On the Various Numerical Techniques for the Optimization of Bone Scaffold
Abstract
1. Introduction
2. Current Methods in the Optimization of Bone Scaffold
2.1. Solid Isotropic Material with Penalization (SIMP) Method
2.2. The Voronoi Method
2.3. The Machine Learning Method
2.4. The Genetic Algorithm (GA)
2.5. Other Methods
3. Current Settings in the Optimization of Bone Scaffold
3.1. Setting of Objective Function
3.2. Setting of Design Variables
3.3. Setting of Constraints
3.3.1. Pore Size
3.3.2. Porosity
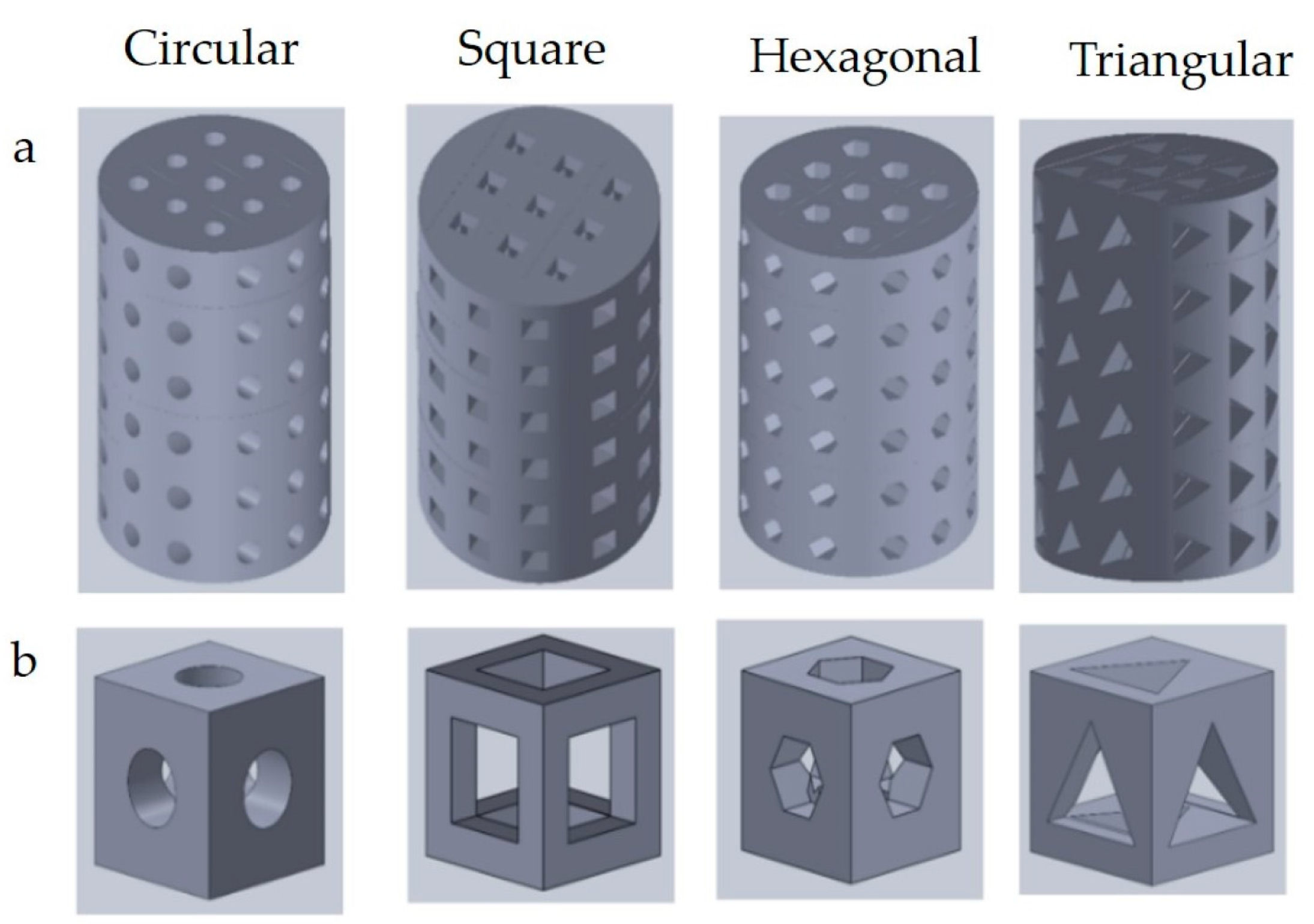
3.3.3. Pore Shapes
3.3.4. Additive Manufacturing Constraints
- Overhead Structural Constraints
- The Accuracy Constraints
- Connectivity Constraints
4. Challenges in the Optimization of Bone Scaffolds
4.1. Design of Bionic Bone Scaffolds
4.2. Validation of Numerical Simulations
4.3. Multi-Objective Optimization
4.4. Integrated Design and Manufacturing Performance
4.5. Incorporating Degradable Behavior into the Optimization Process
5. Conclusions and Future Prospects
- (1)
- Currently, the research and application of digital twin technology are still in their infancy. There are various possibilities for its combined application with additive manufacturing and topology optimization, such as modeling techniques using digital twins and the application of additive manufacturing technology. The integration of these new technologies with different optimization methods will lead to breakthroughs in the field of optimization of the bone scaffold.
- (2)
- The manufacturing and clinical application of personalized implants are still in their early stages, owing to the process of developing the medical 3D printing materials required for implant manufacturing. The current bone implants are mostly based on metal materials. More high-performance materials that conform to the elastic modulus of human bones need to be investigated and developed.
- (3)
- Optimization techniques based on finite element analysis have been used to simulate the mechanical interaction between bones and implants outside the human body. However, there is a lack of evidence to support in vivo studies. Therefore, biomechanical research work in the human body is needed in the future to make the optimization method more clinically relevant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and Properties of 3D Scaffolds for Bone Tissue Engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Sanan, A.; Haines, S.J. Repairing Holes in the Head: A History of Cranioplasty. Neurosurgery 1997, 40, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [PubMed]
- Croteau, S.; Rauch, F.; Silvestri, A.; Hamdy, R.C. Bone Morphogenetic Proteins in Orthopedics: From Basic Science to Clinical Practice. Orthop. (Thorofare N.J.) 1999, 22, 686–695. [Google Scholar]
- Jones, J.R.; Ehrenfried, L.M.; Hench, L.L. Optimising Bioactive Glass Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, H. Matrices and Scaffolds for Delivery of Bioactive Molecules in Bone and Cartilage Tissue Engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid Freeform Fabrication of Three-Dimensional Scaffolds for Engineering Replacement Tissues and Organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef]
- Shen, S.; Chen, M.; Guo, W.; Li, H.; Li, X.; Huang, S.; Luo, X.; Wang, Z.; Wen, Y.; Yuan, Z.; et al. Three Dimensional Printing-Based Strategies for Functional Cartilage Regeneration. Tissue Eng. Part B Rev. 2019, 25, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The Status, Challenges, and Future of Additive Manufacturing in Engineering. Comput. Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, Y.; Meng, L.; Wu, J.; Gong, T.; Zou, J.; Bosiakov, S.; Cheng, L. A Critical Review on the Design, Manufacturing and Assessment of the Bone Scaffold for Large Bone Defects. Front. Bioeng. Biotechnol. 2021, 9, 753715. [Google Scholar] [CrossRef] [PubMed]
- Weigel, T.; Schinkel, G.; Lendlein, A. Design and Preparation of Polymeric Scaffolds for Tissue Engineering. Expert Rev. Med. Devices 2006, 3, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A Review of Preparation Methods of Porous Skin Tissue Engineering Scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal Material, Properties and Design Methods of Porous Biomedical Scaffolds for Additive Manufacturing: A Review. Front. Bioeng. Biotechnol. 2021, 9, 641130. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Dubruel, P.; Möller, L.; Dräger, G.; Chichkov, B. Laser Fabrication of Three-Dimensional CAD Scaffolds from Photosensitive Gelatin for Applications in Tissue Engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef]
- Naing, M.W.; Chua, C.K.; Leong, K.F.; Wang, Y. Fabrication of Customised Scaffolds Using Computer-aided Design and Rapid Prototyping Techniques. Rapid Prototyp. J. 2005, 11, 249–259. [Google Scholar] [CrossRef]
- Cheah, C.-M.; Chua, C.-K.; Leong, K.-F.; Cheong, C.-H.; Naing, M.-W. Automatic Algorithm for Generating Complex Polyhedral Scaffold Structures for Tissue Engineering. Tissue Eng. 2004, 10, 595–610. [Google Scholar] [CrossRef]
- Sudarmadji, N.; Tan, J.Y.; Leong, K.F.; Chua, C.K.; Loh, Y.T. Investigation of the Mechanical Properties and Porosity Relationships in Selective Laser-Sintered Polyhedral for Functionally Graded Scaffolds. Acta Biomater. 2011, 7, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of Growth Factors Using a Smart Porous Nanocomposite Scaffold to Repair a Mandibular Bone Defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, X. Topological Design of 3D Chiral Metamaterials Based on Couple-Stress Homogenization. J. Mech. Phys. Solids 2019, 131, 372–386. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, Y.; Yan, Z.; Wang, Y.-X. Topological Design of a Trabecular Bone Structure With Morphology and Mechanics Control for Additive Manufacturing. IEEE Access 2021, 9, 11123–11133. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kikuchi, N.; Hollister, S.J. A Novel Method for Biomaterial Scaffold Internal Architecture Design to Match Bone Elastic Properties with Desired Porosity. J. Biomech. 2004, 37, 623–636. [Google Scholar] [CrossRef]
- Guest, J.K.; Prévost, J.H. Design of Maximum Permeability Material Structures. Comput. Methods Appl. Mech. Eng. 2007, 196, 1006–1017. [Google Scholar] [CrossRef]
- Guest, J.K.; Prévost, J.H. Optimizing Multifunctional Materials: Design of Microstructures for Maximized Stiffness and Fluid Permeability. Int. J. Solids Struct. 2006, 43, 7028–7047. [Google Scholar] [CrossRef]
- Sturm, S.; Zhou, S.; Mai, Y.-W.; Li, Q. On Stiffness of Scaffolds for Bone Tissue Engineering—A Numerical Study. J. Biomech. 2010, 43, 1738–1744. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, W.; Du, R.; Wang, S.; Wang, Y. Improved Proportional Topology Optimization Algorithm for Solving Minimum Compliance Problem. Struct. Multidiscip. Optim. 2020, 62, 475–493. [Google Scholar] [CrossRef]
- Xiao, F.; Yin, X. Geometry Models of Porous Media Based on Voronoi Tessellations and Their Porosity–Permeability Relations. Comput. Math. Appl. (1987) 2016, 72, 328–348. [Google Scholar] [CrossRef]
- Fantini, M.; Curto, M.; De Crescenzio, F. A Method to Design Biomimetic Scaffolds for Bone Tissue Engineering Based on Voronoi Lattices. Virtual Phys. Prototyp. 2016, 11, 77–90. [Google Scholar] [CrossRef]
- Lei, H.-Y.; Li, J.-R.; Xu, Z.-J.; Wang, Q.-H. Parametric Design of Voronoi-Based Lattice Porous Structures. Mater. Des. 2020, 191, 108607. [Google Scholar] [CrossRef]
- Zhao, H.; Han, Y.; Pan, C.; Yang, D.; Wang, H.; Wang, T.; Zeng, X.; Su, P. Design and Mechanical Properties Verification of Gradient Voronoi Scaffold for Bone Tissue Engineering. Micromachines 2021, 12, 664. [Google Scholar] [CrossRef]
- Kechagias, S.; Oosterbeek, R.N.; Munford, M.J.; Ghouse, S.; Jeffers, J.R.T. Controlling the Mechanical Behaviour of Stochastic Lattice Structures: The Key Role of Nodal Connectivity. Addit. Manuf. 2022, 54, 102730. [Google Scholar] [CrossRef]
- Du, Y.; Liang, H.; Xie, D.; Mao, N.; Zhao, J.; Tian, Z.; Wang, C.; Shen, L. Design and Statistical Analysis of Irregular Porous Scaffolds for Orthopedic Reconstruction Based on Voronoi Tessellation and Fabricated via Selective Laser Melting (SLM). Mater. Chem. Phys. 2020, 239, 121968. [Google Scholar] [CrossRef]
- Michalski, R.S.; Carbonell, J.G.; Mitchell, T.M. Machine Learning: An Artificial Intelligence Approach; Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 978-3-662-12407-9. [Google Scholar]
- Cilla, M.; Borgiani, E.; Martínez, J.; Duda, G.N.; Checa, S. Machine Learning Techniques for the Optimization of Joint Replacements: Application to a Short-Stem Hip Implant. PLoS ONE 2017, 12, e0183755. [Google Scholar] [CrossRef]
- Soize, C. Design Optimization under Uncertainties of a Mesoscale Implant in Biological Tissues Using a Probabilistic Learning Algorithm. Comput. Mech. 2017, 62, 477–497. [Google Scholar] [CrossRef]
- Gu, G.X.; Chen, C.-T.; Richmond, D.J.; Buehler, M.J. Bioinspired Hierarchical Composite Design Using Machine Learning: Simulation, Additive Manufacturing, and Experiment. Mater. Horiz. 2018, 5, 939–945. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Da, D.; Fuge, M.; Rai, R. IH-GAN: A Conditional Generative Model for Implicit Surface-Based Inverse Design of Cellular Structures. Comput. Methods Appl. Mech. Eng. 2022, 396, 115060. [Google Scholar] [CrossRef]
- Mairpady, A.; Mourad, A.-H.I.; Mozumder, M.S. Accelerated Discovery of the Polymer Blends for Cartilage Repair through Data-Mining Tools and Machine-Learning Algorithm. Polymers 2022, 14, 1802. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.A.; Bates, R.A. Robust Optimization of Cardiovascular Stents: A Comparison of Methods. Eng. Optim. 2004, 36, 207–217. [Google Scholar] [CrossRef]
- Heljak, M.K.; Kurzydlowski, K.J.; Swieszkowski, W. Computer Aided Design of Architecture of Degradable Tissue Engineering Scaffolds. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 1623–1632. [Google Scholar] [CrossRef]
- Yin, H.; Zheng, X.; Wen, G.; Zhang, C.; Wu, Z. Design Optimization of a Novel Bio-Inspired 3D Porous Structure for Crashworthiness. Compos. Struct. 2021, 255, 112897. [Google Scholar] [CrossRef]
- You, F.; Yao, Y.; Hu, Q. Generation and Evaluation of Porous Structure of Bionic Bone Scaffold. Ji Xie Gong Cheng Xue Bao 2011, 47, 138–144. [Google Scholar] [CrossRef]
- Dos Reis, F.; Karathanasopoulos, N. Inverse Metamaterial Design Combining Genetic Algorithms with Asymptotic Homogenization Schemes. Int. J. Solids Struct. 2022, 250, 111702. [Google Scholar] [CrossRef]
- Chanda, S.; Gupta, S.; Kumar Pratihar, D. A Genetic Algorithm Based Multi-Objective Shape Optimization Scheme for Cementless Femoral Implant. J. Biomech. Eng. 2015, 137, 34502. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, F.; Roner, S.; von Atzigen, M.; Schweizer, A.; Nagy, L.; Vlachopoulos, L.; Snedeker, J.G.; Fürnstahl, P. An Automatic Genetic Algorithm Framework for the Optimization of Three-Dimensional Surgical Plans of Forearm Corrective Osteotomies. Med. Image Anal. 2020, 60, 101598. [Google Scholar] [CrossRef]
- Chanda, S.; Gupta, S.; Pratihar, D.K. A Combined Neural Network and Genetic Algorithm Based Approach for Optimally Designed Femoral Implant Having Improved Primary Stability. Appl. Soft Comput. 2016, 38, 296–307. [Google Scholar] [CrossRef]
- Osher, S.; Sethian, J.A. Fronts Propagating with Curvature-Dependent Speed: Algorithms Based on Hamilton-Jacobi Formulations. J. Comput. Phys. 1988, 79, 12–49. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, X.; Guo, D. A Level Set Method for Structural Topology Optimization. Comput. Methods Appl. Mech. Eng. 2003, 192, 227–246. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Q. A Variational Level Set Method for the Topology Optimization of Steady-State Navier–Stokes Flow. J. Comput. Phys. 2008, 227, 10178–10195. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Catledge, S.A.; Clem, W.C.; Shrikishen, N.; Chowdhury, S.; Stanishevsky, A.V.; Koopman, M.; Vohra, Y.K. An Electrospun Triphasic Nanofibrous Scaffold for Bone Tissue Engineering. Biomed. Mater. (Bristol) 2007, 2, 142–150. [Google Scholar] [CrossRef]
- Augat, P.; Hollensteiner, M.; von Rüden, C. The Role of Mechanical Stimulation in the Enhancement of Bone Healing. Injury 2021, 52, S78–S83. [Google Scholar] [CrossRef]
- Glatt, V.; Evans, C.H.; Tetsworth, K. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front. Physiol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.; Peach, C.; Fernandes, P.R.; Cseke, A.; Bartolo, P.J.D.S. Topology Optimization to Reduce the Stress Shielding Effect for Orthopedic Applications. Procedia CIRP 2017, 65, 202–206. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. Stiffness and Strength Tailoring of Cobalt Chromium Graded Cellular Structures for Stress-Shielding Reduction. Mater. Des. 2017, 114, 633–641. [Google Scholar] [CrossRef]
- Liverani, E.; Rogati, G.; Pagani, S.; Brogini, S.; Fortunato, A.; Caravaggi, P. Mechanical Interaction between Additive-Manufactured Metal Lattice Structures and Bone in Compression: Implications for Stress Shielding of Orthopaedic Implants. J. Mech. Behav. Biomed. Mater. 2021, 121, 104608. [Google Scholar] [CrossRef]
- Narra, N.; Valášek, J.; Hannula, M.; Marcián, P.; Sándor, G.K.; Hyttinen, J.; Wolff, J. Finite Element Analysis of Customized Reconstruction Plates for Mandibular Continuity Defect Therapy. J. Biomech. 2014, 47, 264–268. [Google Scholar] [CrossRef]
- Li, P.; Shen, L.; Li, J.; Liang, R.; Tian, W.; Tang, W. Optimal Design of an Individual Endoprosthesis for the Reconstruction of Extensive Mandibular Defects with Finite Element Analysis. J. Cranio-Maxillofac. Surg. 2014, 42, 73–78. [Google Scholar] [CrossRef]
- Luo, D.; Rong, Q.; Chen, Q. Finite-Element Design and Optimization of a Three-Dimensional Tetrahedral Porous Titanium Scaffold for the Reconstruction of Mandibular Defects. Med. Eng. Phys. 2017, 47, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Rahchamani, R.; Soheilifard, R. Three-Dimensional Structural Optimization of a Cementless Hip Stem Using a Bi-Directional Evolutionary Method. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Matsoukas, G.; Kim, I.Y. Design Optimization of a Total Hip Prosthesis for Wear Reduction. J. Biomech. Eng. 2009, 131, 051003. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, W.; Zhong, W. Doing Topology Optimization Explicitly and Geometrically—A New Moving Morphable Components Based Framework. J. Appl. Mech. 2014, 81, 081009. [Google Scholar] [CrossRef]
- Dias, M.R.; Guedes, J.M.; Flanagan, C.L.; Hollister, S.J.; Fernandes, P.R. Optimization of Scaffold Design for Bone Tissue Engineering: A Computational and Experimental Study. Med. Eng. Phys. 2014, 36, 448–457. [Google Scholar] [CrossRef]
- Xiao, D.; Yang, Y.; Su, X.; Wang, D.; Luo, Z. Topology Optimization of Microstructure and Selective Laser Melting Fabrication for Metallic Biomaterial Scaffolds. Trans. Nonferr. Met. Soc. China 2012, 22, 2554–2561. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D Printed Porous Ti6Al4V Implants with Different Pore Sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of Porous Ti Implants with a Channel Structure Fabricated by Selective Laser Melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The Effect of Pore Size on Tissue Ingrowth and Neovascularization in Porous Bioceramics of Controlled Architecture in Vivo. Biomed. Mater. (Bristol) 2011, 6, 015007. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hao, L.; Hussein, A.; Young, P. Ti–6Al–4V Triply Periodic Minimal Surface Structures for Bone Implants Fabricated via Selective Laser Melting. J. Mech. Behav. Biomed. Mater. 2015, 51, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, Q.; Han, X.; Feng, Q.; Song, J.; Setchi, R.; Liu, Y.; Liu, Y.; Goulas, A.; Engstrøm, D.S.; et al. Manufacturability, Mechanical Properties, Mass-Transport Properties and Biocompatibility of Triply Periodic Minimal Surface (TPMS) Porous Scaffolds Fabricated by Selective Laser Melting. Mater. Des. 2020, 195, 109034. [Google Scholar] [CrossRef]
- Inglam, S.; Chantarapanich, N.; Suebnukarn, S.; Vatanapatimaku, N.; Sucharitpwatskul, S.; Sitthiseripratip, K. Biomechanical Evaluation of a Novel Porous-Structure Implant: Finite Element Study. Int. J. Oral Maxillofac. Implant. 2013, 28, E48–E56. [Google Scholar] [CrossRef]
- Ferlin, K.M.; Prendergast, M.E.; Miller, M.L.; Kaplan, D.S.; Fisher, J.P. Influence of 3D Printed Porous Architecture on Mesenchymal Stem Cell Enrichment and Differentiation. Acta Biomater. 2016, 32, 161–169. [Google Scholar] [CrossRef]
- Gong, B.; Cui, S.; Zhao, Y.; Sun, Y.; Ding, Q. Strain-Controlled Fatigue Behaviors of Porous PLA-Based Scaffolds by 3D-Printing Technology. J. Biomater. Sci. Polym. Ed. 2017, 28, 2196–2204. [Google Scholar] [CrossRef]
- Jahir-Hussain, M.J.; Maaruf, N.A.; Esa, N.E.F.; Jusoh, N. The Effect of Pore Geometry on the Mechanical Properties of 3D-Printed Bone Scaffold Due to Compressive Loading. IOP Conf. Series. Mater. Sci. Eng. 2021, 1051, 12016. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Liu, R.; Xiao, D.; Sun, J. Study on the Designing Rules and Processability of Porous Structure Based on Selective Laser Melting (SLM). J. Mater. Process. Technol. 2013, 213, 1734–1742. [Google Scholar] [CrossRef]
- Cooper, K.; Steele, P.; Cheng, B.; Chou, K. Contact-Free Support Structures for Part Overhangs in Powder-Bed Metal Additive Manufacturing. Inventions 2018, 3, 2. [Google Scholar] [CrossRef]
- Ameen, W.; Mohammed, M.K.; Al-Ahmari, A.; Ahmed, N.; Mian, S.H. Investigation of Support Structure Parameters and Their Affects during Additive Manufacturing of Ti6Al4V Alloy via Electron Beam Melting. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2020. [Google Scholar] [CrossRef]
- Calignano, F. Design Optimization of Supports for Overhanging Structures in Aluminum and Titanium Alloys by Selective Laser Melting. Mater. Eng. 2014, 64, 203–213. [Google Scholar] [CrossRef]
- Hussein, A.; Hao, L.; Yan, C.; Everson, R.; Young, P. Advanced Lattice Support Structures for Metal Additive Manufacturing. J. Mater. Process. Technol. 2013, 213, 1019–1026. [Google Scholar] [CrossRef]
- El Moumen, A.; Tarfaoui, M.; Lafdi, K. Additive Manufacturing of Polymer Composites: Processing and Modeling Approaches. Compos. Part B-Eng. 2019, 171, 166–182. [Google Scholar] [CrossRef]
- Rane, K.; Strano, M. A Comprehensive Review of Extrusion-Based Additive Manufacturing Processes for Rapid Production of Metallic and Ceramic Parts. Adv. Manuf. 2019, 7, 155–173. [Google Scholar] [CrossRef]
- Wu, W.; Xue, J.; Wang, L.; Zhang, Z.; Hu, Y.; Dong, C. Forming Process, Microstructure, and Mechanical Properties of Thin-Walled 316L Stainless Steel Using Speed-Cold-Welding Additive Manufacturing. Metals 2019, 9, 109. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W. Topology Optimization Method with Elimination of Enclosed Voids. Struct. Multidiscip. Optim. 2019, 60, 117–136. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Chen, W.; Tong, L.; Cheng, G. An Identification Method for Enclosed Voids Restriction in Manufacturability Design for Additive Manufacturing Structures. Front. Mech. Eng. 2015, 10, 126–137. [Google Scholar] [CrossRef]
- Li, Q.; Chen, W.; Liu, S.; Tong, L. Structural Topology Optimization Considering Connectivity Constraint. Struct. Multidiscip. Optim. 2016, 54, 971–984. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Zhou, C.; Fan, Y.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.; Sun, Y.; et al. The Biomimetic Design and 3D Printing of Customized Mechanical Properties Porous Ti6Al4V Scaffold for Load-Bearing Bone Reconstruction. Mater. Des. 2018, 152, 30–39. [Google Scholar] [CrossRef]
- Han, Q.; Wang, C.; Chen, H.; Zhao, X.; Wang, J. Porous Tantalum and Titanium in Orthopedics: A Review. ACS Biomater. Sci. Eng. 2019, 5, 5798–5824. [Google Scholar] [CrossRef]
- Pei, X.; Wang, L.; Zhou, C.; Wu, L.; Lei, H.; Fan, S.; Zeng, Z.; Deng, Z.; Kong, Q.; Jiang, Q.; et al. Ti6Al4V Orthopedic Implant with Biomimetic Heterogeneous Structure via 3D Printing for Improving Osteogenesis. Mater. Des. 2022, 221, 110964. [Google Scholar] [CrossRef]
- Mather, M.L.; Morgan, S.P.; White, L.J.; Tai, H.; Kockenberger, W.; Howdle, S.M.; Shakesheff, K.M.; Crowe, J.A. Image-Based Characterization of Foamed Polymeric Tissue Scaffolds. Biomed. Mater. (Bristol) 2008, 3, 015011. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.Y.; Tan, S.T. A Simple and Effective Geometric Representation for Irregular Porous Structure Modeling. Comput. Aided Des. 2010, 42, 930–941. [Google Scholar] [CrossRef]
- Sancisi, N.; Baldisserri, B.; Parenti-Castelli, V.; Belvedere, C.; Leardini, A. One-Degree-of-Freedom Spherical Model for the Passive Motion of the Human Ankle Joint. Med. Biol. Eng. Comput. 2014, 52, 363–373. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Wang, Z.; Hu, Y. A Novel Cooperation Multi-Objective Optimization Approach: Multi-Swarm Multi-Objective Evolutionary Algorithm Based on Decomposition (MSMOEA/D). Front. Energy Res. 2022, 10, 925053. [Google Scholar] [CrossRef]
- Iqbal, T.; Wang, L.; Li, D.; Dong, E.; Fan, H.; Fu, J.; Hu, C. A General Multi-Objective Topology Optimization Methodology Developed for Customized Design of Pelvic Prostheses. Med. Eng. Phys. 2019, 69, 8–16. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Wei, Q.; Li, X.; Ji, K.; Zhang, K. Current Researches on Design and Manufacture of Biopolymer-Based Osteochondral Biomimetic Scaffolds. Bio-Des. Manuf. 2021, 4, 541–567. [Google Scholar] [CrossRef]
- Jin, H.; Zhuo, Y.; Sun, Y.; Fu, H.; Han, Z. Microstructure Design and Degradation Performance in Vitro of Three-Dimensional Printed Bioscaffold for Bone Tissue Engineering. Adv. Mech. Eng. 2019, 11, 1687814019883784. [Google Scholar] [CrossRef]
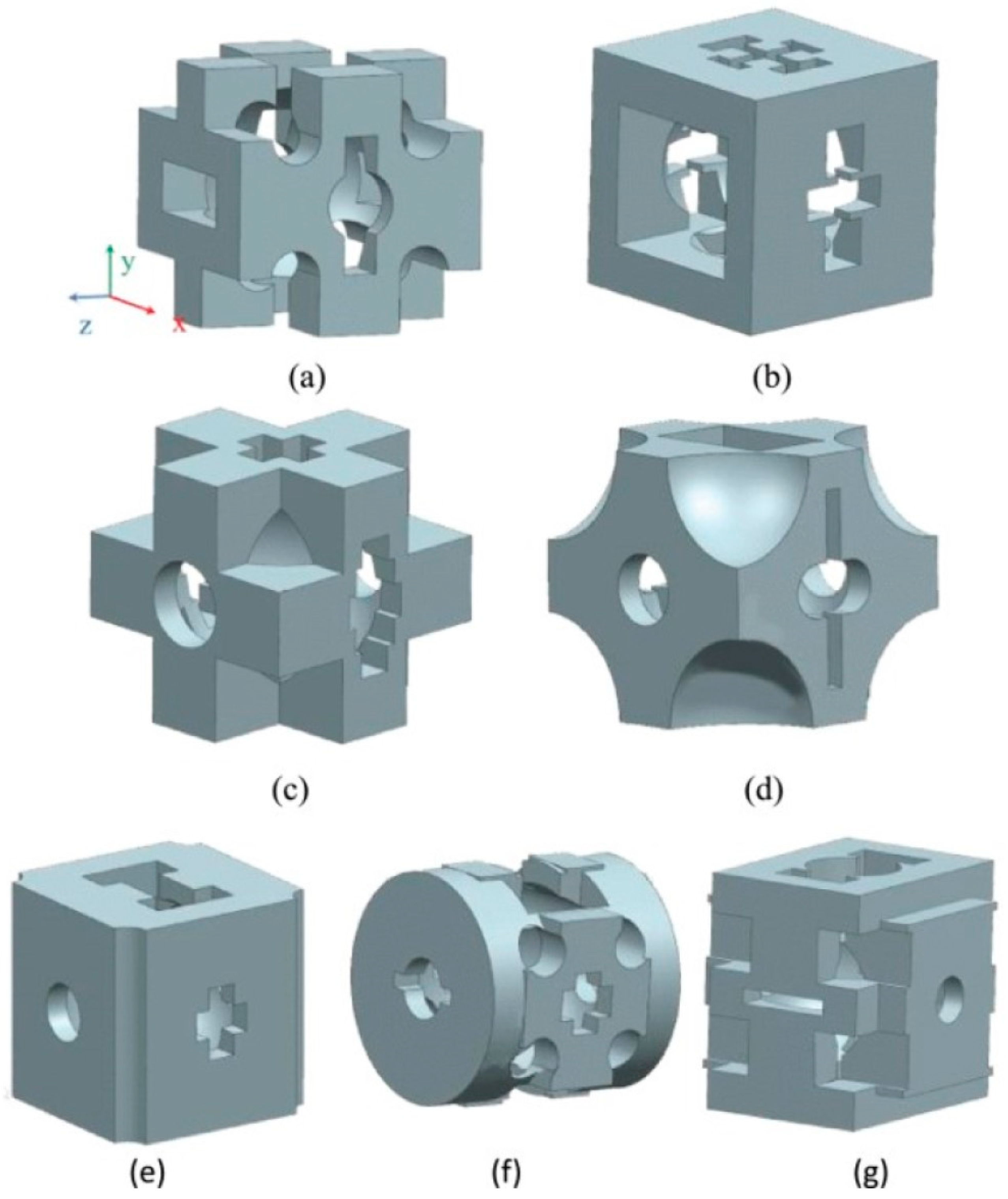

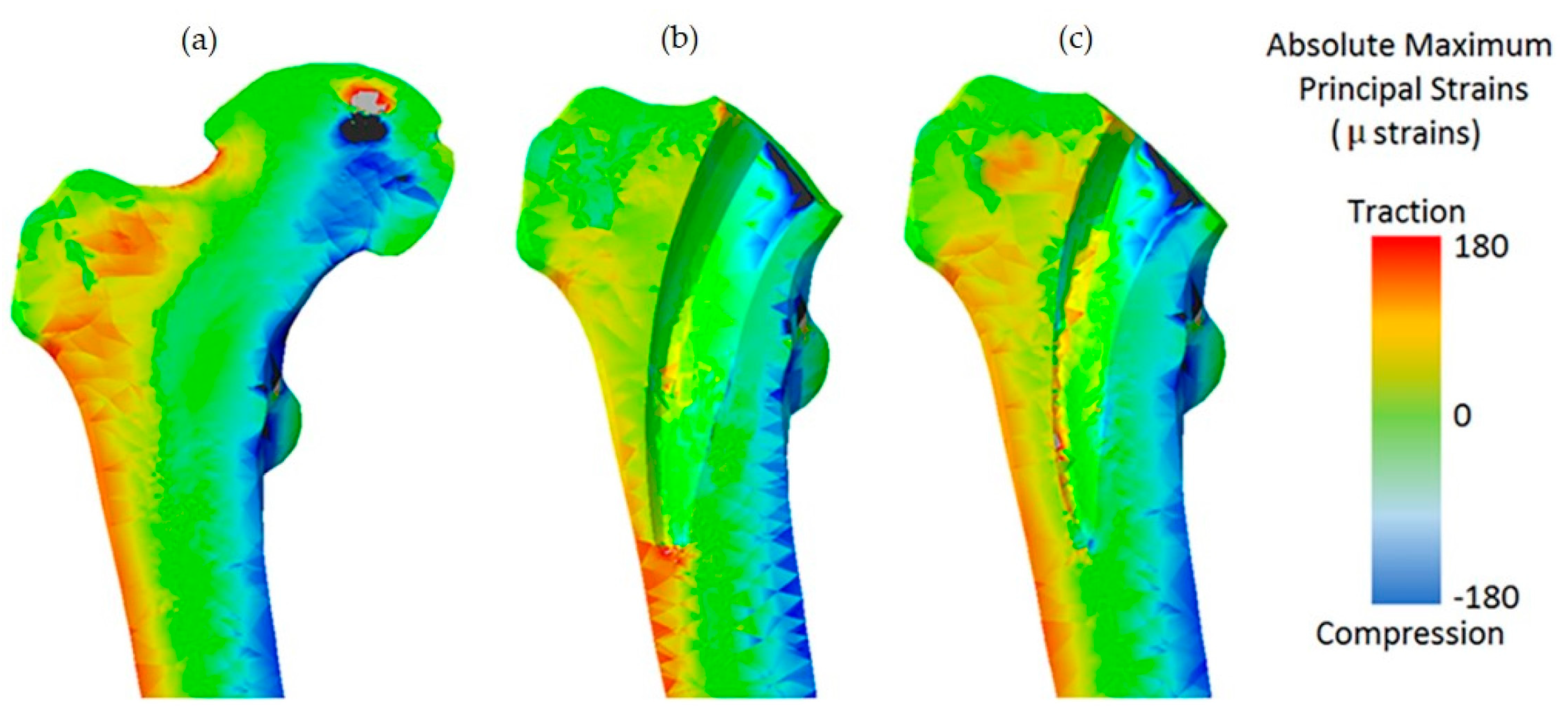
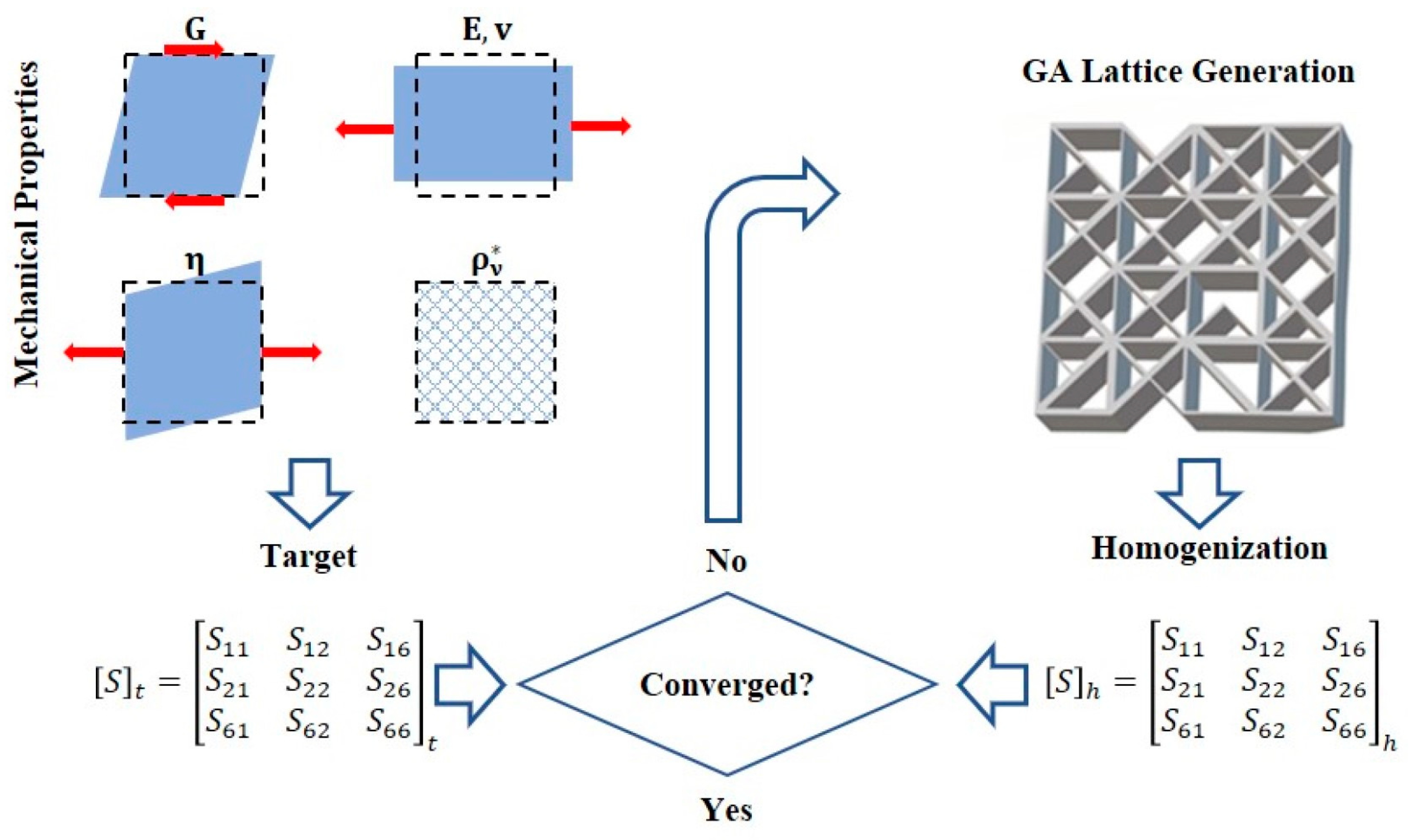
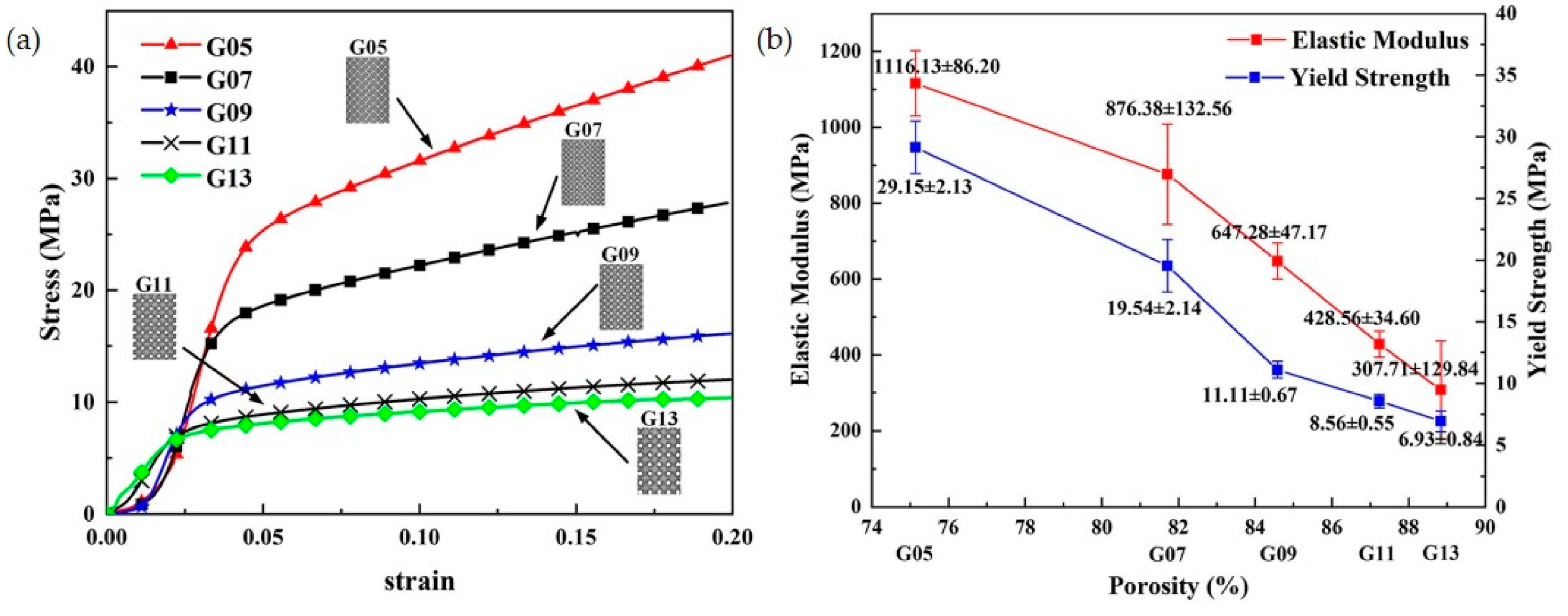
| Advantages | Disadvantages | References | |
|---|---|---|---|
| The Voronoi method | Excellent structure, good distribution of voids | Complex structure, complex relationship between parameters | [31,32,33,35] |
| The Machine learning method | Low calculation cost | High demand for training data | [38,40,41] |
| Genetic algorithm | Good scalability, simple process, fast convergence | Complex programming, high experience requirement for parameter selection, slow speed | [43,44,47,48] |
| The SIMP method | Good mechanical properties | High calculation cost, complex programming, slow calculation speed | [24,25,26,27] |
| Design Variables (Examples) | Advantages | References |
|---|---|---|
| Number of seeds (n), polyhedrons faces scale factor Sf, polyhedrons volume scale factor Sv | Provide geometrical heterogeneity Really biomimetic shape | [31] |
| Total stem length L, radius of the lateral side cross section R1, radius of the medial side cross section R2, internal length D | Low calculation cost | [37] |
| Finite element mesh or node | Widely used Simple and reliable | [17,68] |
| Geometric parameters of the transverse section of the stem | Multi-objective optimization A lot of options | [47] |
| Constraints | Possible Issues if Higher Than the Recommended Value | Possible Issues if Below the Recommended Value | Recommended Value | ||
|---|---|---|---|---|---|
| Pore size | Low intensity, weak osseointegration ability | Slow transfer of nutrients, slow excretion of metabolic waste, not conducive to cell growth | 500–1000 μm | ||
| Porosity | Reduced mechanical properties, high manufacturing difficulty | Unfavorable for new bone growth | 60–80% | ||
| Pore shapes | Round | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhang, Y.; Lyu, Y.; Cheng, L. On the Various Numerical Techniques for the Optimization of Bone Scaffold. Materials 2023, 16, 974. https://doi.org/10.3390/ma16030974
Wu J, Zhang Y, Lyu Y, Cheng L. On the Various Numerical Techniques for the Optimization of Bone Scaffold. Materials. 2023; 16(3):974. https://doi.org/10.3390/ma16030974
Chicago/Turabian StyleWu, Jiongyi, Youwei Zhang, Yongtao Lyu, and Liangliang Cheng. 2023. "On the Various Numerical Techniques for the Optimization of Bone Scaffold" Materials 16, no. 3: 974. https://doi.org/10.3390/ma16030974
APA StyleWu, J., Zhang, Y., Lyu, Y., & Cheng, L. (2023). On the Various Numerical Techniques for the Optimization of Bone Scaffold. Materials, 16(3), 974. https://doi.org/10.3390/ma16030974








