [Google Scholar] [CrossRef]
- Balbi, T.; Montagna, M.; Fabbri, R.; Carbone, C.; Franzellitti, S.; Fabbri, E.; Canesi, L. Diclofenac affects early embryo development in the marine bivalve Mytilus galloprovincialis. Sci. Total. Environ. 2018, 642, 601–609. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ. Monit. Assess. 2017, 189, 348–359. [Google Scholar] [CrossRef]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Aleman, R.M.; Acena, J.; Barcelo, D.; Lopez de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.-P. Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Tran, N.H.; Gin, K.Y. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine dis-rupters in a full-scale water reclamation plant. Sci. Total Environ. 2017, 599–600, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total. Environ. 2020, 698, 134057. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, K.; Aras, E. Evaluation of the Removal Efficiency of Diclofenac in the Aquatic Environment by Combined Coagula-tion and Adsorption Processes. JOTCSB 2022, 5, 173–182. [Google Scholar]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Cherik, D.D.; Benali, M.; Louhab, K. Occurrence, ecotoxicology, removal of diclofenac by adsorption on activated carbon and biodegradation and its effect on bacterial community: A review. WSN 2015, 16, 96–124. [Google Scholar]
- Zhang, H.; Tu, Y.-J.; Duan, Y.-P.; Liu, J.; Zhi, W.; Tang, Y.; Xiao, L.-S.; Meng, L. Production of biochar from waste sludge/leaf for fast and efficient removal of diclofenac. J. Mol. Liq. 2020, 299, 112193. [Google Scholar] [CrossRef]
- Angosto, J.M.; Roca, M.J.; Fernández-López, J.A. Removal of Diclofenac in Wastewater Using Biosorption and Advanced Oxidation Techniques: Comparative Results. Water 2020, 12, 3567. [Google Scholar] [CrossRef]
- Ghorbani, L.; Caschera, D.; Shokri, B. Effect of Oxygen Plasma Pre-Treatment on the Surface Properties of Si-Modified Cotton Membranes for Oil/Water Separations. Materials 2022, 15, 8551. [Google Scholar] [CrossRef]
- Alobaidi, R.A.K.; Ulucan-Altuntas, K.; Mhemid, R.K.S.; Manav-Demir, N.; CINAR, O. Biodegradation of Emerging Pharma-ceuticals from Domestic Wastewater by Membrane Bioreactor: The Effect of Solid Retention Time. Int. J. Environ. Res. Public Health 2021, 18, 3395. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A review on pharmaceuticals re-moval from waters by single and combined biological, membrane filtration and ultrasound systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Y.; Song, Q.; Wang, L.; Luo, T.; Gao, C.; Liu, L.; Yang, S. Removal of Pharmaceuticals and Personal Care Products (PPCPs) by Free Radicals in Advanced Oxidation Processes. Materials 2022, 15, 8152. [Google Scholar] [CrossRef]
- Jabbari, F.; Eslami, A.; Mahmoudian, J. Degradation of Diclofenac in Water Using the O3/UV/S2O8 Advanced Oxidation Process. Health Scope 2020, 9, e99436. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, N.; Dai, C.; Duan, Y.; Tu, Y. Zero-Valent Iron Nanoparticles and Its Combined Process for Diclofenac Degradation under Various Experimental Conditions. Pol. J. Environ. Stud. 2021, 30, 1279–1288. [Google Scholar] [CrossRef]
- Nabgan, W.; Jalil, A.A.; Nabgan, B.; Ikram, M.; Ali, M.W.; Kumar, A.; Lakshminarayana, P. A state of the art overview of carbon-based composites applications for detecting and eliminating pharmaceuticals containing wastewater. Chemosphere 2022, 288, 132535. [Google Scholar] [CrossRef]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Yun, J.; Zhang, G.; Zabed, H.M.; Qi, X. A review on constructed wetlands-based removal of pharmaceutical contaminants derived from non-point source pollution. Environ. Technol. Innov. 2022, 26, 102504. [Google Scholar] [CrossRef]
- Ilyas, H.; Masih, I.; van Hullebusch, E.D. Pharmaceuticals’ removal by constructed wetlands: A critical evaluation and meta-analysis on performance, risk reduction, and role of physicochemical properties on removal mechanisms. J. Water Health 2020, 18, 253–291. [Google Scholar] [CrossRef]
- Xu, W.; Zou, R.; Jin, B.; Zhang, G.; Su, Y.; Zhang, Y. The ins and outs of pharmaceutical wastewater treatment by microbial electrochemical technologies. Sustain. Horiz. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Stadlmair, L.F.; Letzel, T.; Drewes, J.E.; Grassmann, J. Enzymes in removal of pharmaceuticals from wastewater: A critical review of challenges, applications and screening methods for their selection. Chemosphere 2018, 205, 649–661. [Google Scholar] [CrossRef]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T.; Pinelo, M.; Meyer, A.S.; Iqbal, H.M.; Bilal, M.; Nguyen, L.N.; Nghiem, L.D. Free and immobilized biocatalysts for removing micropollutants from water and wastewater: Recent progress and challenges. Bioresour. Technol. 2022, 344, 126201. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Kakade, A.; Yu, Z.; Khan, A.; Liu, P.; Li, X. Anaerobic membrane bioreactors for treatment of emerging contaminants: A review. J. Environ. Manag. 2020, 270, 110913. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, M. A Study of Pharmaceutical Residues in Wastewater from Small Municipalities in Northern Sweden: E-peroxone as a Complementary Tertiary Removal Technique. Master’s Thesis, University of Umea, Umeå, Sweden, 2021. [Google Scholar]
- Rios-Miguel, A.B.; Jetten, M.S.M.; Welte, C.U. Effect of concentration and hydraulic reaction time on the removal of phar-maceutical compounds in a membrane bioreactor inoculated with activated sludge. Microb. Biotechnol. 2021, 14, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Timis, D.; Taschina, M.; Copolovici, D.; Cioca, G.; Bungau, S. Diclofenac Influence on Photosynthetic Parameters and Volatile Organic Compounds Emision from Phaseolus vulgaris L. Plants Rev. Chim. 2017, 68, 2076–2078. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2022) 5098) (Text with EEA Relevance), Official Journal of the European Union, L197/117. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022D1307 (accessed on 15 January 2023).
- Ozdemir, G.; Aydin, E.; Topuz, E.; Yangin-Gomec, C.; Tas, D.O. Acute and chronic responses of denitrifying culture to diclofenac. Bioresour. Technol. 2015, 176, 112–120. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. Chem. 2007, 387, 1379–1389. [Google Scholar] [CrossRef]
- Okońska, M.; Marciniak, M.; Zembrzuska, J.; Kaczmarek, M. Laboratory investigations of diclofenac migration in saturated porous media—A case study. Geologos 2019, 25, 213–223. [Google Scholar] [CrossRef]
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of selected pharmaceuticals by biosolids from municipal wastewater treatment plants: Importance of modest pH change and degree of mineralization. Water Sci. Technol. 2010, 62, 1084–1089. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 15 January 2023).
- Deng, Y.; Li, B.; Yu, K.; Zhang, T. Biotransformation and adsorption of pharmaceutical and personal care products by activated sludge after correcting matrix effects. Sci. Total. Environ. 2016, 544, 980–986. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, X.; Lin, W.; Yang, C.; Ren, Y. Adsorption behavior of diclofenac-containing wastewater on three kinds of sewage sludge. Water Sci. Technol. 2019, 80, 717–726. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef]
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathways and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005, 39, 2654–2664. [Google Scholar] [CrossRef]
- Anumol, T.; Vijayanandan, A.; Park, M.; Philip, L.; Snyder, S.A. Occurrence and fate of emerging trace organic chemicals in wastewater plants in Chennai, India. Environ. Int. 2016, 92–93, 33–42. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Q.; Zhang, Q.; Ma, L.; Tu, B.; Li, H.; Zhou, Y. Removal of clofibric acid and diclofenac during anaerobic digestion of sewage sludge. Environ. Prot. Eng. 2013, 39, 63–77. [Google Scholar] [CrossRef]
- Lara-Pérez, C.; Leyva, E.; Zermeño, B.; Osorio, I.; Montalvo, C.; Moctezuma, E. Photocatalytic degradation of diclofenac sodium salt: Adsorption and reaction kinetic studies. Environ. Earth Sci. 2020, 79, 277. [Google Scholar] [CrossRef]
- Shi, X.; Leong, K.Y.; Ng, H.Y. Anaerobic treatment of pharmaceutical wastewater: A critical review. Bioresour. Technol. 2017, 245, 1238–1244. [Google Scholar] [CrossRef]
- Bezsenyi, A.; Sági, G.; Makó, M.; Palkó, G.; Tóth, T.; Wojnárovits, L.; Takács, E. The effect of combined cometabolism and gamma irradiation treatment on the biodegradability of diclofenac and sulfamethoxazole. Radiat. Phys. Chem. 2020, 170, 108642. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]


| Cycle Number | Description of the Performed Activities | Established Working Parameters of the System | |
|---|---|---|---|
| 0 | Development and adaptation of activated sludge to laboratory conditions, as well as ensuring constant operating conditions of the system at a specific temperature. | T (°C) pH (-) O2 (mg/L) SRT 1 (d)HRT 2 (d) | 20 ± 2 °C pH: 6.5–7.5 2 ± 1 mg O2/L 12 d 4 d |
| I | Analysis of diclofenac removal efficiency for a specific concentration (CDCL—mg/L). Maintenance of specified operating conditions in both reactors. Dosing of the medium to reactors. | T (°C) pH (-) O2 (mg/L) SRT | 20 ± 2 °C pH: 7.0–7.5 (natural) 2 ± 1 mg O2/L 12 d |
| HRT (d)/(CDCL—mg/L) time period (d) | 4 d for 1 mg/L/5 mg/L/10 mg/L 12 d | ||
| II | Analysis of diclofenac removal efficiency for a specific concentration (CDCL—mg/L). Maintenance of specified operating conditions in both reactors. Dosing of the medium to reactors. | T (°C) pH (-) O2 (mg/L) SRT | 20 ± 2 °C pH: 7.5 2 ± 1 mg O2/L 12 d |
| HRT (d)/(CDCL—mg/L) time period (d) | 4 d for 1 mg/L/5 mg/L/10 mg/L12 d | ||
| III | Analysis of diclofenac removal efficiency for a specific concentration (CDCL—mg/L). Maintenance of specified operating conditions in both reactors. Dosing of the medium to reactors. | T (°C) pH (-) O2 (mg/L) SRT | 20 ± 2 °C pH: 6.5 (natural) 2 ± 1 mg O2/L 12 d |
| HRT (d)/(CDCL—mg/L) time period (d) | 4 d for 1 mg/L/5 mg/L/10 mg/L12 d | ||
| IV | Analysis of diclofenac removal efficiency for a specific concentration (CDCL—mg/L). Maintenance of specified operating conditions in both reactors. Dosing of the medium to reactors. | T (°C) pH (-) O2 (mg/L) SRT | 20 ± 2 °C pH: 6.0 (natural) 2 ± 1 mg O2/L 12 d |
| HRT (d)/(CDCL—mg/L) time period (d) | 4 d for 1 mg/L/5 mg/L/10 mg/L12 d | ||
| V | Analysis of diclofenac removal efficiency for a specific concentration (CDCL—mg/L). This cycle assumed the analysis of the effectiveness of diclofenac removal, with the amount of carbon in the starting medium reduced by 5%. | T (°C) pH (-) O2 (mg/L) SRT | 20 ± 2 °C pH: natural 2 ± 1 mg O2/L 12 d |
| HRT (d)/(CDCL—mg/L) time period (d) | 4 d for 1 mg/L/5 mg/L/10 mg/L12 d | ||
| VI | Analysis of diclofenac removal efficiency for a specific concentration (CDCL—mg/L).Reduction of oxygen concentration in reactors. Dosing of the medium to reactors. | T (°C) pH (-) O2 (mg/L) time period (d) | 20 ± 2 °C pH: natural 0.5–1 mg O2/L 4 d |
| HRT (d)/(CDCL—mg/L) | 4 d/10 mg/L | ||
| Component | Concentration [mg/L Medium] |
|---|---|
| Casein peptone (CAS:91079-40-2) Sigma-Aldrich/Merck (Darmstadt, Germany) | 160 |
| Meat extract (70164), Fluka Chemie (Buchs, Switzerland) | 110 |
| Urea CO(NH2)2 (CAS 57-13-6) Chempur (Piekary Śląskie, Polska) | 30 |
| K2HPO4 (CAS 7758-11-4) Chempur (Piekary Śląskie, Polska) | 28 |
| NaCl (CAS-7647-14-5) Chempur (Piekary Śląskie, Polska) | 11 |
| Glucose—C6H1206 (CAS 50-99,7) Chempur (Piekary Śląskie, Polska) | 114 |
| Diclofenac sodium salt C14H10Cl2NNaO2, (CAS:15307-79-6) Sigma-Aldrich/Merck (Darmstadt, Germany) | Dosed into the R2 reactor in the amount of 1, 5, or 10 mg/L |
| distilled water | refill to 1 L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając-Woźnialis, A.; Kruszelnicka, I.; Zembrzuska, J.; Ginter-Kramarczyk, D.; Ochowiak, M.; Krupińska, A. Efficiency of Diclofenac Removal Using Activated Sludge in a Dynamic System (SBR Reactor) with Variable Parameters of pH, Concentration, and Sludge Oxygenation. Materials 2023, 16, 1422. https://doi.org/10.3390/ma16041422
Zając-Woźnialis A, Kruszelnicka I, Zembrzuska J, Ginter-Kramarczyk D, Ochowiak M, Krupińska A. Efficiency of Diclofenac Removal Using Activated Sludge in a Dynamic System (SBR Reactor) with Variable Parameters of pH, Concentration, and Sludge Oxygenation. Materials. 2023; 16(4):1422. https://doi.org/10.3390/ma16041422
Chicago/Turabian StyleZając-Woźnialis, Anna, Izabela Kruszelnicka, Joanna Zembrzuska, Dobrochna Ginter-Kramarczyk, Marek Ochowiak, and Andżelika Krupińska. 2023. "Efficiency of Diclofenac Removal Using Activated Sludge in a Dynamic System (SBR Reactor) with Variable Parameters of pH, Concentration, and Sludge Oxygenation" Materials 16, no. 4: 1422. https://doi.org/10.3390/ma16041422
APA StyleZając-Woźnialis, A., Kruszelnicka, I., Zembrzuska, J., Ginter-Kramarczyk, D., Ochowiak, M., & Krupińska, A. (2023). Efficiency of Diclofenac Removal Using Activated Sludge in a Dynamic System (SBR Reactor) with Variable Parameters of pH, Concentration, and Sludge Oxygenation. Materials, 16(4), 1422. https://doi.org/10.3390/ma16041422







