Differences in Mechanical and Physicochemical Properties of Several PTFE Membranes Used in Guided Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Specifications
2.2. Scanning Electron Microscopy (SEM)
2.3. Surface Chemical Structure and Composition—X-ray Photoelectron Spectroscopy (XPS)
2.4. Mechanical Properties
2.5. Physical Properties
2.5.1. Wettability
2.5.2. Surface Roughness
2.5.3. Density
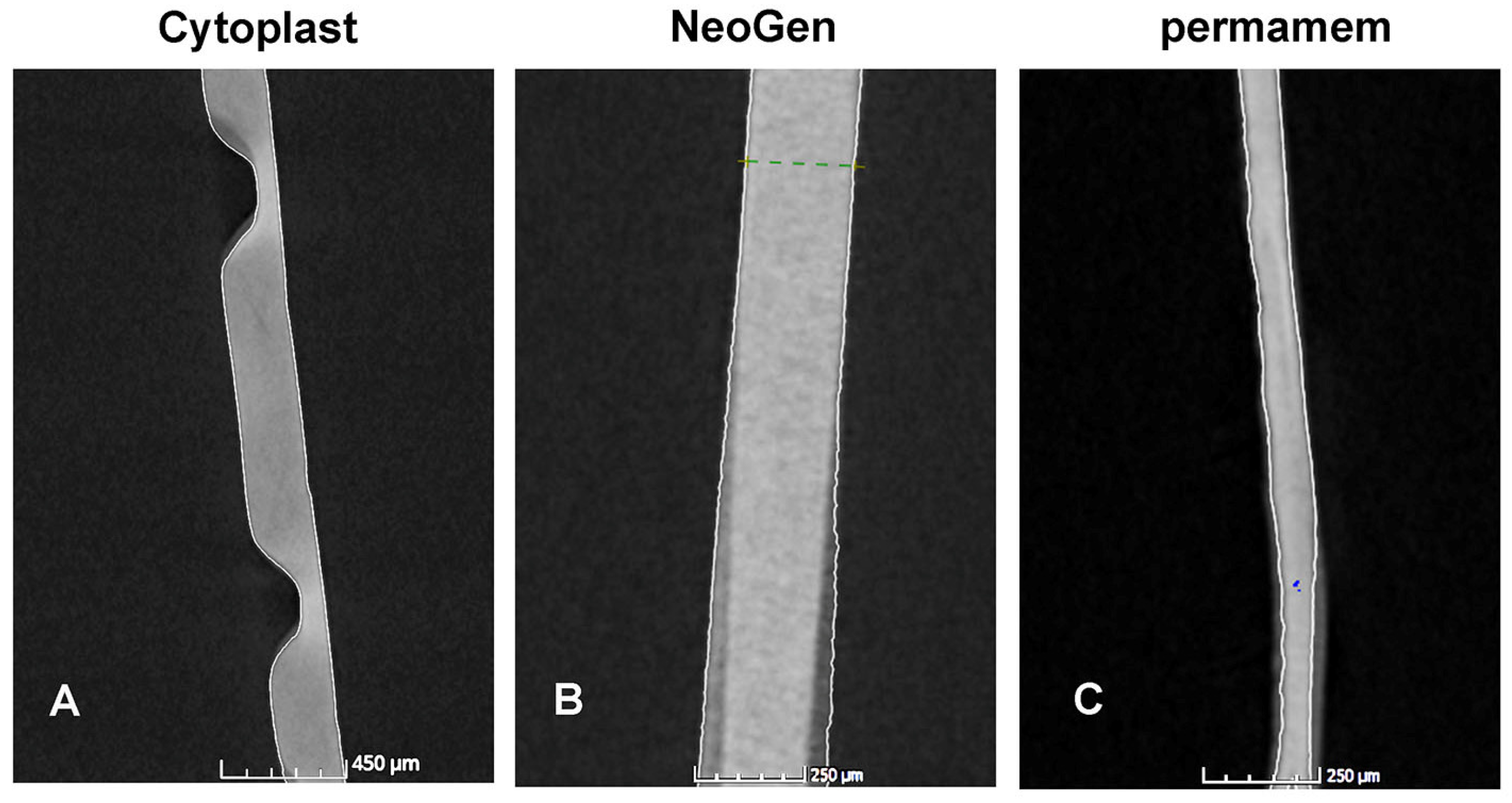
2.5.4. Nano-Computerized Tomography
2.6. Statistical Analysis
3. Results
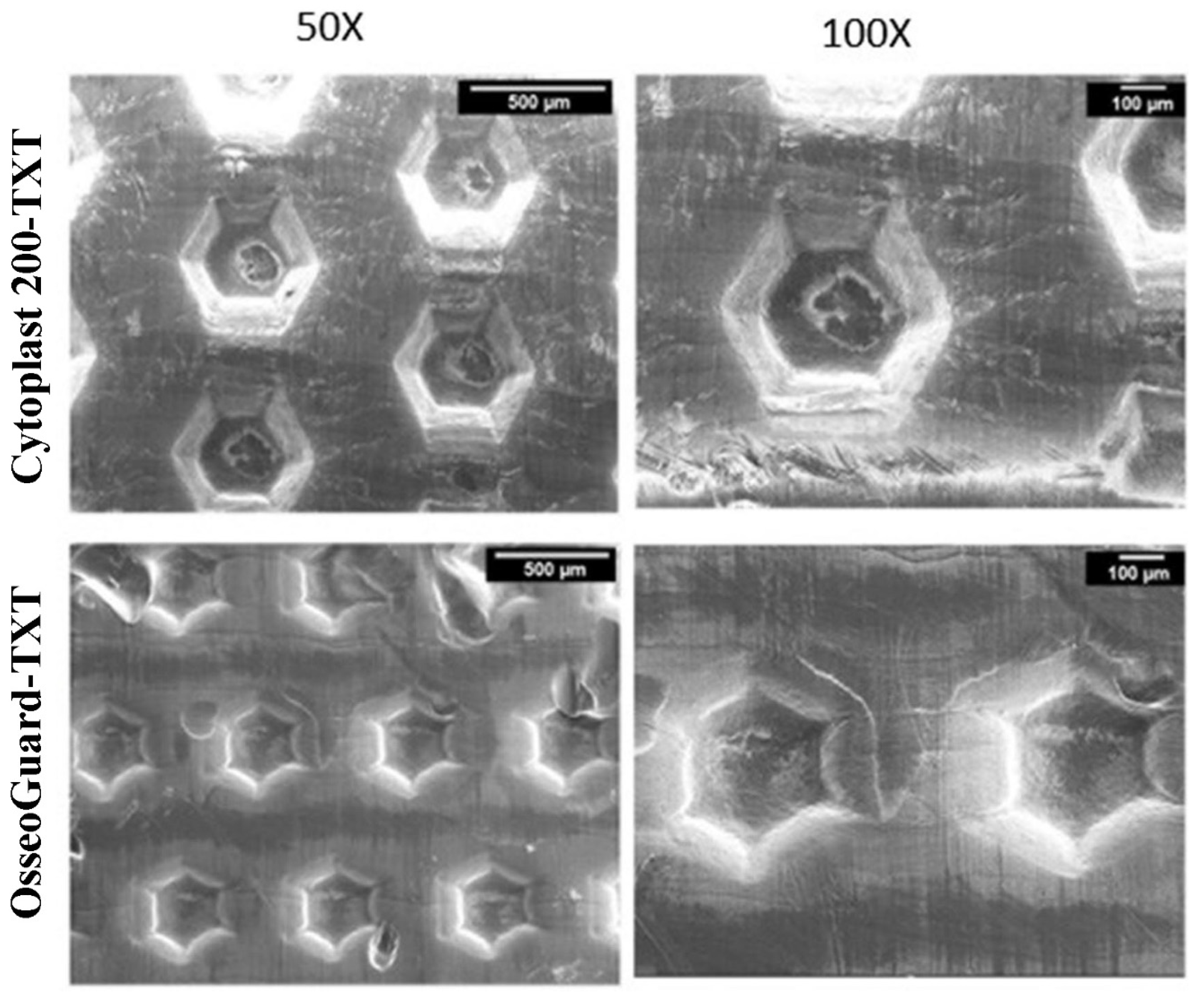
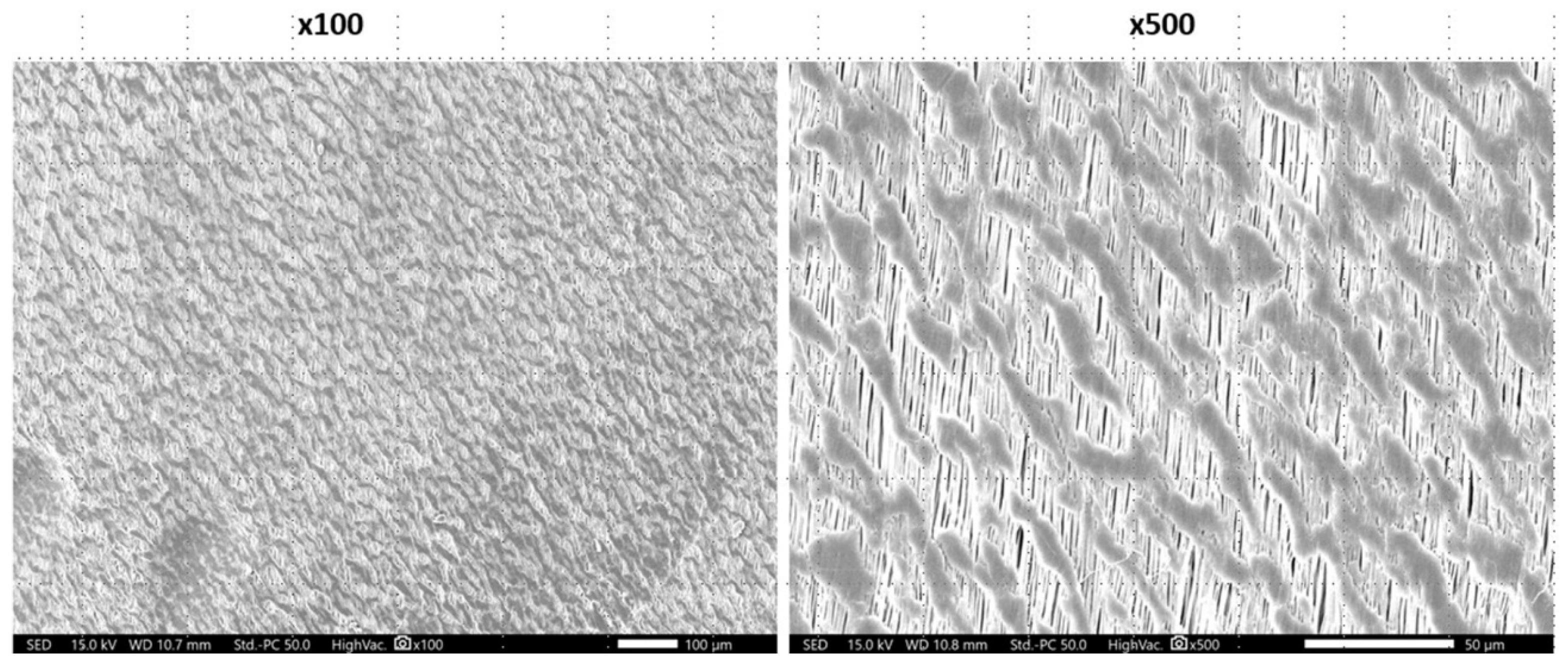
3.1. Scanning Electron Microscopy (SEM)
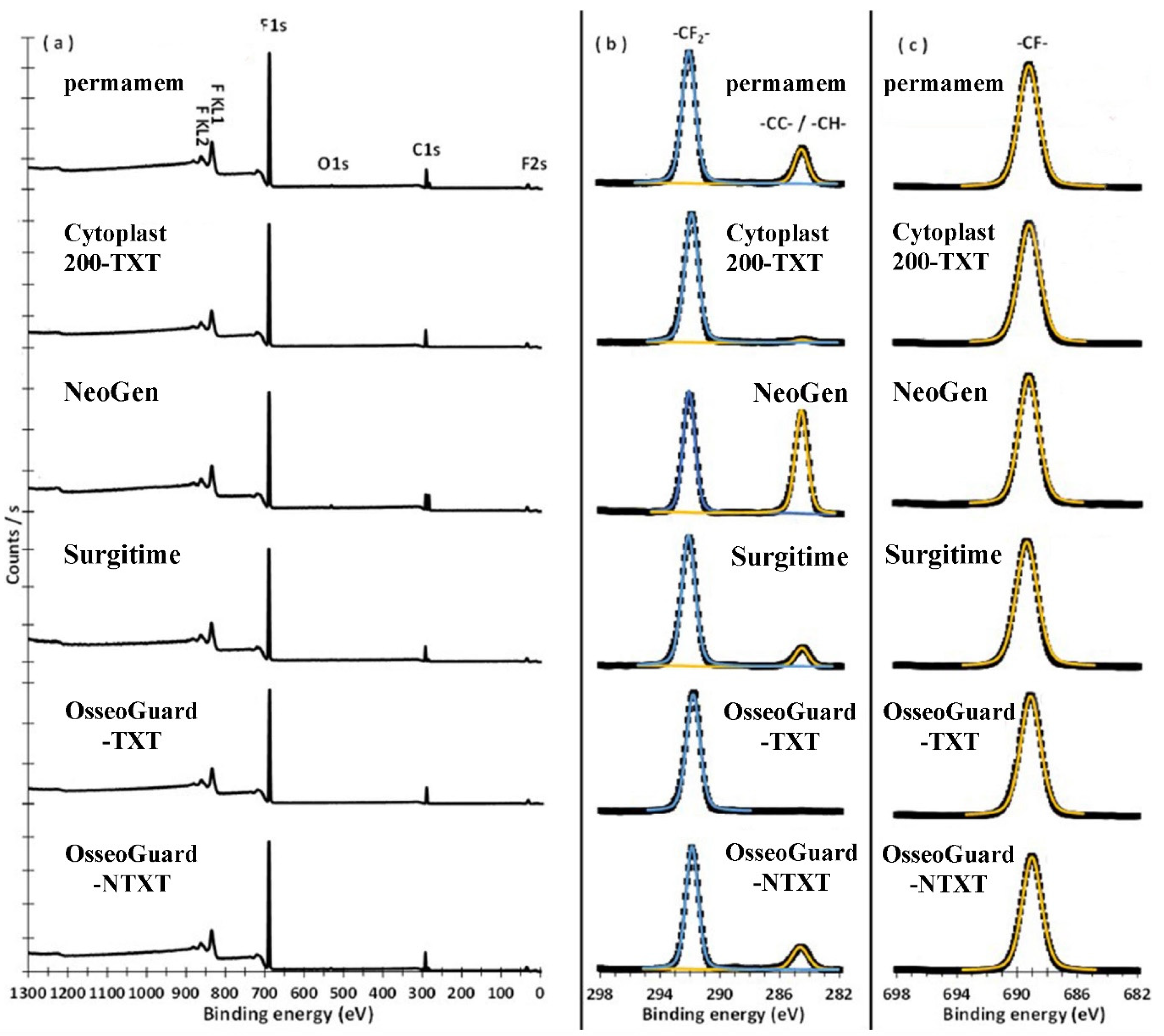
3.2. X-ray Photoelectron Spectroscopy (XPS)
3.3. Mechanical and Physical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol 2019, 46, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Rathnayake, N.; Trajkovski, B.; Rahman, B.; Zafiropoulos, G.G. Clinical applications and outcomes of non-resorbable polytetrafluoroethylene (PTFE) membranes in guided bone regeneration: Review. J. Int. Dent. Med. Res. 2019, 12, 1626–1635. [Google Scholar]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernández–Alfaro, F. Physicochemical characterization of barrier membranes for bone regeneration. J. Mech. Beh. Biomed Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus non-resorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar]
- Turri, A.; Čirgić, E.; Shah, F.A.; Hoffman, M.; Omar, O.; Dahlin, C.; Trobos, M. Early plaque formation on PTFE membranes with expanded or dense surface structures applied in the oral cavity of human volunteers. Clin. Exp. Dent. Res. 2021, 7, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 1–24. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef]
- Bartee, B.K.; Carr, J.A. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J. Oral. Implantol. 1995, 21, 88–95. [Google Scholar]
- Carbonell, J.; Martín, I.; Santos, A.; Pujol, A.; Sanz-Moliner, J.J.N. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral. Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bartee, B.K. Evaluation of a new polytetrafluoroethylene guided tissue regeneration membrane in healing extraction sites. Compend. Contin. Educ. Dent. 1998, 19, 1256–1258. [Google Scholar] [PubMed]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral. Implant Res 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, T.; Ambruster, J. The development of guided regeneration: Making the impossible possible and the unpredictable predictable. Evi Bas Dent Prac 2012, 12, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Qian, Y.; Zhang, B.; Zhang, Z.; Jiang, Z. Surface engineering and self-cleaning properties of the novel TiO2/PAA/PTFE ultrafiltration membranes. Appl. Petrochem. Res. 2016, 6, 225–233. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Glaris, P.; Coulon, J.-F.; Dorget, M.; Poncin-Epaillard, F. Thermal annealing as a new simple method for PTFE texturing. Polymer 2013, 54, 5858–5864. [Google Scholar] [CrossRef]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (PTFE) membranes for guided bone regeneration. Clin. Implant Dent. Relat. Res. 2018, 20, 738–748. [Google Scholar] [CrossRef]
- Begic, G.; Petkovic Didovic, M.; Lucic Blagojevic, S.; Jelovica Badovinac, I.; Žigon, J.; Percic, M.; Cvijanovic Peloza, O.; Gobin, I. Adhesion of oral bacteria to commercial d-PTFE membranes: Polymer microstructure makes a difference. Int. J. Mol. Sci. 2022, 23, 2983. [Google Scholar] [CrossRef]
- Jiang, Y.; Bo Li, B.; Tanabashi, Y. Estimating the relation between surface roughness and mechanical properties of rock joints. Int. J. Rock Mech. Min. 2006, 43, 837–846. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, J.H.; Kim, C.H.; Kim, Y.J. Fabrication of polytetrafluoroethylene nanofibrous membranes for guided bone regeneration. RSC Adv. 2018, 8, 34359–34369. [Google Scholar] [CrossRef]
- Park, H.; Tinh, V.D.C.; Kim, D. Surface hydrophilization toward the proton conductive porous PTFE substrate impregnating SPEEK for polymer electrolyte membranes. Progr. Org. Coat. 2022, 163, 106643. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Tasiopoulos, C.P.; Petronis, S.; Sahlin, H.; Hedhammar, M. Surface functionalization of PTFE membranes intended for guided bone regeneration using recombinant spider silk. ACS Appl. Bio Mater. 2020, 3, 577–583. [Google Scholar] [CrossRef]
- Raz, P.; Brosh, T.; Ronen, G.; Tal, H. Tensile Properties of three selected collagen membranes. Biomed. Res. Int. 2019, 2019, 5163603. [Google Scholar] [CrossRef]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Trajkovski, B.; Jaunich, M.; Müller, W.D.; Beuer, F.; Zafiropoulos, G.G.; Houshmand, A. Hydrophilicity, viscoelastic, and physicochemical properties variations in dental bone grafting substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Elder, S.H.; Jouett, R.; Yang, Y.; Ong, J.L. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Sources 2022, 525, 231121. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Barber, H.D.; Lignelli, J.; Smith, B.M.; Bartee, B.K. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J. Oral Maxillofac. Surg. 2007, 65, 748–752. [Google Scholar] [CrossRef]
- Zellin, G.; Linde, A. Effects of different osteopromotive membrane porosities on experimental bone neogenesis in rats. Biomaterials 1996, 17, 695–702. [Google Scholar] [CrossRef]
- Stamopoulos, A.G.; Tserpes, K.I.; Prucha, P.; Vavrik, D. Evaluation of porosity effects on the mechanical properties of carbon fiber-reinforced plastic unidirectional laminates by X-ray computed tomography and mechanical testing. J. Compos. Mater 2016, 50, 2087–2098. [Google Scholar] [CrossRef]
- Bertoldi, S.; Farè, S.; Tanzi, M.C. Assessment of scaffold porosity: The new route of micro-CT. J. Appl. Biomater. Biomech. 2011, 9, 165–175. [Google Scholar] [CrossRef]
- McGaughey, A.L.; Karandikar, P.; Gupta, M.; Childress, A.E. Hydrophobicity versus pore size: Polymer coatings to improve membrane wetting resistance for membrane distillation. ACS Appl. Polym. Mater. 2020, 23, 1256–1267. [Google Scholar] [CrossRef]
- Kampschulte, M.; Langheinirch, A.C.; Sender, J.; Litzlbauer, H.D.; Althöhn, U.; Schwab, J.D.; Alejandre-Lafont, E.; Martels, G.; Krombach, G.A. Nano-computed tomography: Technique and applications. RoFo 2016, 188, 146–154. [Google Scholar] [CrossRef]
- Gottlow, J. Guided tissue regeneration using bioresorbable and nonresorbable devices: Initial healing and long-term results. J. Periodontol. 1993, 64 (Suppl. S11), 1157–1165. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982-1992: A decade of technology development for guided tissue regeneration. J. Periodontol. 1993, 64 (Suppl. S11), 1129–1137. [Google Scholar] [CrossRef]


| Membranes | Structure | Surface | Manufacturer |
|---|---|---|---|
| permamem® | hd-PTFE | NTXT | botiss biomaterials GmbH, Zossen, Germany |
| CytoplastTM TXT-200 | hd-PTFE | TXT | Osteogenics Biomedical Inc., Lubbock, TX, USA |
| NeoGen® | Dual e-PTFE | NTXT | Neoss Ltd., Harrogate, UK |
| OsseoGuard®-TXT | hd-PTFE | TXT | Zimmer Biomet Dental, Palm Beach Gardens, FL, USA |
| OsseoGuard®-NTXT | hd-PTFE | NTXT | Zimmer Biomet Dental, Palm Beach Gardens, FL, USA |
| Surgitime | PTFE | NTXT | Bionnovation Biomedical, Bauru, Brazil |
| Property | Membrane Type | |||||
|---|---|---|---|---|---|---|
| permamem® | CytoplastTM TXT-200 | NeoGen® | Surgitime | OsseoGuard® TXT | OsseoGuard® NTXT | |
| SEM characteristics | ||||||
| Fibril orientation | multidirectional, heterogenous, fibrillar | hexagonal shaped indentations, non-fibrillar | Monodirectional, fibrillar | heterogenous, non-fibrillar | hexagonal shaped indentations, non-fibrillar s | bi-directional, fibrillar |
| XPS surface characteristics | ||||||
| C1s BE, (FWHM), (a%), CB | 284.63, (1.06), (7.93), -CC-/-CH-292.05, (1.05), (30.13), -CF2- | 284.61, (1.28), (0.90), -CC-/-CH-291.96, (1.06), (32.83), -CF2- | 284.64, (0.96), (22.21), -CC-/-H- 292.09, (0.92), (25.25), -CF2- | 284.61, (1.07), (4.30), -CC-/-CH-292.16, (1.07), (30.12), -CF2- | 291.85, (1.09), (33.92), -CF2- | 284.63, (1.26), (7.37), -CC-/-CH-291.86, (0.98), (31.14), -CF2- |
| F1s BE, (FWHM), (a%), CB | 689.25, (1.62), 61.10), -CF- | 689.21, (1.70), (66.27), -CF- | 689.26, (1.56), (50.88), -CF- | 689.37, (1.70), (65.58), -CF- | 689.11, (1.60), (66.08), -CF- | 689.02, (1.55), (60.15), -CF- |
| O1s BE, (FWHM), (a%), CB | 531.58, (2.04), (0.84), -H/H2O | – | 531.94, (1.96), (1.66), -H/H2O | – | – | 532.02, (2.15), (1.34), -H/H2O |
| -CF2-/F | 0.49 | 0.49 | 0.49 | 0.46 | 0.51 | 0.52 |
| Properties | Permamem® | CytoplastTM TXT-200 | NeoGen® | Surgitime | OsseoGuard® TXT | OsseoGuard® NTXT | p |
|---|---|---|---|---|---|---|---|
| Mechanical properties | |||||||
| UTS (MPa) | 6.0 ± 0.7 | 4.3 ± 1.7 | 14.7 ± 2.0 | 4.8 ± 2.0 | 3.6 ± 0.4 | 3.8 ± 1.8 | <0.001 |
| Strain (%) a | 0.46 ± 0.03 | 0.59 ± 0.27 | 0.48 ± 0.15 | 0.54 ± 0.25 | 2.03 ± 0.94 | 1.30 ± 1.47 | 0.15 |
| E a | 7.4 ± 1.8 | 5.7 ± 2.1 | 34.4 ± 6.6 | 11.7 ± 6.9 | 3.4 ± 0.9 | 18.1 ± 1.3 | <0.001 |
| Physical properties | |||||||
| Roughness (µm) a | 2.1 ± 0.5 | 6.9 ± 3.6 | 3.2 ± 1.1 | 32.2 ± 15.6 | 6.1 ± 6.2 | 0.9 ± 0.3 | <0.001 |
| Density (g/cm3) | 1.57 ± 0.06 | 1.48 ± 0.24 | 1.27 ± 0.06 | 1.62 ± 0.12 | 1.35 ± 0.06 | 1.51 ± 0.20 | 0.09 |
| Wettability (CA) | 107.6 ± 0.5 | 96.8 ± 0.5 | 107.9 ± 0.3 | 139.4 ± 0.6 | 108.7 ± 8.8 | 88.8 ± 5.1 | <0.001 |
| Properties | Non-Textured | Textured | p | CytoplastTM TXT-200 | OsseoGuard®-TXT | p |
|---|---|---|---|---|---|---|
| UTS (MPa) | 7.3 ± 4.7 | 4.0 ± 1.2 | 0.11 | 4.3 ± 1.7 | 3.6 ± 0.4 | 0.53 |
| Strain (%) a | 0.70 ± 0.74 | 1.31 ± 1.00 | 0.99 | 0.59 ± 0.27 | 2.04 ± 0.94 | 0.04 |
| E a | 17.9 ± 11.5 | 4.7 ± 2.0 | 0.008 | 5.7 ± 2.1 | 43.4 ± 0.9 | 0.14 |
| Roughness (µm) a | 9.6 ± 15.2 | 6.5 ± 4.9 | 0.23 | 6.9 ± 3.6 | 6.1 ± 6.2 | 0.20 |
| Density (g/cm3) | 1.49 ± 0.18 | 1.42 ± 0.18 | 0.40 | 1.48 ± 0.24 | 1.35 ± 0.06 | 0.41 |
| Wettability (CA) | 110.9 ± 18.4 | 102.7 ± 8.6 | <0.001 | 96.8 ± 0.5 | 108.7 ± 8.7 | <0.001 |
| Variable | Combined Groups of Membranes | ||
|---|---|---|---|
| Textured | Non-Textured | p-Value | |
| Material volume (μm3) | |||
| Median | 1,310,736 | 1,000,850 | 0.98 |
| IQR | 514,594 | 309,093 | |
| Defect volume (μm3) | |||
| Median | 222,128 | 208,874 | 0.13 |
| IQR | 954,764 | 999,934 | |
| Defect volume ratio (%) | |||
| Median | 13,705 | 1197 | 0.011 |
| IQR | 109.523 | 195.569 | |
| Thickness (μm) | |||
| Median | 216.69 | 218.32 | 0.27 |
| IQR | 38.41 | 17.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasim, S.S.B.; Al-Asfour, A.A.; Abuzayeda, M.; Mohamed, A.M.; Trajkovski, B.; Murray, C.A.; Zafiropoulos, G.-G. Differences in Mechanical and Physicochemical Properties of Several PTFE Membranes Used in Guided Bone Regeneration. Materials 2023, 16, 904. https://doi.org/10.3390/ma16030904
Qasim SSB, Al-Asfour AA, Abuzayeda M, Mohamed AM, Trajkovski B, Murray CA, Zafiropoulos G-G. Differences in Mechanical and Physicochemical Properties of Several PTFE Membranes Used in Guided Bone Regeneration. Materials. 2023; 16(3):904. https://doi.org/10.3390/ma16030904
Chicago/Turabian StyleQasim, Syed Saad Bin, Adel A. Al-Asfour, Moosa Abuzayeda, Ahmed M. Mohamed, Branko Trajkovski, Colin Alexander Murray, and Gregor-Georg Zafiropoulos. 2023. "Differences in Mechanical and Physicochemical Properties of Several PTFE Membranes Used in Guided Bone Regeneration" Materials 16, no. 3: 904. https://doi.org/10.3390/ma16030904
APA StyleQasim, S. S. B., Al-Asfour, A. A., Abuzayeda, M., Mohamed, A. M., Trajkovski, B., Murray, C. A., & Zafiropoulos, G.-G. (2023). Differences in Mechanical and Physicochemical Properties of Several PTFE Membranes Used in Guided Bone Regeneration. Materials, 16(3), 904. https://doi.org/10.3390/ma16030904






