In Vitro Bioelectrochemical Properties of Second-Generation Oxide Nanotubes on Ti–13Zr–13Nb Biomedical Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Treatment
2.2. Production of ONTs on Ti–13Zr–13Nb Alloy
2.3. Physicochemical Characteristics of ONTs on Ti–13Zr–13Nb Alloy
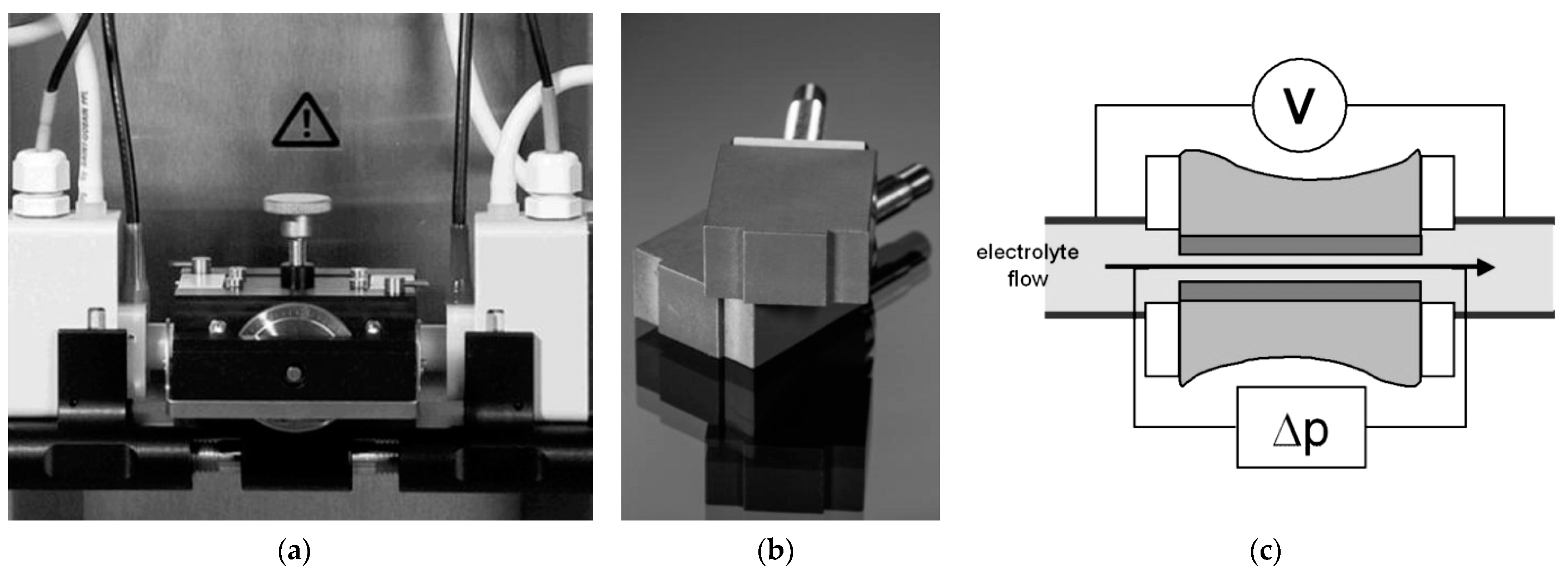
2.4. In Vitro Surface Characteristics of ONTs on Ti–13Zr–13Nb Alloy in Body Fluids
2.5. In Vitro Corrosion Resistance of ONTs on Ti–13Zr–13Nb Alloy in PBS
3. Results and Discussion
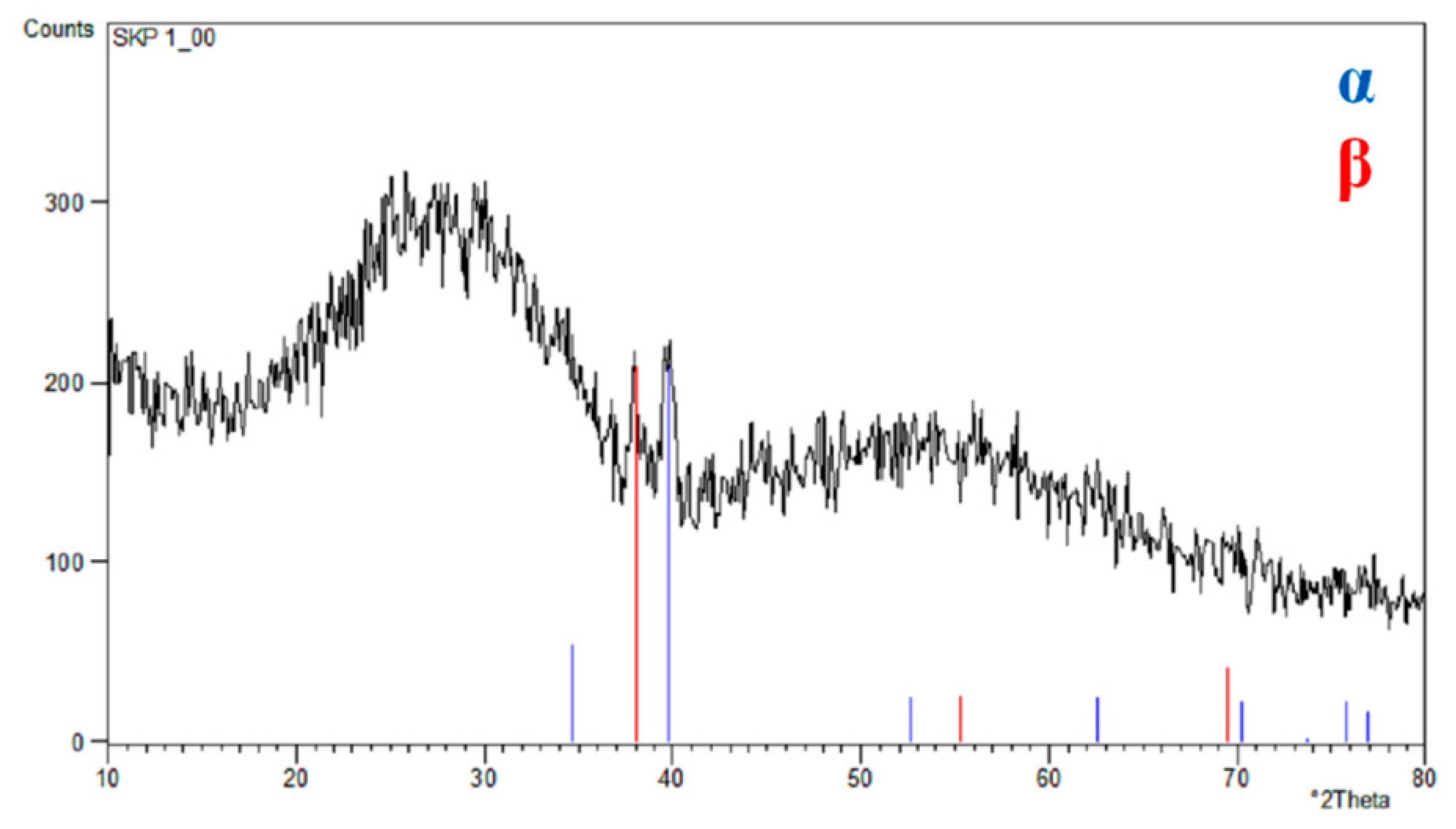
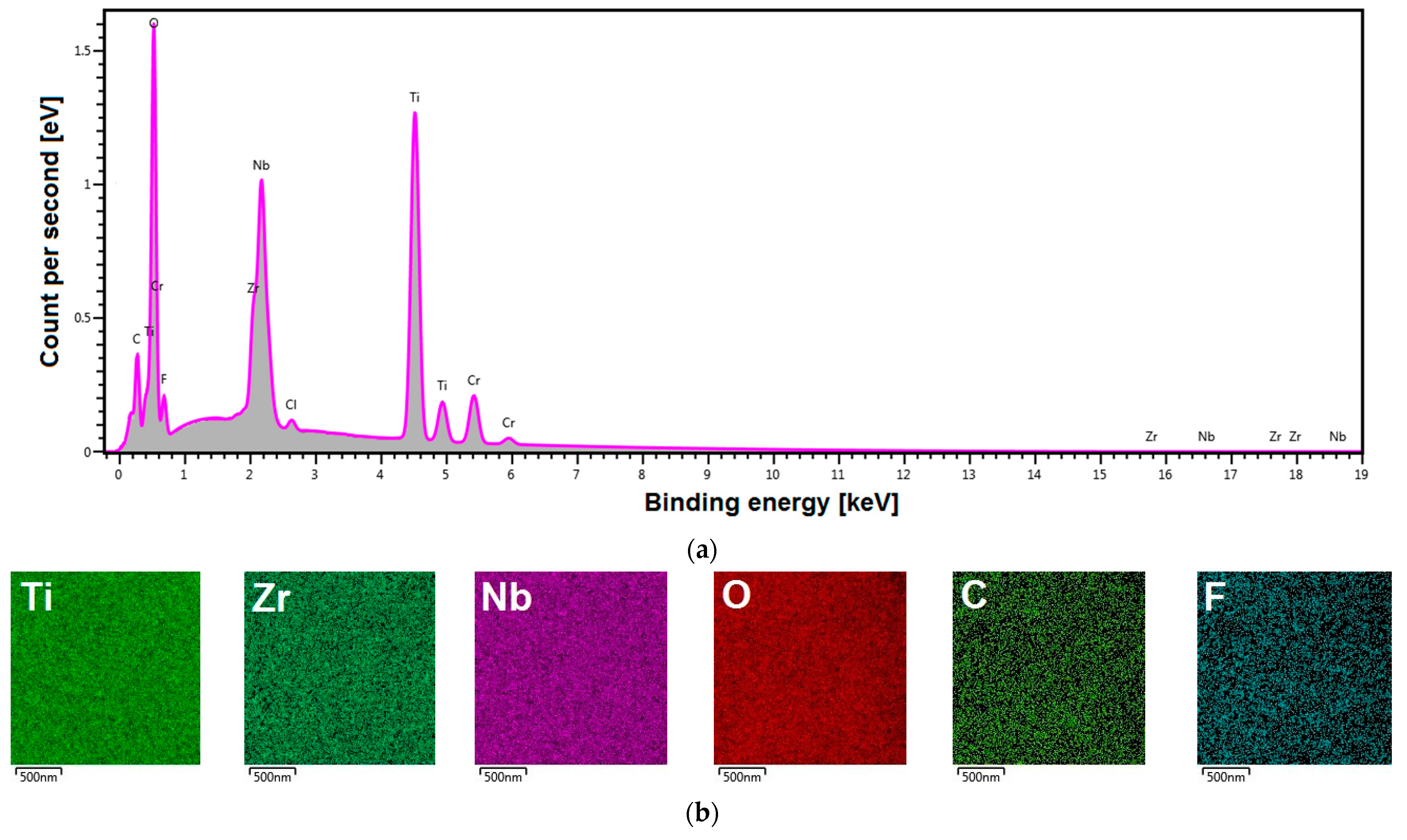
3.1. FE-SEM/EDS Studies of ONTs on Ti–13Zr–13Nb Alloy
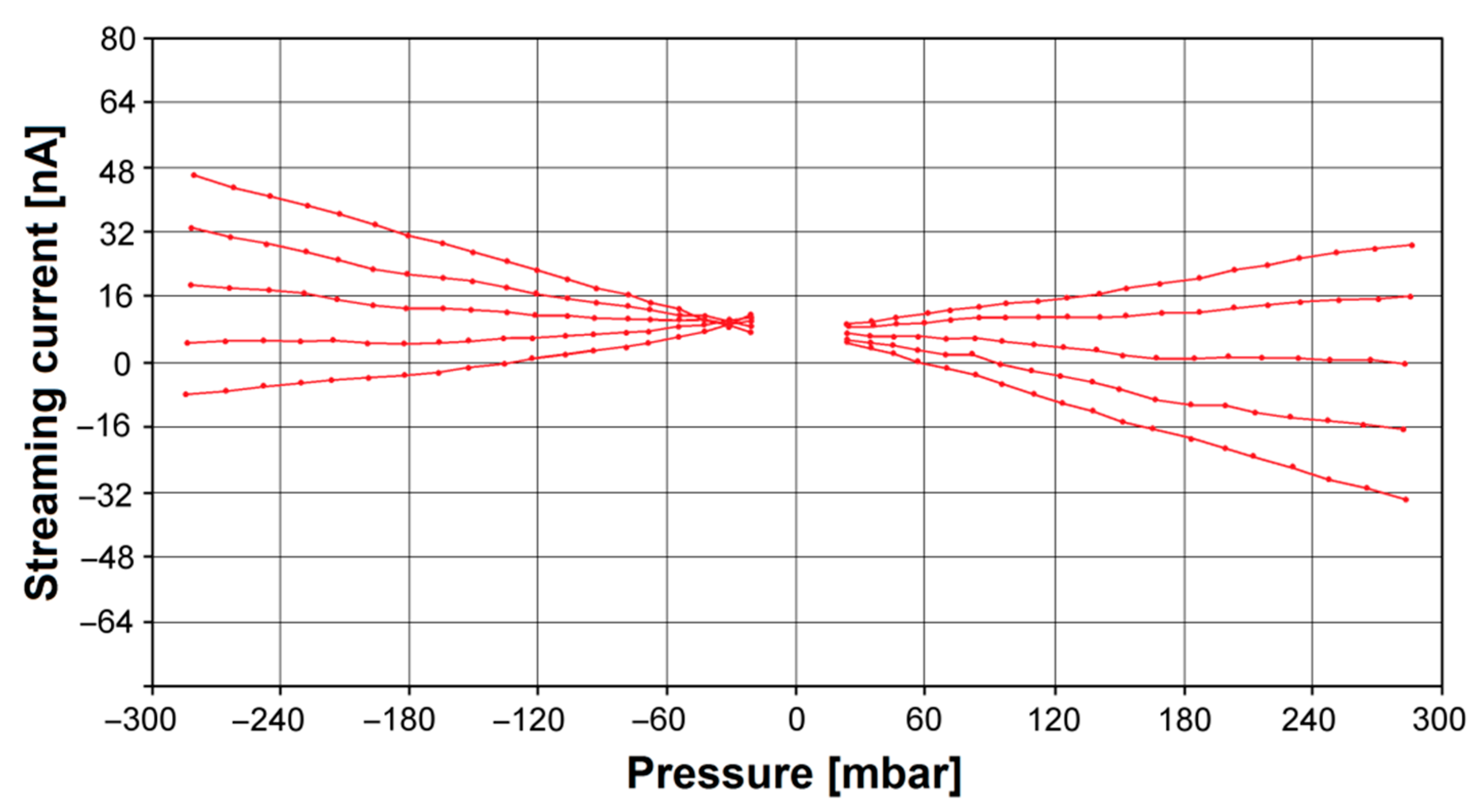
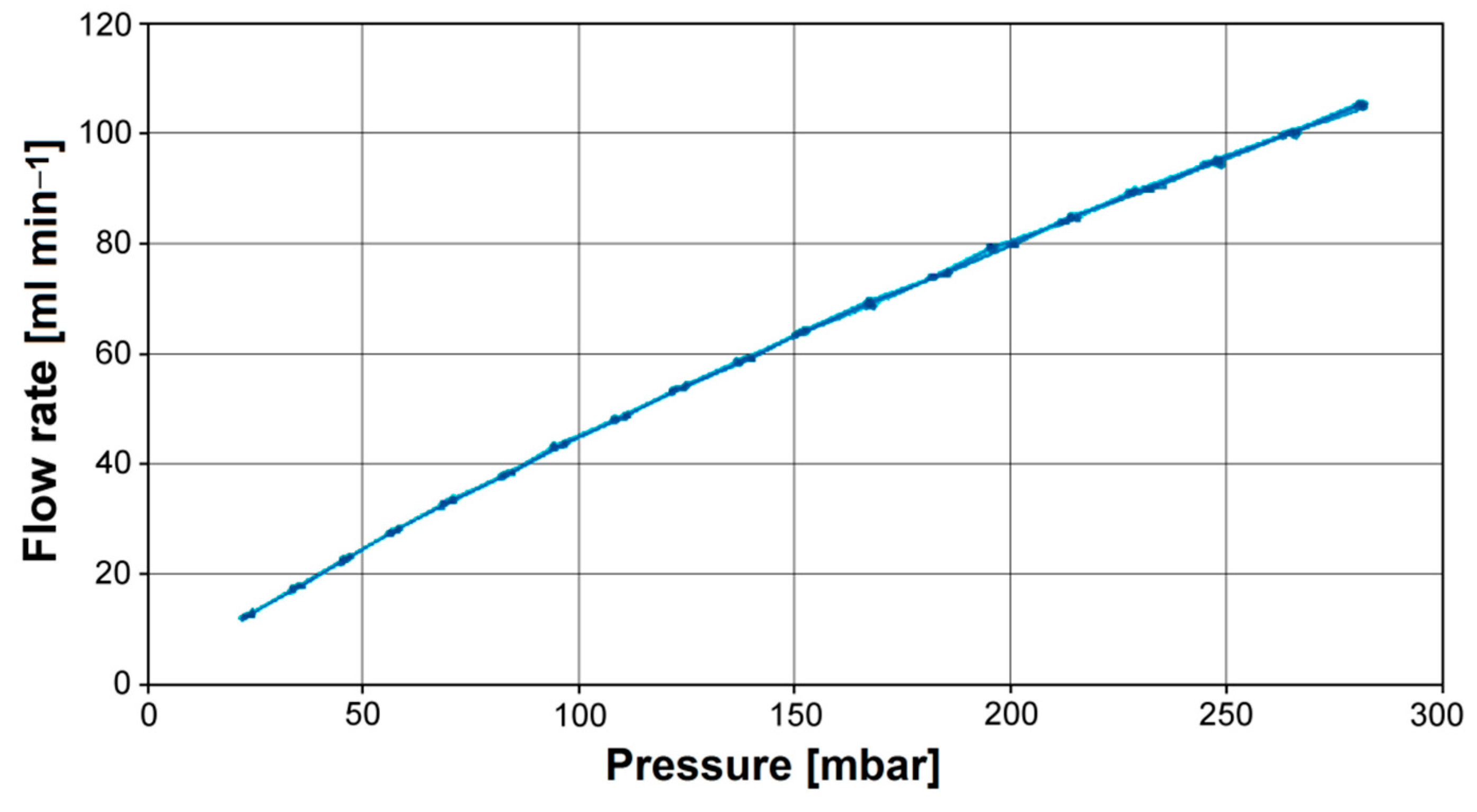
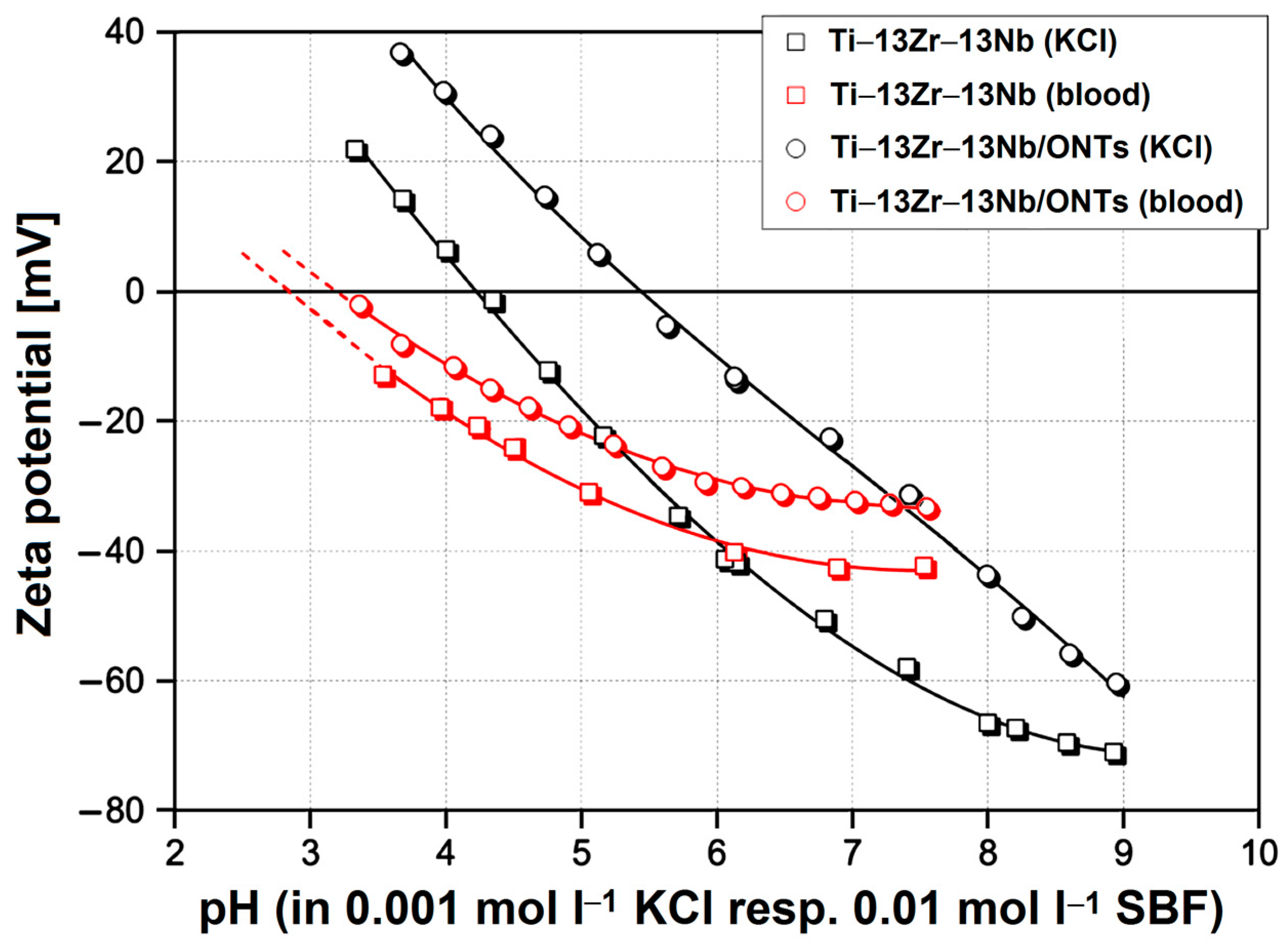
3.2. In Vitro Bioelectrochemical Characteristics in Body Fluids
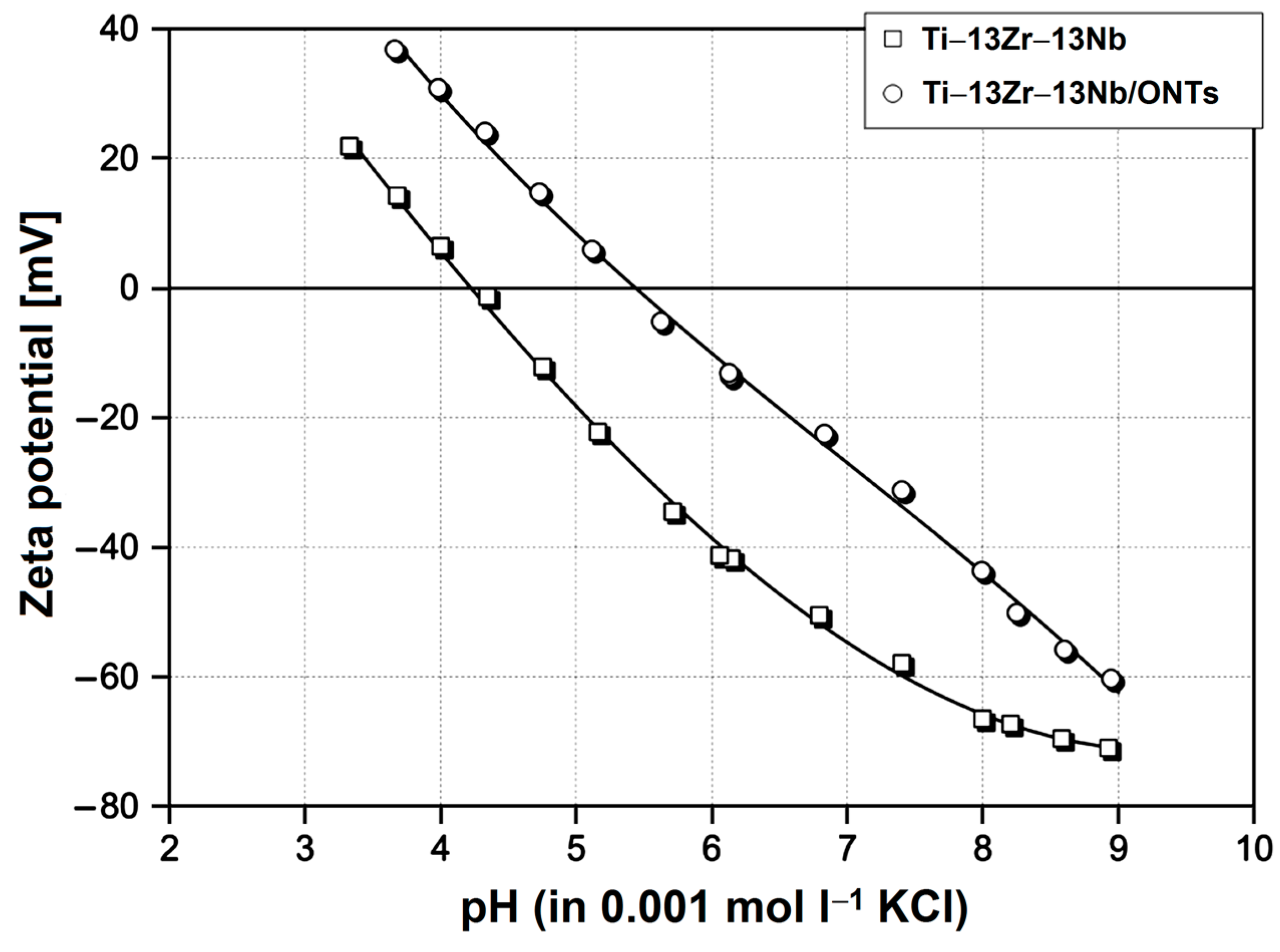
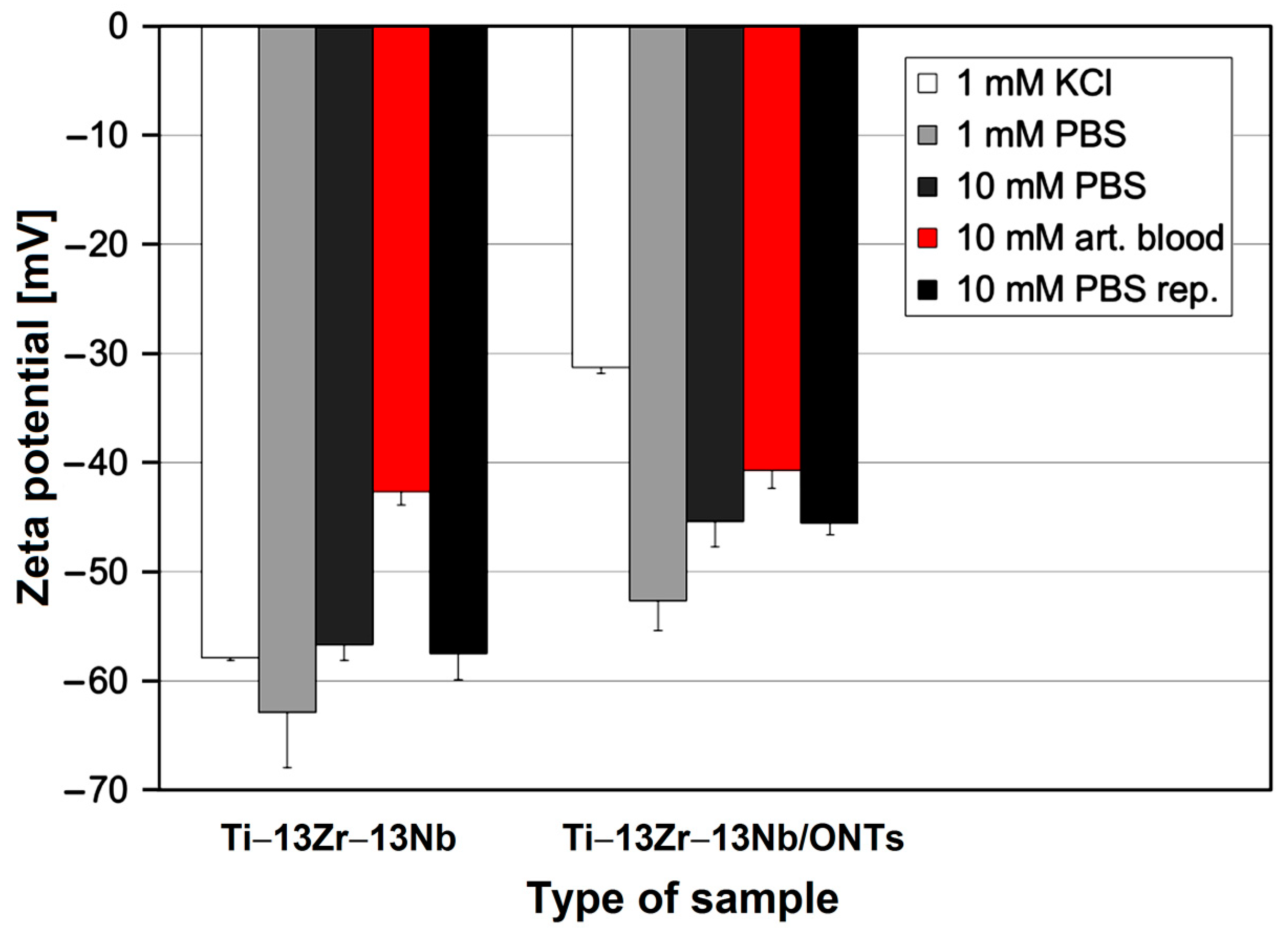
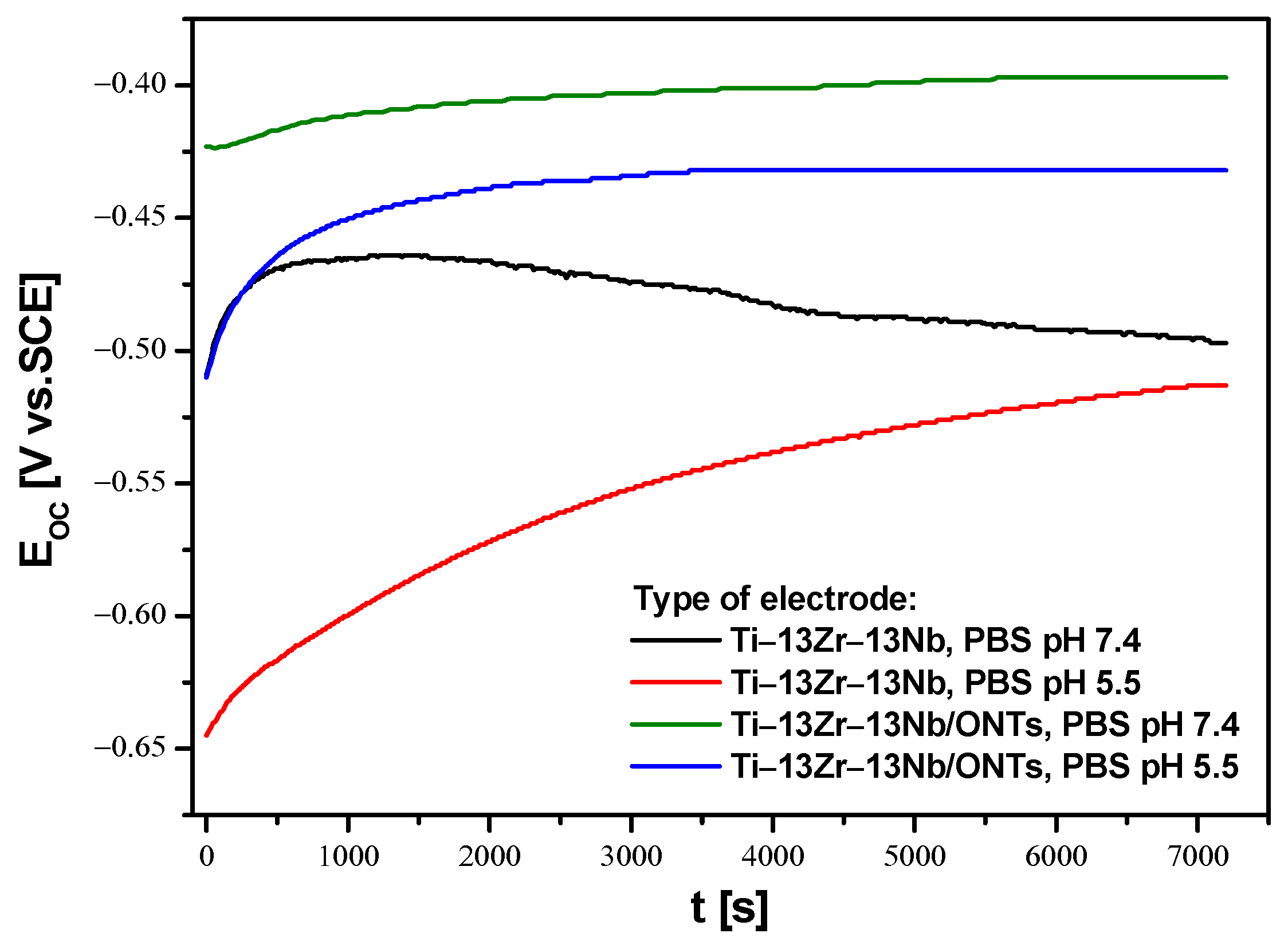
3.3. In Vitro Open Circuit Potential Characteristics in Body Fluids
3.4. In Vitro Susceptibility to Pitting Corrosion in Body Fluids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anene, F.; Jaafar, C.A.; Zainol, I.; Hanim, M.A.; Suraya, M. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2020, 235, 3792–3805. [Google Scholar] [CrossRef]
- Ti-Based Biomaterials; MDPI AG: Basel, Switzerland, 2020.
- Titanium in Medical and Dental Applications; Elsevier BV: Amsterdam, The Netherlands, 2018.
- Ossowska, A.; Zieliński, A.; Supernak, M. Formation of High Corrosion Resistant Nanotubular Layers on Titanium Alloy Ti13Nb13Zr. Solid State Phenom. 2011, 183, 137–142. [Google Scholar] [CrossRef]
- Smołka, A.; Dercz, G.; Rodak, K.; Łosiewicz, B. Evaluation of corrosion resistance of nanotubular oxide layers on the Ti13Zr13Nb alloy in physiological saline solution. Arch. Metall. Mater. 2015, 60, 2681–2686. [Google Scholar] [CrossRef]
- Aïnouche, L.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Bradai, D. Interfacial barrier layer properties of three generations of TiO2 nanotube arrays. Electrochim. Acta 2014, 133, 597–609. [Google Scholar] [CrossRef]
- Luz, A.R.; de Souza, G.B.; Lepienski, C.M.; Siqueira, C.J.M.; Kuromoto, N.K. Tribological properties of nanotubes grown on Ti-35Nb alloy by anodization. Thin Solid Film. 2018, 660, 529–537. [Google Scholar] [CrossRef]
- Sarraf, M.; Zalnezhad, E.; Bushroa, A.R.; Hamouda, A.M.S.; Rafieerad, A.R.; Nasiri-Tabrizi, B. Effect of microstructural evolution on wettability and tribological behavior of TiO2 nanotubular arrays coated on Ti–6Al–4V. Ceram. Int. 2015, 41, 7952–7962. [Google Scholar] [CrossRef]
- Stróż, A.; Goryczka, T.; Łosiewicz, B. Electrochemical formation of self-organized nanotubular oxide layers on niobium (Review). Curr. Nanosci. 2019, 15, 42–48. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Stróż, A.; Osak, P.; Maszybrocka, J.; Gerle, A.; Dudek, K.; Balin, K.; Łukowiec, D.; Gawlikowski, M.; Bogunia, S. Production, Characterization and Application of Oxide Nanotubes on Ti–6Al–7Nb Alloy as a Potential Drug Carrier. Materials 2021, 14, 6142. [Google Scholar] [CrossRef]
- Stróż, A.; Łosiewicz, B.; Zubko, M.; Chmiela, B.; Balin, K.; Dercz, G.; Gawlikowski, M.; Goryczka, T. Production, structure and biocompatible properties of oxide nanotubes on Ti13Nb13Zr alloy for medical applications. Mater. Charact. 2017, 132, 363–372. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Łosiewicz, B. Electrochemical synthesis of oxide nanotubes on biomedical Ti13Nb13Zr alloy with potential use as bone implant. AIP Conf. Proc. 2019, 2083, 030004. [Google Scholar] [CrossRef]
- Smołka, A.; Rodak, K.; Dercz, G.; Dudek, K.; Łosiewicz, B. Electrochemical Formation of Self-Organized Nanotubular Oxide Layers on Ti13Zr13Nb Alloy for Biomedical Applications. Acta Phys. Pol. 2014, 125, 932–935. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Stróż, D.; Łosiewicz, B. Electrochemical formation of second generation TiO2 nanotubes on Ti13Nb13Zr alloy for biomedical applications. Acta Phys. Pol. 2016, 130, 1079–1080. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Skwarek, S.; Stróż, A.; Osak, P.; Dudek, K.; Kubisztal, J.; Maszybrocka, J. Production and Characterization of the Third-Generation Oxide Nanotubes on Ti-13Zr-13Nb Alloy. Materials 2022, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Durdu, S.; Cihan, G.; Yalcin, E.; Altinkok, A. Characterization and mechanical properties of TiO2 nanotubes formed on titanium by anodic oxidation. Ceram. Int. 2021, 47, 10972–10979. [Google Scholar] [CrossRef]
- Ossowska, A.; Olive, J.-M.; Zielinski, A.; Wojtowicz, A. Effect of double thermal and electrochemical oxidation on titanium alloys for medical applications. Appl. Surf. Sci. 2021, 563, 150340. [Google Scholar] [CrossRef]
- Ossowska, A.; Zieliński, A.; Olive, J.-M.; Wojtowicz, A.; Szweda, P. Influence of Two-Stage Anodization on Properties of the Oxide Coatings on the Ti–13Nb–13Zr Alloy. Coatings 2020, 10, 707. [Google Scholar] [CrossRef]
- Handzlik, P.; Gutkowski, K. Synthesis of oxide nanotubes on Ti13Nb13Zr alloy by the electrochemical method. J. Porous Mater. 2019, 26, 1631–1637. [Google Scholar] [CrossRef]
- Stępień, M.; Handzlik, P.; Fitzner, K. Electrochemical synthesis of oxide nanotubes on Ti6Al7Nb alloy and their interaction with the simulated body fluid. J. Solid State Electrochem. 2016, 20, 2651–2661. [Google Scholar] [CrossRef]
- Wu, S.; Wang, S.; Liu, W.; Yu, X.; Wang, G.; Chang, Z.; Wen, D. Microstructure and properties of TiO2 nanotube coatings on bone plate surface fabrication by anodic oxidation. Surf. Coat. Technol. 2019, 374, 362–373. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of autoclaving time on corrosion resistance of sandblasted Ti G4 in artificial saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef]
- Szklarska, M.; Dercz, G.; Simka, W.; Łosiewicz, B.A.c. impedance study on the interfacial properties of passivated Ti13Zr13Nb alloy in physiological saline solution. Surf. Interface Anal. 2014, 46, 698–701. [Google Scholar] [CrossRef]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical properties, corrosion resistance and bioactivity of oxide layers formed by isothermal oxidation of Ti−6Al−7Nb alloy. Coatings 2021, 11, 505. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Ratajczak, P.; Łosiewicz, B. Long-Term Assessment of the In Vitro Corrosion Resistance of Biomimetic ACP Coatings Electrodeposited from an Acetate Bath. J. Funct. Biomater. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Dulski, M.; Łosiewicz, B. Functionalization of the NiTi Shape Memory Alloy Surface by HAp/SiO2/Ag Hybrid Coatings Formed on SiO2-TiO2 Glass Interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Tanji, A.; Feng, R.; Lyu, Z.; Sakidja, R.; Liaw, P.K.; Hermawan, H. Passivity of AlCrFeMnTi and AlCrFeCoNi high-entropy alloys in Hanks’ solution. Corros. Sci. 2023, 210, 110828. [Google Scholar] [CrossRef]
- Costa, B.C.; Tokuhara, C.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Zubko, M.; Rak, J.; Bogunia, S.; Łosiewicz, B. Influence of Sandblasting Process on Tribological Properties of Titanium Grade 4 in Artificial Saliva for Dentistry Applications. Materials 2021, 14, 7536. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Łosiewicz, B. Effect of amorphous calcium phosphate coatings on tribological properties of titanium grade 4 in protein-free artificial saliva. Biotribology 2022, 32, 100219. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, J.; Yang, C.; Zhang, X.; Liu, R. Effect of Zr addition on the microstructure and tribological property of the anodization of Ti-6Al-4V alloy. Surf. Coat. Technol. 2018, 356, 38–48. [Google Scholar] [CrossRef]
- Schneider, S.G.; Nunes, C.A.; Rogero, S.P.; Higa, O.Z.; Bressiani, J.C. Mechanical properties and cytotoxic evaluation of the Ti−13Nb−13Zr alloy. Biomecánica 2000, 8, 84–87. [Google Scholar] [CrossRef]
- Lee, T. Variation in Mechanical Properties of Ti−13Nb−13Zr Depending on Annealing Temperature. Appl. Sci. 2020, 10, 7896. [Google Scholar] [CrossRef]
- Davidson, J.A.; Kovacs, P. New Biocompatible, Low Modulus Titanium Alloy for Medical Implants. U.S. Patent No. 5,169,597, 8 December 1992. [Google Scholar]
- Lee, M.; Kim, I.-S.; Moon, Y.H.; Yoon, H.S.; Park, C.H.; Lee, T. Kinetics of Capability Aging in Ti-13Nb-13Zr Alloy. Crystals 2020, 10, 693. [Google Scholar] [CrossRef]
- ISO 13099-1:2012; Colloidal Systems—Methods for Zeta Potential Determination—Part 1: Electroacoustic and Electrokinetic Phenomena. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- ISO 13099-2:2012; Colloidal Systems—Methods for Zeta Potential Determination—Part 2: Optical Methods. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- ISO 13099-3:2012; Colloidal Systems—Methods for Zeta Potential Determination—Part 3: Acoustic Methods. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- Lorenzetti, M.; Gongadze, E.; Kulkarni, M.; Junkar, I.; Iglič, A. Electrokinetic Properties of TiO2 Nanotubular Surfaces. Nanoscale Res. Lett. 2016, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Luxbacher, T.; Kobe, S.; Novak, S. Electrokinetic behaviour of porous TiO2-coated implants. J. Mater. Sci. Mater. Med. 2015, 26, 191. [Google Scholar] [CrossRef]
- ASTM F1713-08(2021)e1; Standard Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications (UNS R58130). ASTM: West Conshohocken, PA, USA, 2021.
- Gudić, S.; Nagode, A.; Šimić, K.; Vrsalović, L.; Jozić, S. Corrosion Behavior of Different Types of Stainless Steel in PBS Solution. Sustainability 2022, 14, 8935. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Chen, Q.; Zhang, S.M. Corrosion behavior of Zr-based bulk metallic glasses in different artificial body fluids. J. Alloys Compd. 2006, 425, 268–273. [Google Scholar] [CrossRef]
- Ferraris, S.; Cazzola, M.; Peretti, V.; Stella, B.; Spriano, S. Zeta Potential Measurements on Solid Surfaces for in Vitro Biomaterials Testing: Surface Charge, Reactivity upon Contact with Fluids and Protein Absorption. Front. Bioeng. Biotechnol. 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stróż, A.; Luxbacher, T.; Dudek, K.; Chmiela, B.; Osak, P.; Łosiewicz, B. In Vitro Bioelectrochemical Properties of Second-Generation Oxide Nanotubes on Ti–13Zr–13Nb Biomedical Alloy. Materials 2023, 16, 1408. https://doi.org/10.3390/ma16041408
Stróż A, Luxbacher T, Dudek K, Chmiela B, Osak P, Łosiewicz B. In Vitro Bioelectrochemical Properties of Second-Generation Oxide Nanotubes on Ti–13Zr–13Nb Biomedical Alloy. Materials. 2023; 16(4):1408. https://doi.org/10.3390/ma16041408
Chicago/Turabian StyleStróż, Agnieszka, Thomas Luxbacher, Karolina Dudek, Bartosz Chmiela, Patrycja Osak, and Bożena Łosiewicz. 2023. "In Vitro Bioelectrochemical Properties of Second-Generation Oxide Nanotubes on Ti–13Zr–13Nb Biomedical Alloy" Materials 16, no. 4: 1408. https://doi.org/10.3390/ma16041408
APA StyleStróż, A., Luxbacher, T., Dudek, K., Chmiela, B., Osak, P., & Łosiewicz, B. (2023). In Vitro Bioelectrochemical Properties of Second-Generation Oxide Nanotubes on Ti–13Zr–13Nb Biomedical Alloy. Materials, 16(4), 1408. https://doi.org/10.3390/ma16041408







