Shungite (Mineralized Carbon) as a Promising Electrode Material for Electroanalysis
Abstract
1. Introduction
2. Theoretical Section
3. Materials and Methods
3.1. Chemicals and Reagents
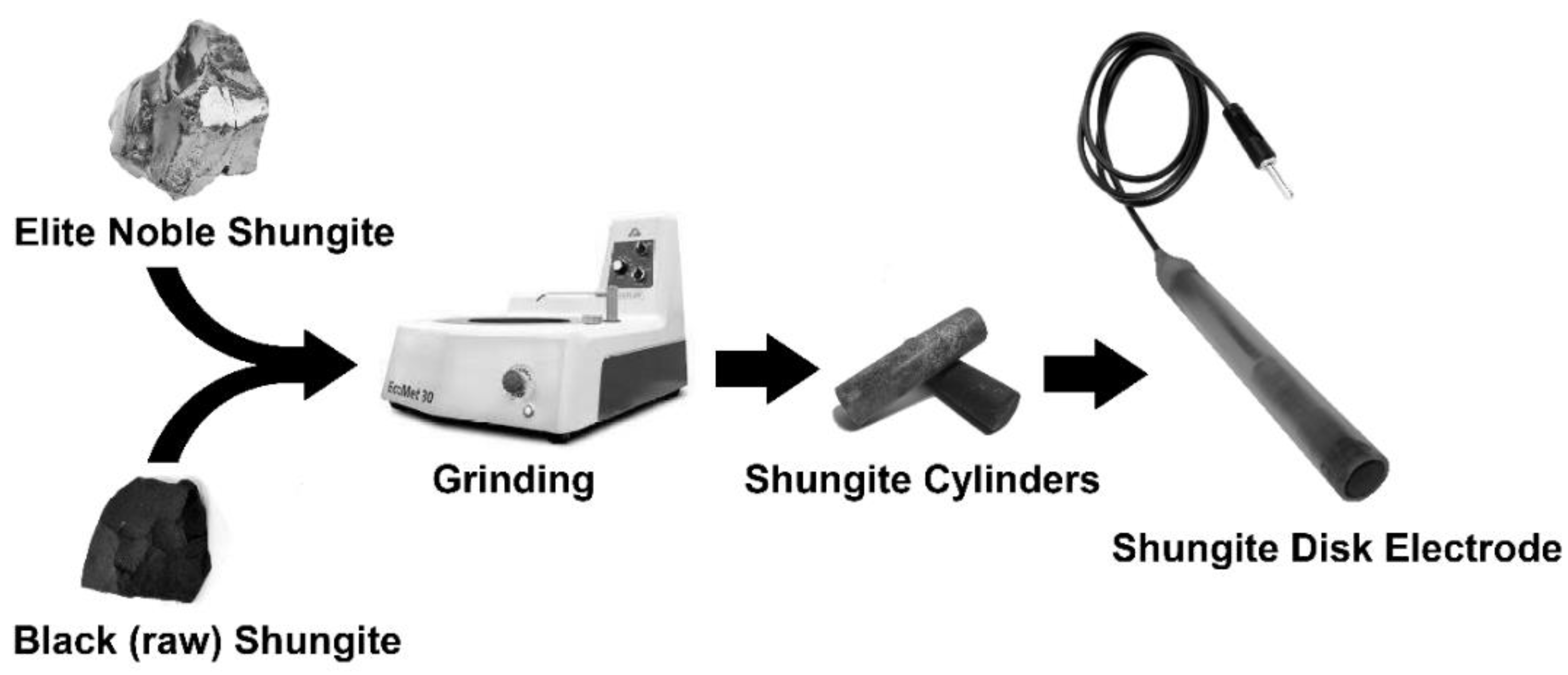
3.2. Electrode Manufacturing
Mechanical-Surface Pretreatment
3.3. Apparatus
3.4. Electrochemical Experiments
4. Results and Discussion
4.1. Microscopic Analysis of Shungite Stones
4.2. Electrochemical Characterisation of Shungite Electrodes
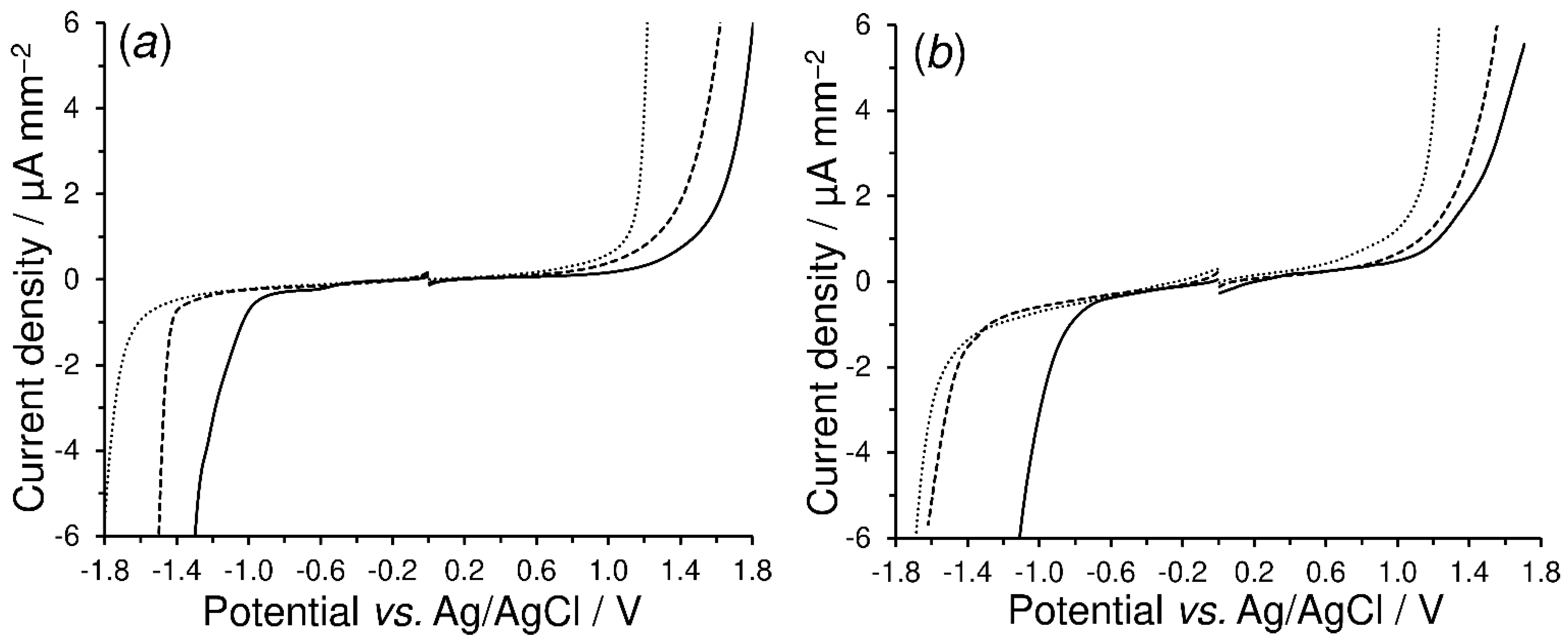
4.2.1. Residual Currents
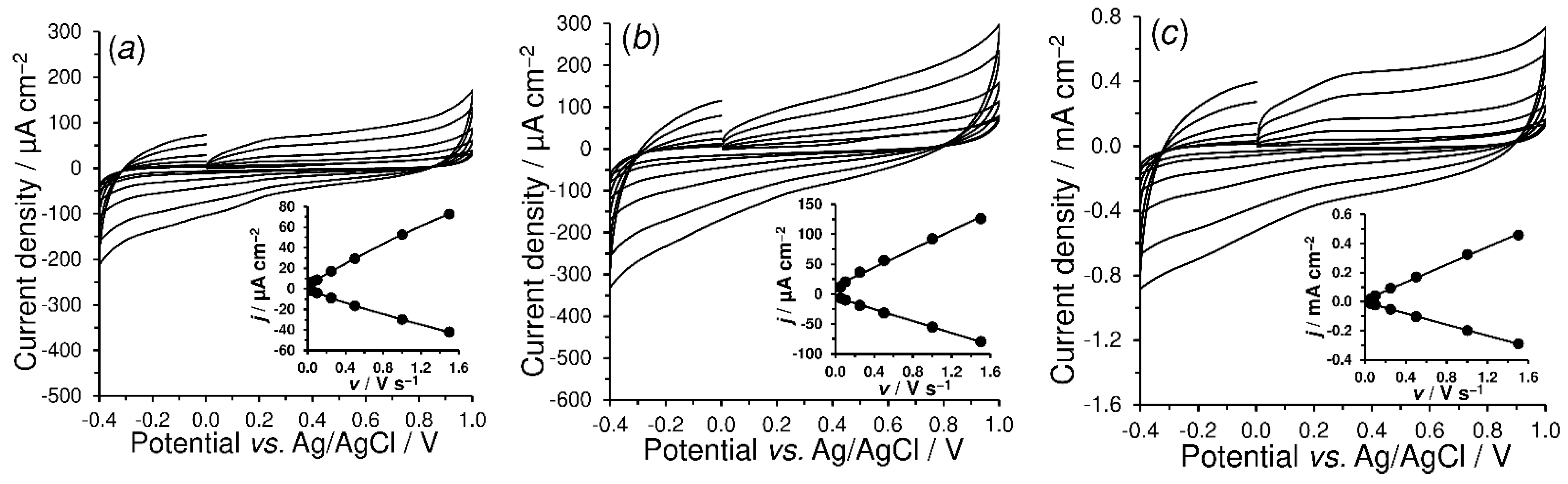
4.2.2. Double-Layer Capacitance
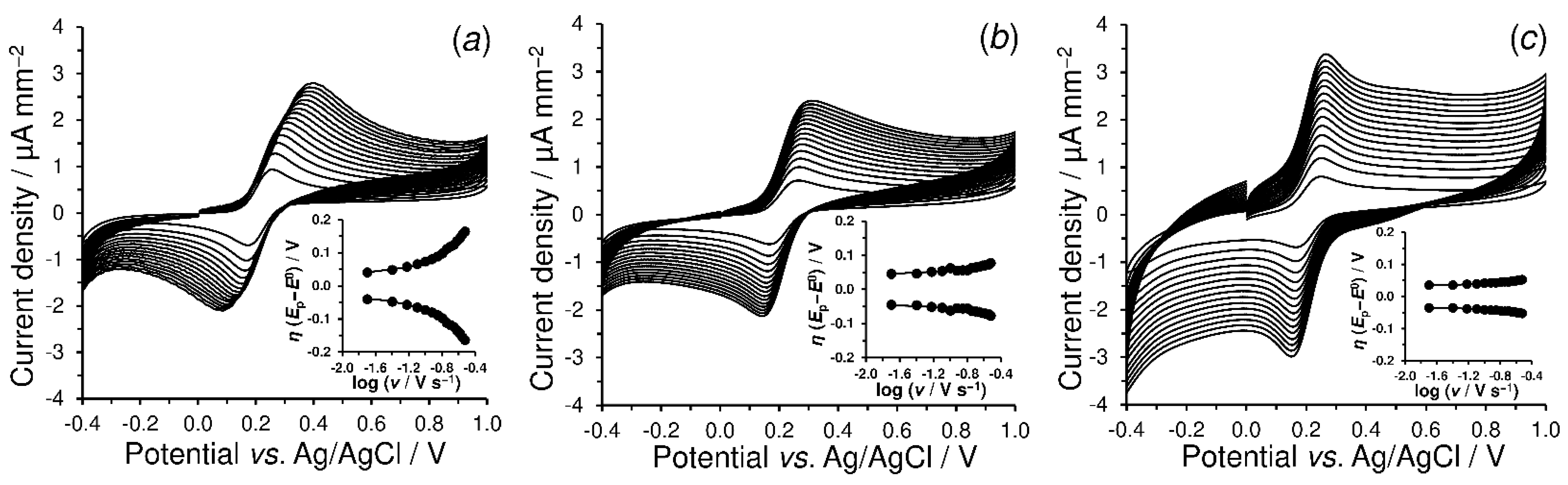
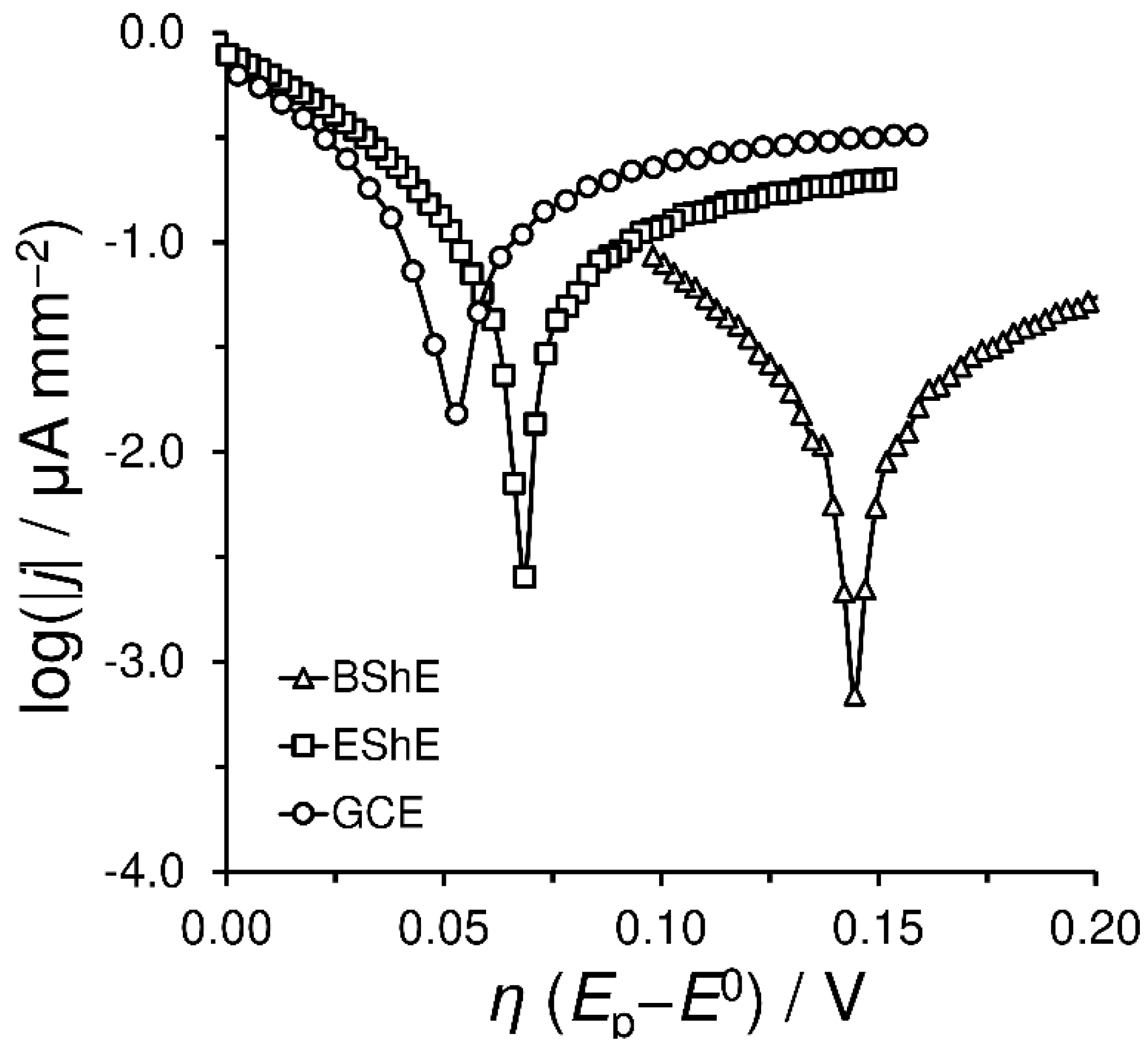
4.2.3. Electrochemical Properties of Shungite Materials
4.3. Electroanalytical Applicability
4.3.1. Potentiometric Indication of the Surfactants
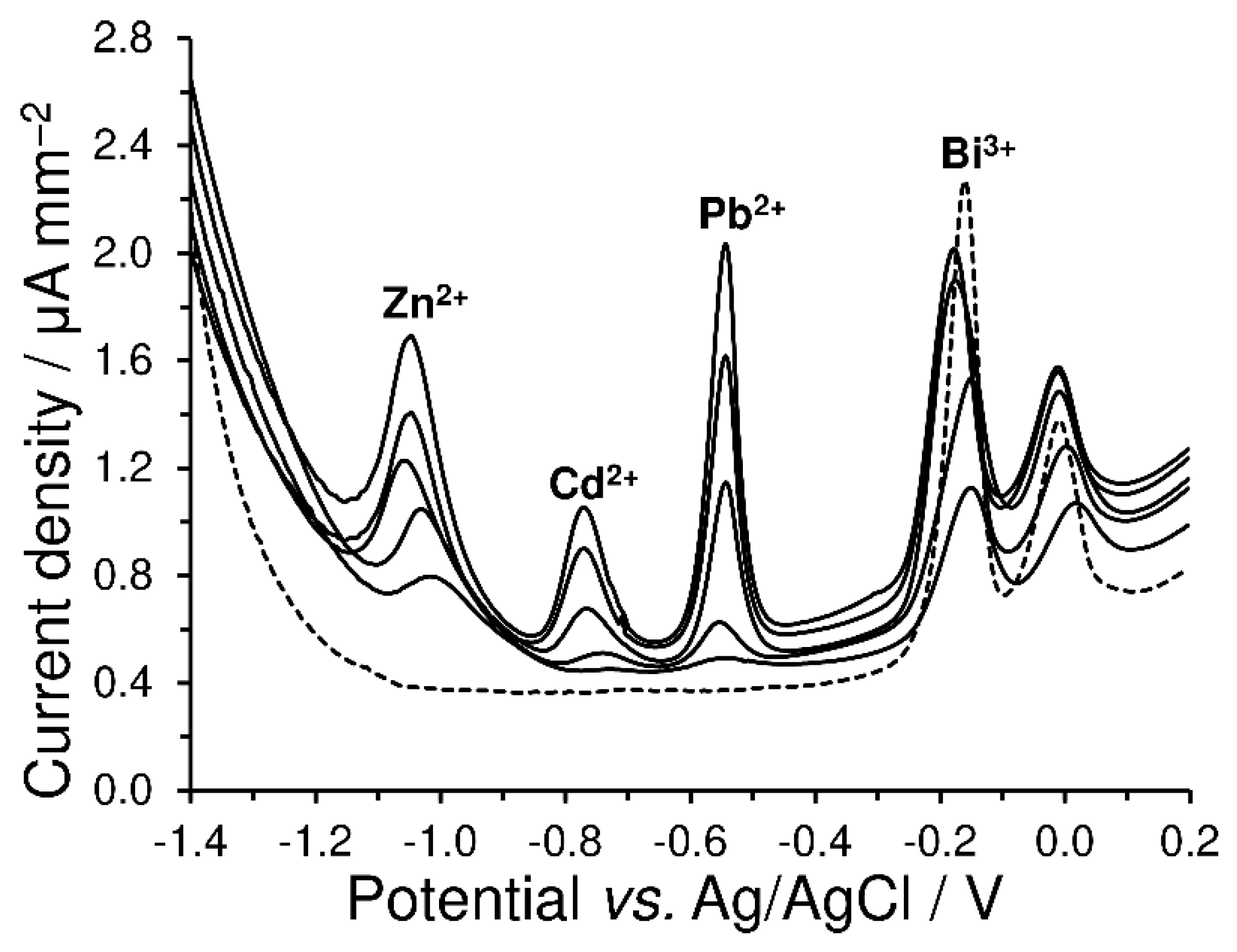
4.3.2. Anodic Stripping Voltammetry of Heavy Metals with a Green Electrode
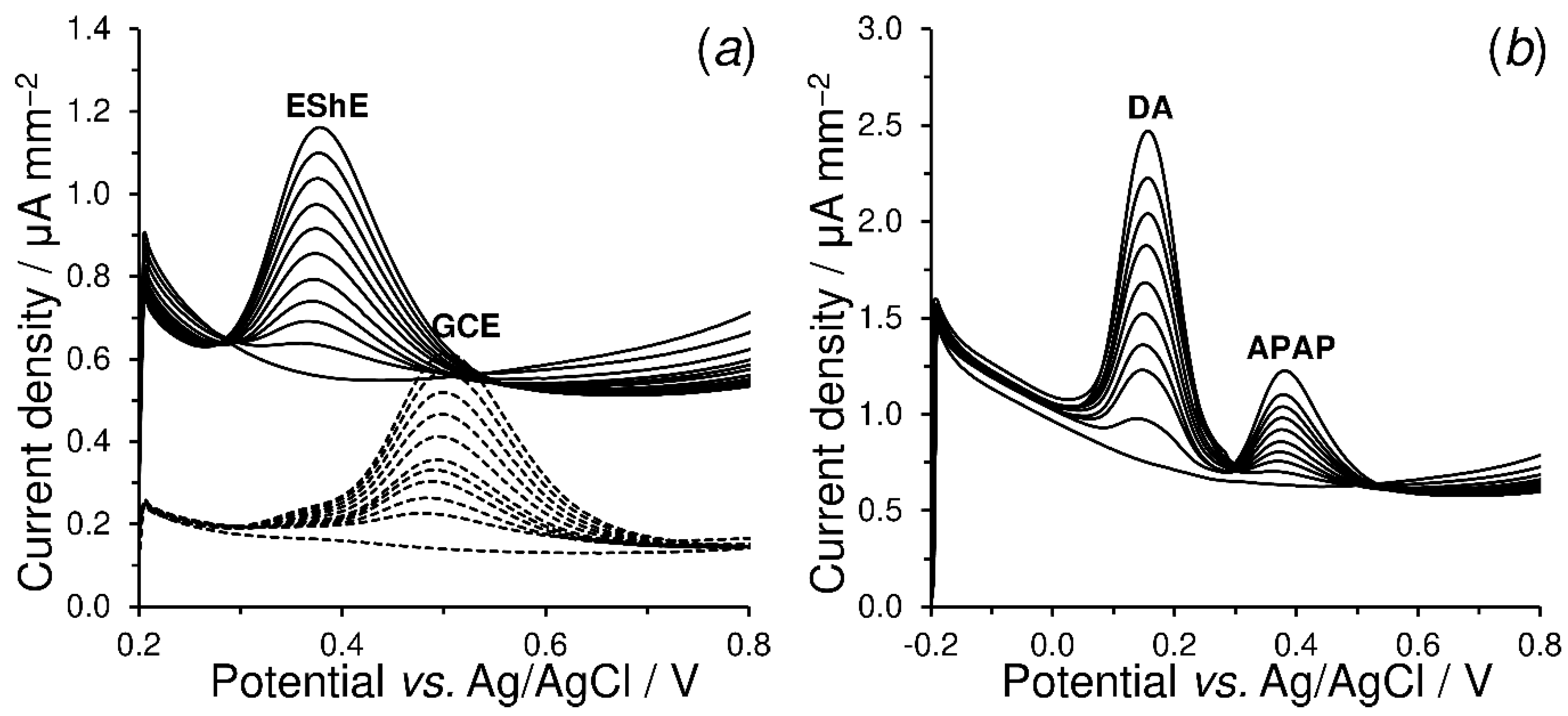
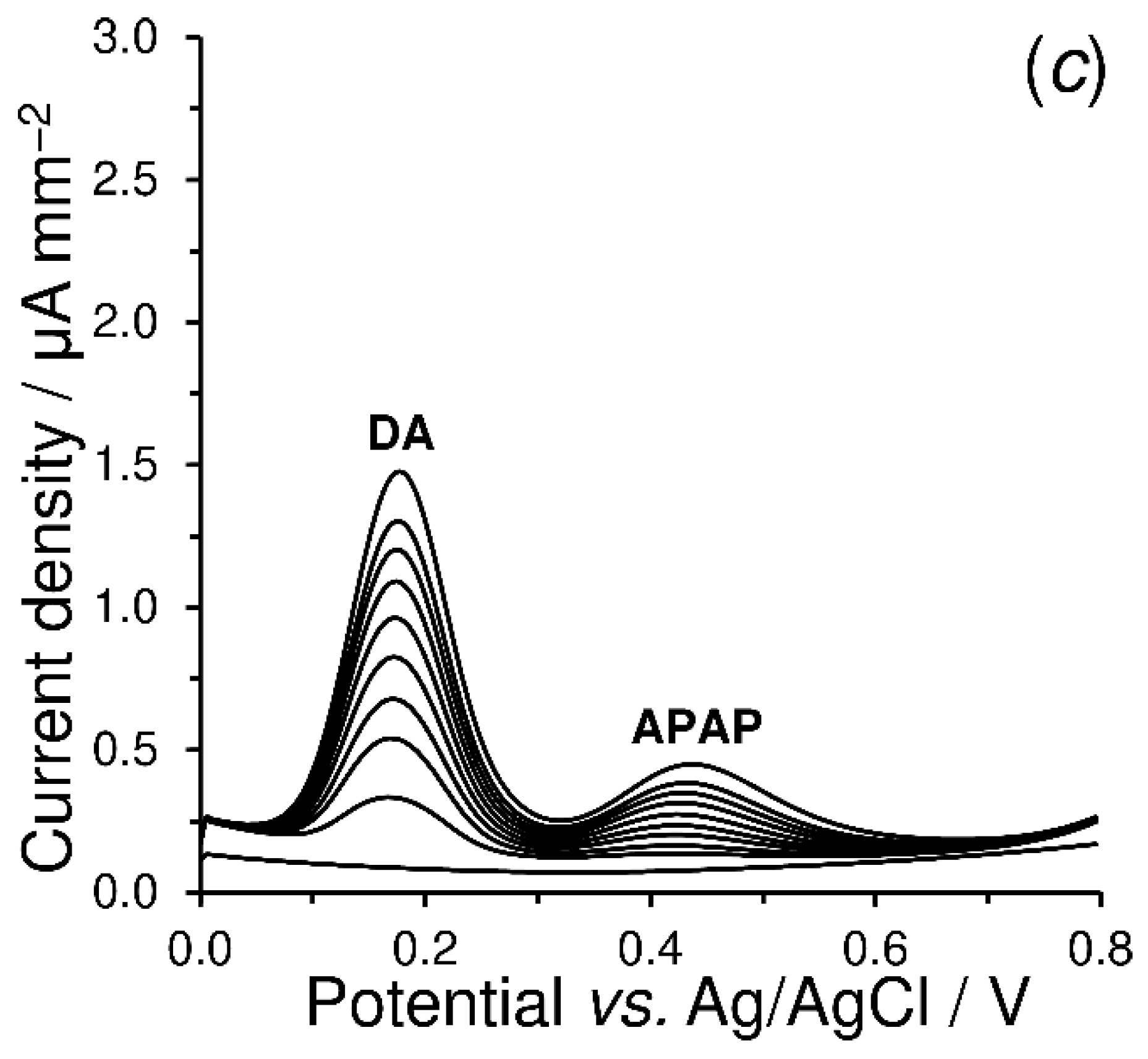
4.3.3. Electrosensing of Acetaminophen and Dopamine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melezhik, V.A.; Filippov, M.M.; Romashkin, A.E. A giant Palaeoproterozoic deposit of shungite in NW Russia: Genesis and practical applications. Ore Geol. Rev. 2004, 24, 135–154. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Antonets, I.V.; Shcheglov, V.I. Static and dynamic conductivity of nanostructured carbonaceous shungite geomaterials. Mater. Chem. Phys. 2019, 226, 195–203. [Google Scholar] [CrossRef]
- Kovalevski, V.V.; Buseck, P.R.; Cowley, J.M. Comparison of carbon in shungite rocks to other natural carbons: An X-ray and TEM study. Carbon 2001, 39, 243–256. [Google Scholar] [CrossRef]
- Reznikov, V.A.; Polekhovskii, Y.S. Amorphous shungite carbon: A natural medium for the formation of fullerenes. Tech. Phys. Lett. 2000, 26, 689–693. [Google Scholar] [CrossRef]
- Ignatov, I.; Popova, T.P.; Petrova, T.; Ignatov, A.I. Physicochemical parameters and in vitro antimicrobial effects of water filtrated with nano-structured carbonaceous shungite. J. Chem. Technol. Metall. 2022, 57, 937–945. [Google Scholar]
- Chou, N.H.; Pierce, N.; Lei, Y.; Perea-López, N.; Fujisawa, K.; Subramanian, S.; Robinson, J.A.; Chen, G.; Omichi, K.; Rozhkov, S.S.; et al. Carbon-rich shungite as a natural resource for efficient Li-ion battery electrodes. Carbon 2018, 130, 105–111. [Google Scholar] [CrossRef]
- Sajo, E.J.; Kim, C.S.; Kim, S.K.; Shim, K.Y.; Kang, T.Y.; Lee, K.J. Antioxidant and anti-inflammatory effects of shungite against ultraviolet B irradiation-induced skin damage in hairless mice. Oxid. Med. Cell Longev. 2017, 2017, 7340143. [Google Scholar] [CrossRef]
- Jushkin, N.P. Globular supramolecular structure shungite: Data scanning tunneling microscopy. Reports Acad. Sci. USSR. 1994, 337, 800–803. [Google Scholar]
- Frąc, M.; Szudek, W.; Szołdra, P.; Pichór, W. The applicability of shungite as an electrically conductive additive in cement composites. J. Build. Eng. 2022, 45, 103469. [Google Scholar] [CrossRef]
- Jurgelane, I.; Locs, J. Shungite application for treatment of drinking water—Is it the right choice? J. Water Health 2021, 19, 89–96. [Google Scholar] [CrossRef]
- Kazimova, N.; Ping, K.; Alam, M.; Danilson, M.; Merisalu, M.; Aruväli, J.; Paiste, P.; Käärik, M.; Mikli, V.; Leis, J.; et al. Shungite-derived graphene as a carbon support for bifunctional oxygen electrocatalysts. J. Catal. 2021, 395, 178–187. [Google Scholar] [CrossRef]
- Ignatov, I.I.; Mosin, O.V. The structure and composition of carbonaceous fullerene containing mineral shungite and microporous crystalline aluminosilicate mineral zeolite. Nanotech. Res. Pract. 2004, 1, 30–42. [Google Scholar] [CrossRef]
- Mosin, O.; Ignatov, I.I. The structure and composition of natural carbonaceous fullerene containing mineral shungite. Int. J. Adv. Sci. Tech. Res. 2013, 6, 9–21. [Google Scholar]
- Atchabarova, A.A.; Tokpayev, R.; Kabulov, A.T.; Nechipurenko, S. New electrodes prepared from mineral and plant raw materials of Kazakhstan. Eurasian Chem. Technol. J. 2016, 18, 141–147. [Google Scholar] [CrossRef]
- Xia, L.; Song, H.; Li, X.; Zhang, X.; Gao, B.; Zheng, Y.; Huo, K.; Chu, P.K. Hierarchical 0D−2D Co/Mo selenides as superior bifunctional electrocatalysts for overall water splitting. Front. Chem. 2020, 8, 382. [Google Scholar] [CrossRef]
- Morales, D.M.; Risch, M. Seven steps to reliable cyclic voltammetry measurements for the determination of double layer capacitance. J. Phys. Energy 2021, 3, 034013. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Moldenhauer, J.; Meier, M.; Paul, D.W. Rapid and direct determination of diffusion coefficients using microelectrode arrays. J. Electrochem. Soc. 2016, 163, H672. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Pumera, M.; Miyahara, Y. What amount of metallic impurities in carbon nanotubes is small enough not to dominate their redox properties? Nanoscale 2009, 1, 260–265. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Khataee, A.; Zarei, M.; Zhang, Y. Two-electron oxygen reduction on fullerene C60-carbon nanotubes covalent hybrid as a metal-free electrocatalyst. Sci. Rep. 2019, 9, 13780. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Švancara, I.; Kalcher, K.; Walcarius, A.; Vytřas, K. Electroanalysis with Carbon Paste Electrodes; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Sýs, M.; Farag, A.S.; Švancara, I. Extractive stripping voltammetry at carbon paste electrodes for determination of biologically active organic compounds. Monatsh. Chem. 2019, 150, 373–386. [Google Scholar] [CrossRef]
- Wang, J.; Kirgöz, Ü.A.; Mo, J.W.; Lu, J.; Kawde, A.N.; Muck, A. Glassy carbon paste electrodes. Electrochem. Commun. 2001, 3, 203–208. [Google Scholar] [CrossRef]
- Vinay, M.M.; Nayaka, Y.A. Iron oxide (Fe2O3) nanoparticles modified carbon paste electrode as an advanced material for elec-trochemical investigation of paracetamol and dopamine. J. Sci. Adv. Mater. Devices 2019, 4, 442–450. [Google Scholar] [CrossRef]
- Tarasevich, Y.I.; Bondarenko, S.V.; Polyakov, V.E.; Zhukova, A.I.; Ivanova, Z.G.; Lukyanov, V.V.; Malysh, G.N. The study of the structural, sorption, and electrochemical properties of a natural composite shungite. Colloid. J. 2008, 70, 349–355. [Google Scholar] [CrossRef]
- James, H.; Carmack, G.; Freiser, H. Coated wire ion-selective electrodes. Anal. Chem. 1972, 44, 856–857. [Google Scholar] [CrossRef]
- Vytřas, K. Coated wire electrodes in the analysis of surfactants of various types: An overview. Electroanalysis 1991, 3, 343–347. [Google Scholar] [CrossRef]
- Vytřas, K. Determination of some pharmaceuticals using simple potentiometric sensors of coated-wire type. Mikrochim. Acta 1984, 84, 139–148. [Google Scholar] [CrossRef]
- Anastasiadou, Z.D.; Sipaki, I.; Jannakoudakis, P.D.; Girousi, S.T. Square-wave anodic stripping voltammetry (SWASV) for the determination of ecotoxic metals, using a bismuth-film electrode. Anal. Lett. 2011, 44, 761–777. [Google Scholar] [CrossRef]
- Economou, A. Bismuth-film electrodes: Recent developments and potentialities for electroanalysis. Trends Anal. Chem. 2005, 24, 334–340. [Google Scholar] [CrossRef]
- Sýs, M.; Metelka, R.; Korecká, L.; Pokorná, H.; Švancara, I. Comparison of various bismuth film electrodes in simultaneous electrochemical detection of heavy metals for application in quantum dot-linked immunoassays. Monatsh. Chem. 2017, 148, 505–510. [Google Scholar] [CrossRef]
- Frangu, A.; Pravcová, K.; Šilarová, P.; Arbneshi, T.; Sýs, M. Flow injection tyrosinase biosensor for direct determination of acetaminophen in human urine. Anal. Bioanal. Chem. 2019, 411, 2415–2424. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M.; Pournaghi-Azar, M.H. Simultaneous determination of acetaminophen and dopamine using SWCNT modified carbon–ceramic electrode by differential pulse voltammetry. Electrochim. Acta 2011, 56, 2888–2894. [Google Scholar] [CrossRef]
- Chatraei, F.; Zare, H.R. A comparative study of the electrochemical characteristics and simultaneous determination of dopamine, acetaminophen, and aspirin at a ruthenium oxide nanoparticles modified glassy carbon electrode versus a bare one. Anal. Methods 2012, 4, 2940. [Google Scholar] [CrossRef]
- Silva, L.A.J.; Stefano, J.S.; Cardoso, R.M.; Prado, N.S.; Soares, P.H.T.; Nossol, E.; Munoz, R.A.A.; Angnes, L.; Richter, E.M. Evaluation of graphite sheets for production of high-quality disposable sensors. J. Electroanal. Chem. 2019, 833, 560–567. [Google Scholar] [CrossRef]
- Carvalho, J.H.S.; Gogola, J.L.; Bergamini, M.F.; Marcolino-Junior, L.H.; Janegitz, B.C. Disposable and low-cost lab-made screen-printed electrodes for voltammetric determination of L-dopa. Sens. Actuator Rep. 2021, 3, 100056. [Google Scholar] [CrossRef]
- Švancara, I.; Mikysek, T.; Stočes, M.; Ludvík, J. Graphite Powder and Related Material as the Principal Component of Carbon Paste Electrodes. In Graphite: Properties, Occurence and Use; Campbell, Q.C., Ed.; NOVA Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 163–188. ISBN 978-1-62618-577-7. [Google Scholar]
- Mooste, M.; Tkesheliadze, T.; Kozlova, J.; Kikas, A.; Kisand, V.; Treshchalov, A.; Tamm, A.; Aruväli, J.; Zagal, J.H.; Kannan, A.M.; et al. Transition metal phthalocyanine-modified shungite-based cathode catalysts for alkaline membrane fuel cell. Int. J. Hydrog. Energy 2021, 46, 4365–4377. [Google Scholar] [CrossRef]


| Shungites | C | O | Si | Fe | S | Al | Cl | K | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Noble elite | 90.6–94.1 | 8–5.9 | 0.2 | 0.6 | 0.4 | — | 0.2 | — | — |
| Black raw | 62.3–64.2 | 24.7–55.2 | 8.8–32.3 | 0–20.4 | 0.2–7.1 | 0.6–1.1 | — | 0.5–2.1 | 0–0.3 |
| Sensor | R/Ω cm | Ageo/cm2 | ECSA/cm2 | CDL/μF | k0/cm s−1 | α | j0/A cm−2 |
|---|---|---|---|---|---|---|---|
| EShE | 0.3 | 0.502 | 0.311 | 45.7 | 0.0078 | 0.47 | 4.3 × 10−6 |
| BShE | 9.6 | 0.636 | 0.472 | 192.0 | 0.0159 | 0.45 | 8.7 × 10−7 |
| GCE | 4.5 × 10−3 | 0.071 | 0.047 | 27.6 | 0.0069 | 0.49 | 5.5 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sýs, M.; Bártová, M.; Bartoš, M.; Švancara, I.; Mikysek, T. Shungite (Mineralized Carbon) as a Promising Electrode Material for Electroanalysis. Materials 2023, 16, 1217. https://doi.org/10.3390/ma16031217
Sýs M, Bártová M, Bartoš M, Švancara I, Mikysek T. Shungite (Mineralized Carbon) as a Promising Electrode Material for Electroanalysis. Materials. 2023; 16(3):1217. https://doi.org/10.3390/ma16031217
Chicago/Turabian StyleSýs, Milan, Michaela Bártová, Martin Bartoš, Ivan Švancara, and Tomáš Mikysek. 2023. "Shungite (Mineralized Carbon) as a Promising Electrode Material for Electroanalysis" Materials 16, no. 3: 1217. https://doi.org/10.3390/ma16031217
APA StyleSýs, M., Bártová, M., Bartoš, M., Švancara, I., & Mikysek, T. (2023). Shungite (Mineralized Carbon) as a Promising Electrode Material for Electroanalysis. Materials, 16(3), 1217. https://doi.org/10.3390/ma16031217







