Aluminum Alloy Anode with Various Iron Content Influencing the Performance of Aluminum-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Al Batteries
2.2. Electrochemical Measurements
2.3. Material Characterization
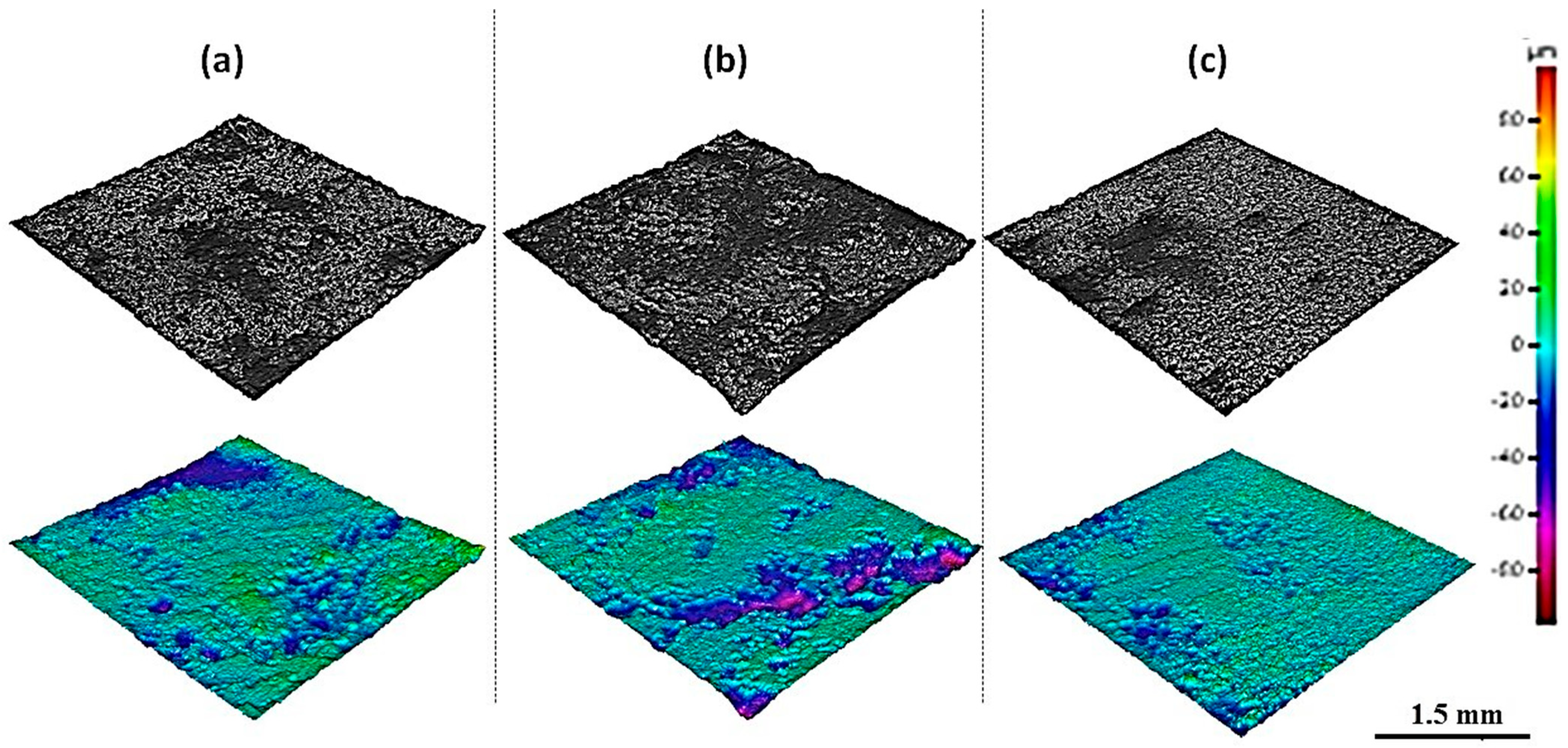
3. Results and Discussion
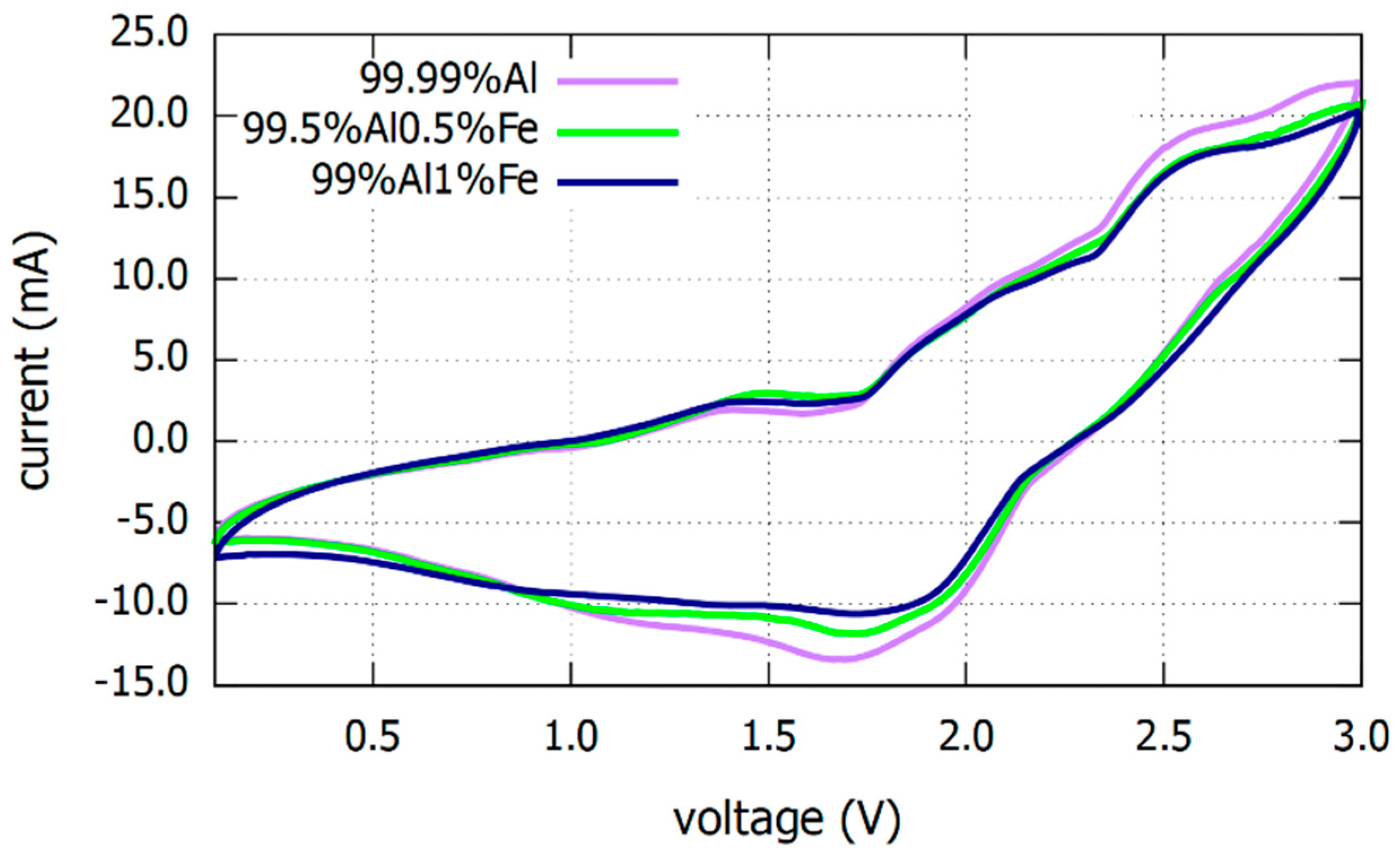
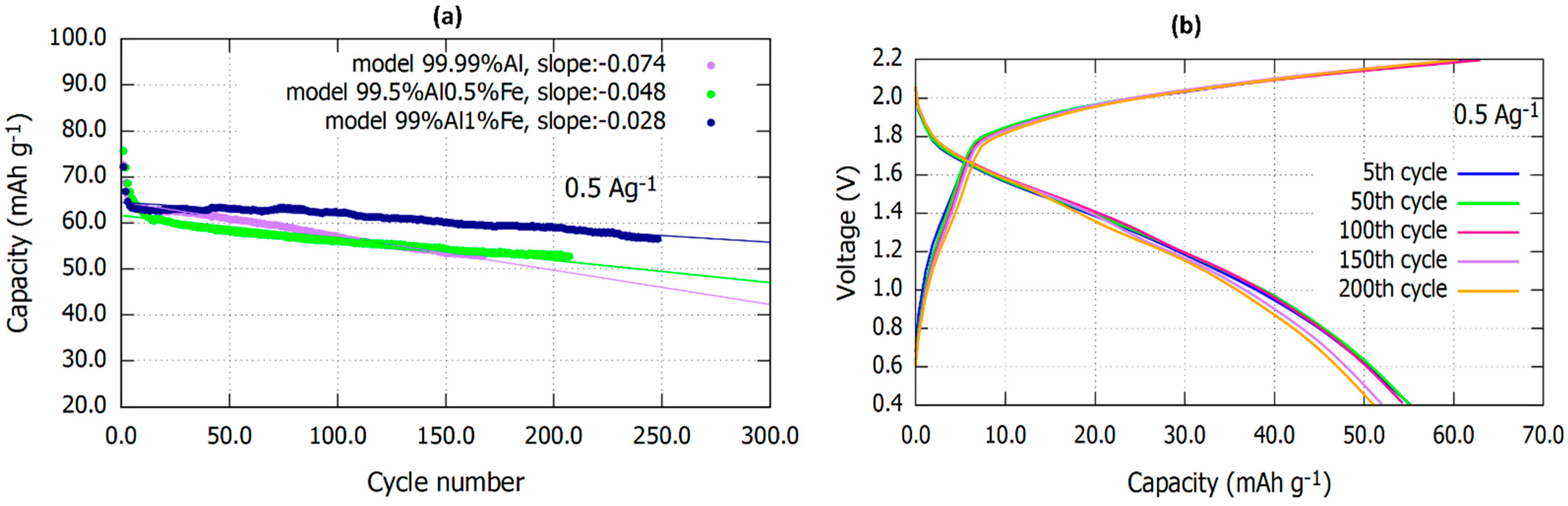
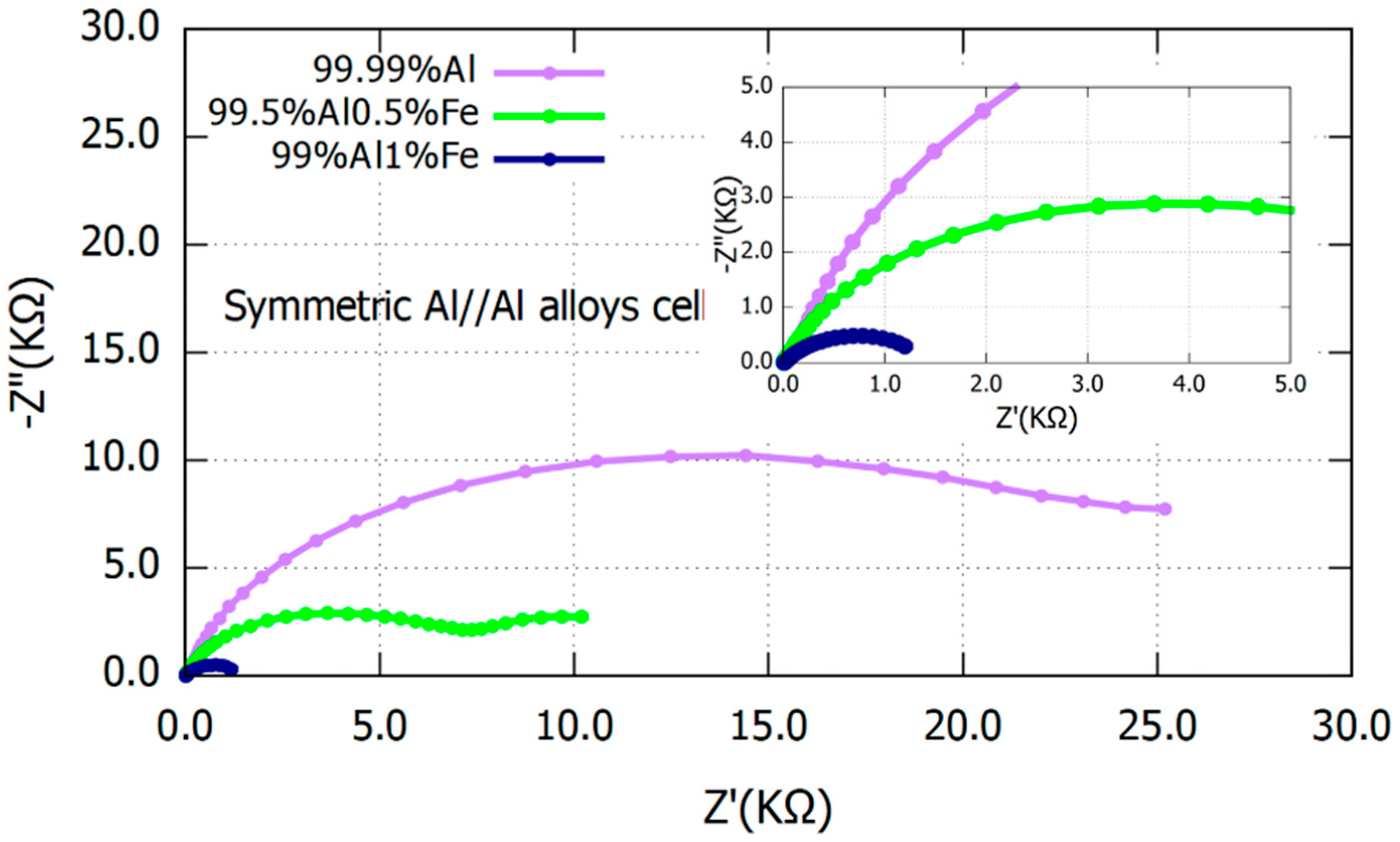
3.1. Electrochemical Analysis
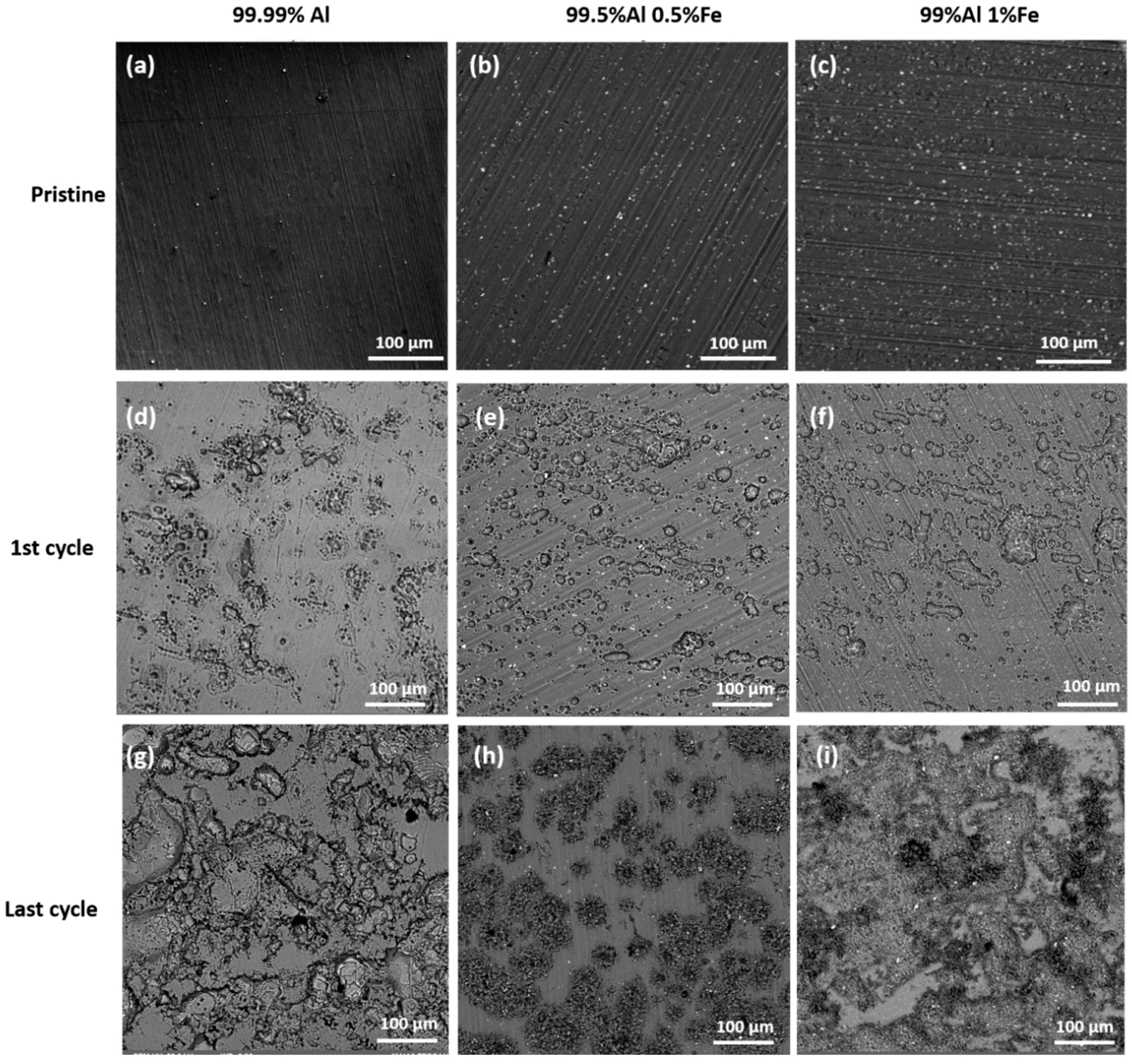
3.2. Material Analysis of Aluminum Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.M.; Li, F. The rechargeable aluminum battery: Opportunities and challenges. Angew. Chem. Int. Ed. 2019, 58, 11978–11996. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Zheng, S.; Xue, H.; Pang, H. Different positive electrode materials in organic and aqueous systems for aluminium ion batteries. J. Mater. Chem. A 2019, 7, 14391–14418. [Google Scholar] [CrossRef]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Meyer, D.C. The aluminum-ion battery: A sustainable and seminal concept? Front. Chem. 2019, 7, 268. [Google Scholar] [CrossRef]
- Faegh, E.; Ng, B.; Hayman, D.; Mustain, W.E. Practical assessment of the performance of aluminium battery technologies. Nature Energy 2021, 6, 21–29. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.L.; Lei, H.; Yu, Z.; Chen, L.L.; Wang, M.; Jiao, S. Nonaqueous rechargeable aluminum batteries: Progresses, challenges, and perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef]
- Ejigu, A.; Le Fevre, L.W.; Elgendy, A.; Spencer, B.F.; Bawn, C.; Dryfe, R.A. Optimization of Electrolytes for High-Performance Aqueous Aluminum-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 25232–25245. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, J.; Manalastas Jr, W.; Kumar, S.; Srinivasan, M. Emerging rechargeable aqueous aluminum ion battery: Status, challenges, and outlooks. Nano Mater. Sci. 2020, 2, 248–263. [Google Scholar] [CrossRef]
- Mckerracher, R.D.; Holland, A.; Cruden, A.; Wills, R.G.A. Comparison of carbon materials as cathodes for the aluminium-ion battery. Carbon 2019, 144, 333–341. [Google Scholar] [CrossRef]
- Jiang, M.; Fu, C.; Meng, P.; Ren, J.; Wang, J.; Bu, J.; Sun, B. Challenges and Strategies of Low-Cost Aluminum Anodes for High-Performance Al-Based Batteries. Adv. Mater. 2022, 34, 2102026. [Google Scholar] [CrossRef]
- Kim, J.; Raj, M.R.; Lee, G. High-Defect-Density Graphite for Superior-Performance Aluminum-Ion Batteries with Ultra-Fast Charging and Stable Long Life. Nano-Micro Lett. 2021, 13, 171. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Bai, Y.; Wu, C. Toward better electrode/electrolyte interfaces in the ionic-liquid-based rechargeable aluminum batteries. J. Energy Chem. 2020, 45, 98–102. [Google Scholar] [CrossRef]
- Long, Y.; Li, H.; Ye, M.; Chen, Z.; Wang, Z.; Tao, Y.; Yang, Q.H. Suppressing Al dendrite growth towards a long-life Al-metal battery. Energy Storage Mater. 2021, 34, 194–202. [Google Scholar] [CrossRef]
- Guo, M.; Fu, C.; Jiang, M.; Bai, Y.; Zhang, J.; Sun, B. High performance aluminum foam-graphite dual-ion batteries and failure analysis. J. Alloys Compd. 2020, 838, 155640. [Google Scholar] [CrossRef]
- Dong, T.; Ng, K.L.; Wang, Y.; Voznyy, O.; Azimi, G. Solid electrolyte interphase engineering for aqueous aluminum metal batteries: A critical evaluation. Adv. Energy Mater. 2021, 11, 2100077. [Google Scholar] [CrossRef]
- Zhao, Q.; Zachman, M.J.; Al Sadat, W.I.; Zheng, J.; Kourkoutis, L.F.; Archer, L. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 2018, 4, eaau8131. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, N.; Bai, Y.; Gao, Y.; Wu, C. An interface-reconstruction effect for rechargeable aluminum battery in ionic liquid electrolyte to enhance cycling performances. Green Energy Environ. 2018, 3, 71–77. [Google Scholar] [CrossRef]
- Wang, H.; Gu, S.; Bai, Y.; Chen, S.; Wu, F.; Wu, C. High-voltage and noncorrosive ionic liquid electrolyte used in rechargeable aluminum battery. ACS Appl. Mater. Interfaces 2016, 8, 27444–27448. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Nann, T. Trends in aluminium-based intercalation batteries. Adv. Energy Mater. 2017, 7, 1602093. [Google Scholar] [CrossRef]
- Choi, S.; Go, H.; Lee, G.; Tak, Y. Electrochemical properties of an aluminum anode in an ionic liquid electrolyte for rechargeable aluminum-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 8653–8656. [Google Scholar] [CrossRef]
- Go, H.; Michael, R.R.; Tak, Y.; Lee, G. Electrochemically surface-modified aluminum electrode enabling high performance and ultra-long cycling life Al-ion batteries. Electroanalysis 2022, 38, 1308–1317. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zheng, B.; Wang, S.; Huang, T.; Guo, F.; Gao, C. Oxide film efficiently suppresses dendrite growth in aluminum-ion battery. ACS Appl. Mater. Interfaces 2017, 9, 22628–22634. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R. Aluminum and Aluminum Alloys; ASM International Handbook: Materials Park, OH, USA, 1993; p. 547. [Google Scholar]
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–181. [Google Scholar]
- Ambat, R.; Davenport, A.J.; Scamans, G.M.; Afseth, A. Effect of iron-containing intermetallic particles on the corrosion behaviour of aluminium. Corros. Sci. 2006, 48, 3455–3471. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, C.; Hashimoto, T.; Yuan, Y.; Kang, D.; Hunter, J.; Zhou, X. The behaviour of iron-containing intermetallic particles in aluminium alloys in alkaline solution. Corros. Sci. 2021, 179, 109134. [Google Scholar] [CrossRef]
- Seri, O. Surface Treatment for Corrosion Resistant Aluminium Alloys by Removing Intermetallic Phases. Mater. Sci. Forum 2006, 519, 729–734. [Google Scholar] [CrossRef]
- Li, J.; Dang, J. A summary of corrosion properties of Al-rich solid solution and secondary phase particles in Al alloys. Metals 2017, 7, 84. [Google Scholar] [CrossRef]
- Rastabi, S.A.; Razaz, G.; Hummelgård, M.; Carlberg, T.; Blomquist, N.; Örtegren, J.; Olin, H. Metallurgical investigation of aluminum anode behavior in water-in-salt electrolyte for aqueous aluminum batteries. J. Power Sources 2022, 523, 231066. [Google Scholar]
- Blomquist, N.; Engström, A.C.; Hummelgård, M.; Andres, B.; Forsberg, S.; Olin, H. Large-scale production of nanographite by tube-shear exfoliation in water. PLoS ONE. 2016, 11, e0154686. [Google Scholar] [CrossRef]
- Blomquist, N.; Koppolu, R.; Dahlström, C.; Toivakka, M.; Olin, H. Influence of substrate in roll-to-roll coated nanographite electrodes for metal-free supercapacitors. Sci. Rep. 2020, 10, 5282. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wei, C.Y.; Lin, M.C.; Pan, C.J.; Chou, H.L.; Chen, H.A.; Dai, H. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Song, X.; Wang, Y.; Zhang, W.; Chhowalla, M. Microwave-Reduced Graphene Oxide for Aluminum Batteries. ACS Appl. Nano Mater. 2022, 5, 14347–14355. [Google Scholar] [CrossRef]
- Sanad, M.M.; Azab, A.A.; Taha, T. A Introduced oxygen vacancies in cadmium ferrite anode materials via Zn2+ incorporation for high performance lithium-ion batteries. Mater. Sci. Semicond. Process. 2022, 143, 106567. [Google Scholar] [CrossRef]
- Nandi, S.; Das, S.K. Realizing a low-cost and sustainable rechargeable aqueous aluminum-metal battery with exfoliated graphite cathode. ACS Sustain. Chem. Eng. 2019, 7, 19839–19847. [Google Scholar] [CrossRef]
- Lim, J.; Jeong, G.; Seo, K.; Lim, J.; Park, S.; Ju, W.; Sim, U. Controlled optimization of Mg and Zn in Al alloys for improved corrosion resistance via uniform corrosion. Mater. Adv. 2022, 3, 4813–4823. [Google Scholar] [CrossRef]
- Ran, Q.; Shi, H.; Meng, H.; Zeng, S.P.; Wan, W.B.; Zhang, W.; Jiang, Q. Aluminum-copper alloy anode materials for high-energy aqueous aluminum batteries. Nat. Commun. 2022, 13, 576. [Google Scholar] [CrossRef]
- Razaz, G.; Carlberg, T. On the Dissolution Process of Manganese and Iron in Molten Aluminum. Metall. Mater. Trans. A 2019, 50, 1873–1887. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, I.J.; Lee, H.J.; Kim, J.G. Aluminum anode for aluminum–air battery–Part I: Influence of aluminum purity. J. Power Sources 2015, 277, 370–378. [Google Scholar] [CrossRef]
- Basariya, M.R.; Roy, R.K.; Pramanick, A.K.; Srivastava, V.C.; Mukhopadhyay, N.K. Structural transition and softening in Al–Fe intermetallic compounds induced by high energy ball milling. Mater. Sci. Eng. A 2015, 638, 282–288. [Google Scholar] [CrossRef]
- Bendjeddou, L.; Debili, M.Y.; Fekrache, A.; Boulkhessaim, S. Structure and phase transformation in HF melted Al–Fe–Ti alloys. Phys. Procedia 2009, 2, 1113–1118. [Google Scholar] [CrossRef]

| Al Alloy Electrode | Lifetime (Cycle #) | Al3Fe Particle (#/cm2) | Surface Area Corrosion (%) | Volume Corrosion (%) | Average Corrosion Depth (µm) | Capacity Degradation Slope | Initial Capacity Cycle#5 (mAh g−1) | Final Capacity (mAh g−1) |
|---|---|---|---|---|---|---|---|---|
| 99.99% Al | 167 | ~8 (105) | 76.2 | 10.6 | ~10 | 0.074 | 64.1 | 52.8 |
| 99.5% Al 0.5% Fe | 207 | ~107 | 77.6 | 8.2 | ~8 | 0.048 | 65.3 | 52.7 |
| 99% Al 1% Fe | 248 | ~4 (107) | 87.5 | 7 | ~5 | 0.028 | 63.3 | 56.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razaz, G.; Arshadirastabi, S.; Blomquist, N.; Örtegren, J.; Carlberg, T.; Hummelgård, M.; Olin, H. Aluminum Alloy Anode with Various Iron Content Influencing the Performance of Aluminum-Ion Batteries. Materials 2023, 16, 933. https://doi.org/10.3390/ma16030933
Razaz G, Arshadirastabi S, Blomquist N, Örtegren J, Carlberg T, Hummelgård M, Olin H. Aluminum Alloy Anode with Various Iron Content Influencing the Performance of Aluminum-Ion Batteries. Materials. 2023; 16(3):933. https://doi.org/10.3390/ma16030933
Chicago/Turabian StyleRazaz, Ghadir, Shahrzad Arshadirastabi, Nicklas Blomquist, Jonas Örtegren, Torbjörn Carlberg, Magnus Hummelgård, and Håkan Olin. 2023. "Aluminum Alloy Anode with Various Iron Content Influencing the Performance of Aluminum-Ion Batteries" Materials 16, no. 3: 933. https://doi.org/10.3390/ma16030933
APA StyleRazaz, G., Arshadirastabi, S., Blomquist, N., Örtegren, J., Carlberg, T., Hummelgård, M., & Olin, H. (2023). Aluminum Alloy Anode with Various Iron Content Influencing the Performance of Aluminum-Ion Batteries. Materials, 16(3), 933. https://doi.org/10.3390/ma16030933







