Enzymatic Transesterification of Atlantic Salmon (Salmo salar) Oil with Isoamyl Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodologies for the Study of Oil and Biodiesel Properties
2.3. Transesterification of Salmon Oil
2.4. Optimization and Statistical Analysis of the Transesterification Process
- Y—predicted response,
- Xi, Xj—independent variables,
- β0, βi, βii and βj, βij—interaction constant coefficients.
3. Results and Discussions
3.1. Oil Properties
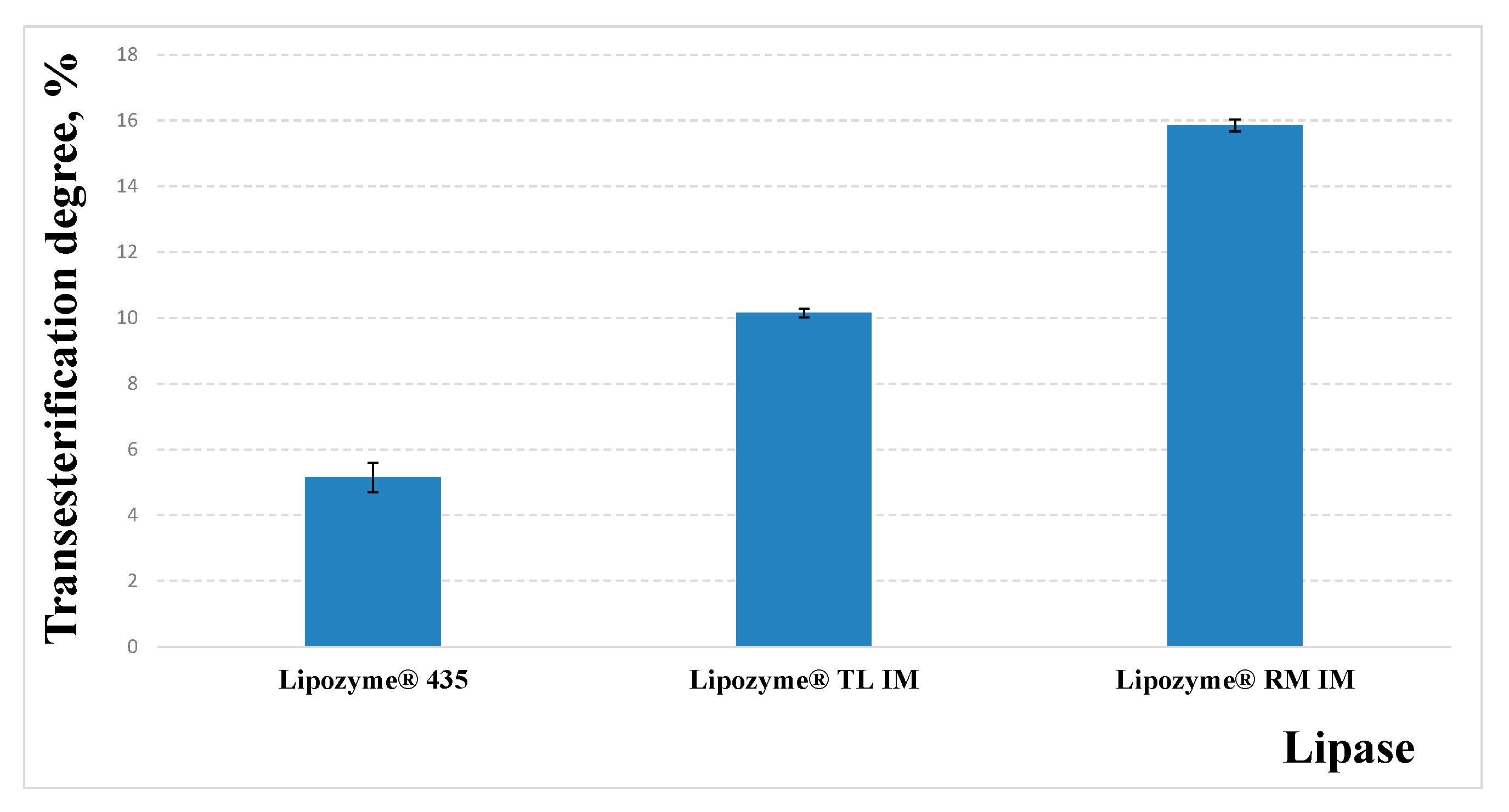
3.2. Selection of Biocatalyst for Transesterification Process
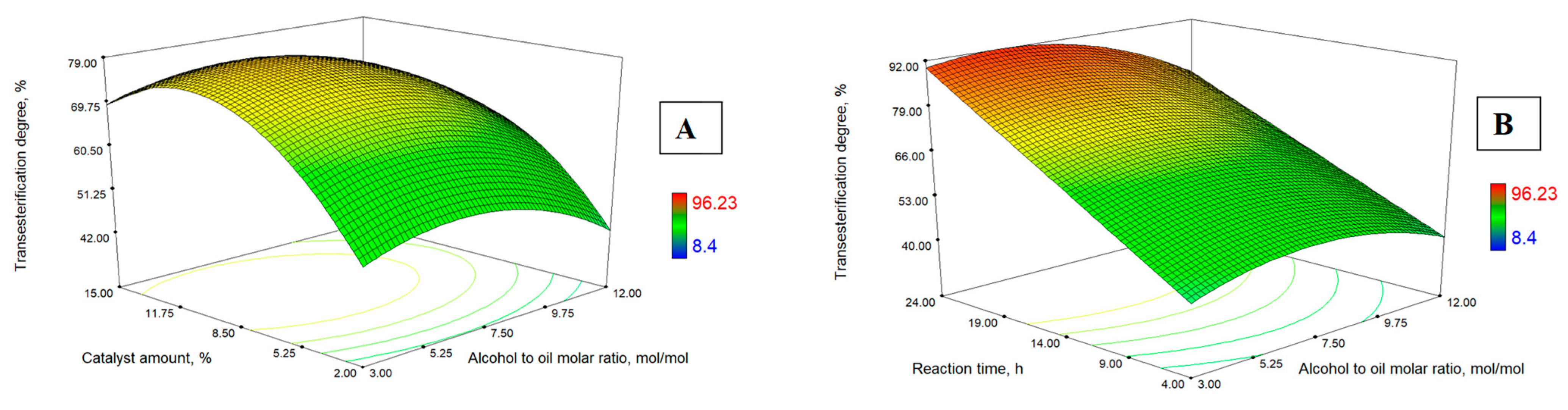
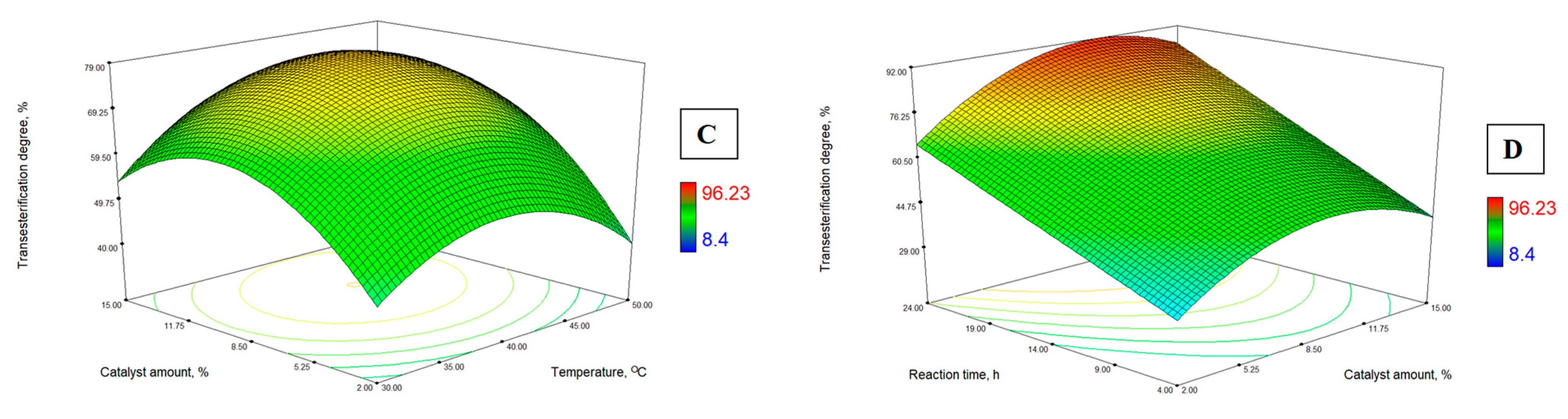
3.3. Response Surface Analysis
- Y—transesterification degree, %;
- the alcohol-to-oil molar ratio, mol/mol;
- temperature, the catalyst (snail shells) amount, °C;
- lipase amount, wt. %;
- the process duration, h.
3.4. Determination of Optimal Conditions and Their Validation
3.5. Gradual Addition of Isoamyl Alcohol
3.6. Determination of Optimal Conditions and Their Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production–A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Makoure, D.; Arhaliass, A.; Echchelh, A.; Legrand, J. Fish oil chemical composition for biodiesel production. J. Mater. Environ. Sci. 2019, 10, 1221–1229. [Google Scholar]
- Ghosh, N.; Halder, G. Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Reyes, J.F.; Sepúlveda, M.A. PM-10 emissions and power of a Diesel engine fueled with crude and refined Biodiesel from salmon oil. Fuel 2006, 85, 1714–1719. [Google Scholar] [CrossRef]
- Costa, E.; Almeida, M.F.; Alvim-Ferraz, C.; Dias, J.M. Otimization of Crambe abyssinica enzymatic transesterification using response surface methodology. Renew. Energy 2021, 174, 444–452. [Google Scholar] [CrossRef]
- Kumar, S.A.A.; Sakthinathan, G.; Vignesh, R.; Banu, J.R.; Al-Muhtaseb, A.H. Optimized transesterification reaction for efficient biodiesel production using Indian oil sardine fish as feedstock. Fuel 2019, 253, 921–929. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of dolomite as solid base catalyst for transesterification of rapeseed oil with butanol. Sustain. Energy Technol. Assess. 2022, 52, 102278. [Google Scholar] [CrossRef]
- Nayak, S.K.; Hoang, A.T.; Nayak, B.; Mishra, P.C. Influence of fish oil and waste cooking oil as post mixed binary biodiesel blends on performance improvement and emission reduction in diesel engine. Fuel 2021, 289, 119948. [Google Scholar] [CrossRef]
- Viswanathan, K.; Wang, S. Experimental investigation on the application of preheated fish oil ethyl ester as a fuel in diesel engine. Fuel 2021, 285, 119244. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V. Catalytic biodiesel synthesis under supercritical conditions. Mendeleev Commun. 2021, 31, 442–450. [Google Scholar] [CrossRef]
- Costa, J.F.; Almeida, M.F.; Alvim-Ferraz, M.C.M.; Dias, J.M. Biodiesel production using oil from fish canning industry wastes. Energy Convers. Manag. 2013, 74, 17–23. [Google Scholar] [CrossRef]
- Cerón, A.A.; Vilas Boas, R.N.; Biaggio, F.C.; de Castro, H.F. Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: Batch and continuous processes. Biomass. Bioenerg. 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Kiehbadroudinezhad, M.; Merabet, A.; Abo-Khalil, A.G.; Salameh, T.; Ghenai, C. Intelligent and Optimized Microgrids for Future Supply Power from Renewable Energy Resources: A Review. Energies 2022, 15, 3359. [Google Scholar] [CrossRef]
- Kiehbadroudinezhad, M.; Merabet, A.; Hosseinzadeh-Bandbafha, H. A life cycle assessment perspective on biodiesel production from fish wastes for green microgrids in a circular bioeconomy. Bioresour. Technol. Rep. 2023, 21, 101303. [Google Scholar] [CrossRef]
- Pham, E.C.; Le, T.V.T.; Le, K.C.T.; Ly, H.H.H.; Vo, B.N.T.; Nguyen, D.V.; Truong, T.N. Optimization of microwave-assisted biodiesel production from waste catfish using response surface methodology. Energy Rep. 2022, 8, 5739–5752. [Google Scholar] [CrossRef]
- Ramakrishnan, V.V.; Dave, D.; Liu, Y.; Routray, W.; Murphy, W. Statistical Optimization of Biodiesel Production from Salmon Oil via Enzymatic Transesterification: Investigation of the Effects of Various Operational Parameters. Processes 2021, 9, 700. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Ahmed, A.I. Ethanolysis of fish oil via optimized protocol and purification by dry washing of crude ethyl esters. J. Taiwan Inst. Chem. Eng. 2016, 58, 71–83. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Carvalho, W.C.A.; Luiz, J.H.H.; Fernandez-Lafuente, R.; Hirata, D.B.; Mendes, A.A. Eco-friendly production of trimethylolpropane triesters from refined and used soybean cooking oils using an immobilized low-cost lipase (Eversa>® Transform 2.0) as heterogeneous catalyst. Biomass Bioenerg. 2021, 155, 106302. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Karčauskiene, D.; Sendžikiene, E.; Makarevičiene, V.; Zaleckas, E.; Repšiene, R.; Ambrazaitiene, D. False flax (Camelina sativa L.) as an alternative source for biodiesel production. Žemdirbystė 2014, 101, 161–168. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E. Effectiveness of Eggshells as Natural Heterogeneous Catalysts for Transesterification of Rapeseed Oil with Methanol. Catalysts 2022, 12, 246. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.; de Almeida, F.N.C.; Souza, G.K.; Asanome, I.Y.; Pereira, N.C. Synthesis and optimization of ethyl esters from fish oil waste for biodiesel production. Renew. Energy 2019, 133, 743–748. [Google Scholar] [CrossRef]
- Gotovuša, M.; Pucko, I.; Racar, M.; Faraguna, F. Biodiesel Produced from Propanol and Longer Chain Alcohols—Synthesis and Properties. Energies 2022, 15, 4996. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, X.; Mishra, P.; Chong, C.B. Metabolically engineered Saccharomyces cerevisiae for enhanced isoamyl alcohol production. Appl. Microbiol. Biotechnol. 2017, 101, 465–474. [Google Scholar] [CrossRef]
- Pando, M.E.; Bravo, B.; Berrios, M.; Galdames, A.; Rojas, C.; Romero, N.; Camilo, C.; Encina, C.; Rivera, M.; Rodríguez, A.; et al. Concentrating n-3 fatty acids from crude and refined commercial salmon oil. Czech J. Food Sci. 2014, 32, 169–176. [Google Scholar] [CrossRef]
- Moreira, K.S.; Moura Júnior, L.S.; Monteiro, R.R.C.; de Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; de Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef]
- Gonçalves, M.C.P.; Amaral, J.C.; Fernandez-Lafuente, R.; Sousa Junior, R.d.; Tardioli, P.W. Lipozyme 435-Mediated Synthesis of Xylose Oleate in Methyl Ethyl Ketone. Molecules 2021, 26, 3317. [Google Scholar] [CrossRef]
- Park, S.B.; Endo, Y.; Maruyama, K.; Fujimoto, K. Enzymatic Synthesis of Ethyl Ester of Highly Unsaturated Fatty Acids from Fish Oils Using Immobilized Lipase. Food Sci. Technol. Res. 2000, 6, 192–195. [Google Scholar] [CrossRef]
- Yadav, G.D.; Trivedi, A.H. Kinetic modeling of immobilized-lipase catalyzed transesterification of n-octanol with vinyl acetate in non-aqueous media. Enzyme Microb. Technol. 2003, 32, 783–789. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem. 2007, 42, 409–414. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Ami, D.; de Divitiis, M.; Brocca, S.; Catelani, T.; Natalello, A.; Lotti, M. Short-chain alcohols inactivate an immobilized industrial lipase through two different mechanisms. Biotechnol. J. 2022, 17, e2100712. [Google Scholar] [CrossRef]
- Ungcharoenwiwat, P.; Canyuk, B.; Kittikun, H.A. Synthesis of jatropha oil based wax esters using an immobilized lipase from Burkholderia sp. EQ3 and Lipozyme RM IM. Process Biochem. 2016, 51, 392–398. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Pessela, B.C.C.; Volpato, G.; Fernandez-Lafuente, R.; Guisan, J.M.; Ayub, M.A.Z. Two step ethanolysis: A simple and efficient way to improve the enzymatic biodiesel synthesis catalyzed by an immobilized–stabilized lipase from Thermomyces lanuginosus. Process Biochem. 2010, 45, 1268–1273. [Google Scholar] [CrossRef]
- Ungcharoenwiwat, P.; Kittikun, A.H. Enzymatic synthesis of coconut oil based wax esters by immobilized lipase EQ3 and commercial lipozyme RMIM. Electron. J. Biotechnol. 2020, 47, 10–16. [Google Scholar] [CrossRef]
- Deng, L.; Xu, X.; Haraldsson, G.G.; Tan, T.; Wang, F. Enzymatic production of alkyl esters through alcoholysis: A critical evaluation of lipases and alcohols. J. Am. Oil Chem. Soc. 2005, 82, 341–347. [Google Scholar] [CrossRef]
- Amini, Z.; Ong, H.C.; Harrison, M.D.; Kusumo, F.; Mazaheri, H.; Ilham, Z. Biodiesel production by lipase-catalyzed transesterification of Ocimum basilicum L. (sweet basil) seed oil. Energy Convers. Manag. 2017, 132, 82–90. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, V.P.; Srivastava, A. Quality biodiesel via biotransesterification from inedible renewable sources. J. Clean. Prod. 2022, 379, 134653. [Google Scholar] [CrossRef]
- Kanaveli, I.P.; Atzemi, M.; Lois, E. Predicting the viscosity of diesel/biodiesel blends. Fuel 2017, 199, 248–263. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
| Factors | Name | Units | Low Actual | High Actual |
|---|---|---|---|---|
| A: | Alcohol-to-oil molar ratio | mol/mol | 3 | 10 |
| B: | Reaction temperature | °C | 30 | 50 |
| C: | Amount of catalyst (from oil mass) | % | 2 | 15 |
| D: | Process duration | h | 4 | 24 |
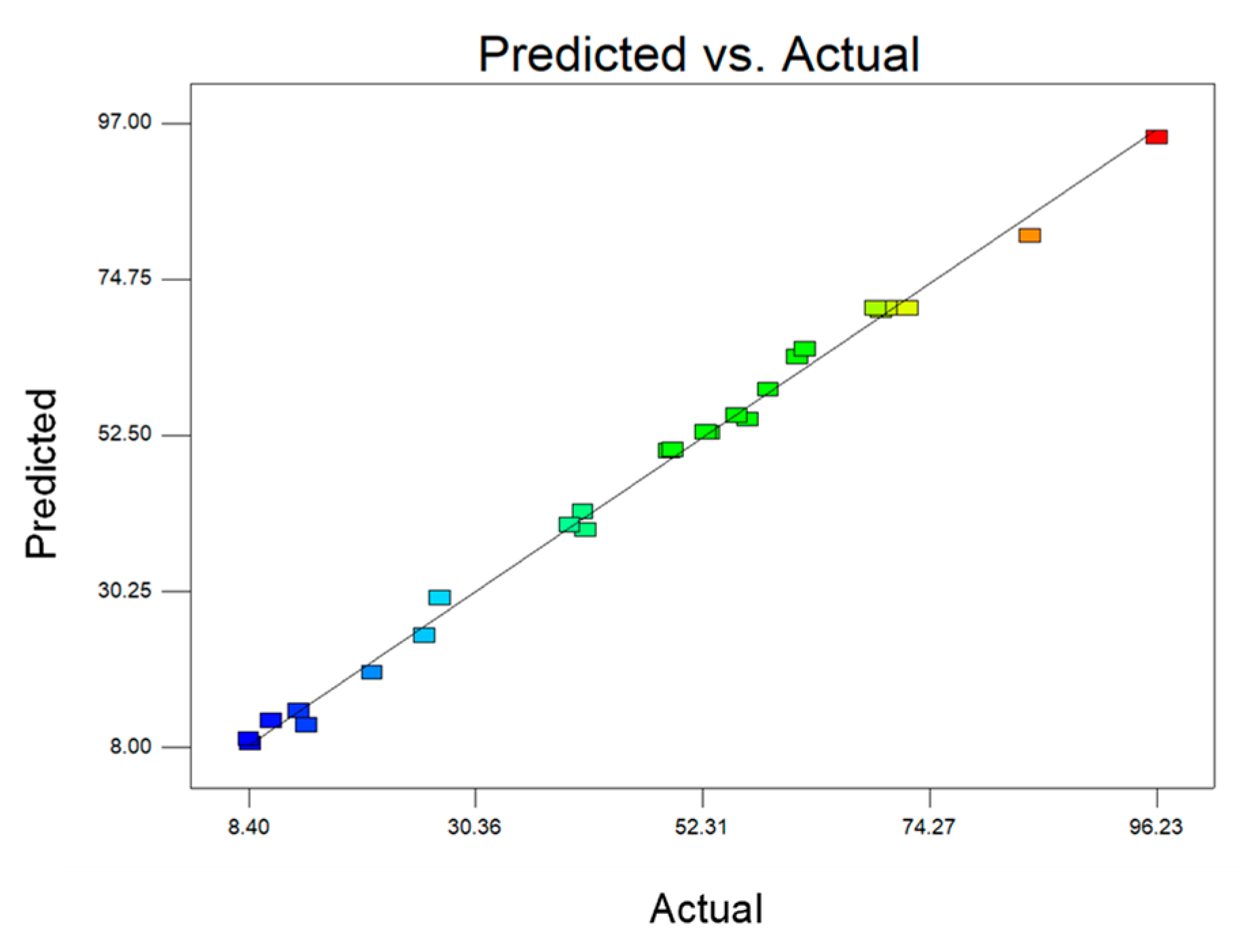
| No | Alcohol-to-Oil Molar Ratio, mol/mol | Reaction Temperature, °C | Amount of Catalyst, % (from Oil Mass) | Process Duration, h | Transesterification Degree, % | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| 1 | 3.00 | 30.00 | 2.00 | 4.00 | 14.01 | 11.23 |
| 2 | 12.00 | 30.00 | 2.00 | 4.00 | 10.54 | 11.83 |
| 3 | 3.00 | 50.00 | 2.00 | 4.00 | 8.57 | 8.62 |
| 4 | 12.00 | 50.00 | 2.00 | 4.00 | 8.40 | 9.22 |
| 5 | 3.00 | 30.00 | 15.00 | 4.00 | 20.36 | 18.68 |
| 6 | 12.00 | 30.00 | 15.00 | 4.00 | 13.28 | 13.29 |
| 7 | 3.00 | 50.00 | 15.00 | 4.00 | 26.93 | 29.37 |
| 8 | 12.00 | 50.00 | 15.00 | 4.00 | 25.40 | 23.98 |
| 9 | 3.00 | 30.00 | 2.00 | 24.00 | 52.99 | 52.91 |
| 10 | 12.00 | 30.00 | 2.00 | 24.00 | 40.72 | 41.64 |
| 11 | 3.00 | 50.00 | 2.00 | 24.00 | 49.08 | 50.30 |
| 12 | 12.00 | 50.00 | 2.00 | 24.00 | 40.97 | 39.02 |
| 13 | 3.00 | 30.00 | 15.00 | 24.00 | 69.57 | 70.25 |
| 14 | 12.00 | 30.00 | 15.00 | 24.00 | 52.66 | 52.98 |
| 15 | 3.00 | 50.00 | 15.00 | 24.00 | 84.00 | 80.94 |
| 16 | 12.00 | 50.00 | 15.00 | 24.00 | 61.52 | 63.67 |
| 17 | 2.10 | 40.00 | 8.50 | 14.00 | 62.21 | 64.80 |
| 18 | 12.90 | 40.00 | 8.50 | 14.00 | 56.66 | 54.80 |
| 19 | 7.50 | 28.00 | 8.50 | 14.00 | 49.47 | 50.50 |
| 20 | 7.50 | 52.00 | 8.50 | 14.00 | 55.64 | 55.34 |
| 21 | 7.50 | 40.00 | 0.70 | 14.00 | 39.44 | 39.79 |
| 22 | 7.50 | 40.00 | 16.30 | 14.00 | 58.66 | 59.04 |
| 23 | 7.50 | 40.00 | 8.50 | 2.00 | 32.52 | * |
| 24 | 7.50 | 40.00 | 8.50 | 26.00 | 96.23 | 95.00 |
| 25 | 7.50 | 40.00 | 8.50 | 14.00 | 70.48 | 70.59 |
| 26 | 7.50 | 40.00 | 8.50 | 14.00 | 72.12 | 70.59 |
| 27 | 7.50 | 40.00 | 8.50 | 14.00 | 69.08 | 70.59 |
| Parameter | Value |
|---|---|
| Acid value, mg KOH/g | 14.56 ± 0.23 |
| Acidity, % | 7.64 ± 0.42 |
| Moisture content, % | 3.1 ± 0.05 |
| Iodine value, g L2/100 g | 122.92 ± 0.95 |
| Density at 15 °C, kg/m3 | 930 ± 2.00 |
| Viscosity at 40 °C, mm2/s | 37.42 ± 0.21 |
| a* colour value | −1.74 ± 0.03 |
| b* colour value | 3.88 ± 0.02 |
| L* colour value | 3.79 ± 0.05 |
| Saturated fatty acids, %: | 20.26 |
| Butyric acid (C4:0) | 0.02 ± 0.0009 |
| Caproic acid (C6:0) | 0.06 ± 0.01 |
| Caprylic acid (C8:0) | 0.23 ± 0.05 |
| Capric acid (C10:0) | 0.29 ± 0.07 |
| Undecylic acid (C11:0) | 0.04 ± 0.0002 |
| Lauric acid (C12:0) | 0.04 ± 0.0003 |
| Tridecanoic acid (C13:0) | 1.46 ± 0.15 |
| Myristic acid (C14:0) | 2.63 ± 0.13 |
| Pentadecanoic acid (C15:0) | 0.2 ± 0.015 |
| Palmitic acid (C16:0) | 9.7 ± 1.78 |
| Margaric acid (C17:0) | 0.13 ± 0.045 |
| Stearic acid (C18:0) | 2.66 ± 0.26 |
| Arachidic acid (C20:0) | 1.07 ± 0.026 |
| Heneicosylic acid (C21:0) | 0.89 ± 0.033 |
| Behenic acid (C22:0) | 0.76 ± 0.05 |
| Tricosylic acid (C23:0) | 0.03 ± 0.0013 |
| Lignoceric acid (C24:0) | 0.05 ± 0.001 |
| Unsaturated fatty acids, %: | 79.76 |
| Monounsaturated fatty acids, %: | 52.92 |
| Myristoleic acid (C14:1 cis-9) | 0.41 ± 0.05 |
| Palmitoleic acid (C16:1 cis-9) | 2.86 ± 0.23 |
| Heptadecenoic acid (C17:1 cis-10) | 0.23 ± 0.044 |
| Oleic acid (C18:1) | 41.05 ± 0.58 |
| Paullinic acid (C20:1) | 3.85 ± 0.046 |
| Erucic acid (C22:1) | 2.92 ± 0.65 |
| Nervonic acid (C24:1) | 1.61 ± 0.25 |
| Poliunsaturated fatty acids, %: | 26.83 |
| Linoleic acid (C18:2) | 14.21 ± 0.95 |
| Linolenic acid (C18:3) | 5.28 ± 0.45 |
| Eicosadienoic acid (C20:2) | 0.04 ± 0.0001 |
| Dihomo-gamma-linolenic acid (C20:3 8,11,14) | 0.04 ± 0.0002 |
| Eicosatrienoic acid (C20:3 11,14,17) | 0.41 ± 0.11 |
| Arachidonic acid (C20:4) | 0.2 ± 0.05 |
| Eicosapentaenoic acid (C20:5) | 3.18 ± 0.47 |
| Brassic acid (C22:2) | 0.02 ± 0.001 |
| Docosahexaenoic acid (C22:6) | 3.45 ± 0.33 |
| Oil molecular mass, g/mol | 878.45 ± 2.05 |
| Source of Variation | Sum of Squares | Degrees of Freedom (df) | Mean Squares | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 14,964.06 | 11 | 1360.37 | 321.38 | <0.0001 | significant |
| A-molar ratio | 327.89 | 1 | 327.89 | 77.46 | <0.0001 | |
| B-temperature | 77.06 | 1 | 77.06 | 18.21 | 0.0008 | |
| C-catalyst | 1215.76 | 1 | 1215.76 | 287.21 | <0.0001 | |
| D-duration | 7109.11 | 1 | 7109.11 | 1679.47 | <0.0001 | |
| AC | 35.94 | 1 | 35.94 | 8.49 | 0.0113 | |
| AD | 141.13 | 1 | 141.13 | 33.34 | <0.0001 | |
| BC | 177.02 | 1 | 177.02 | 41.82 | <0.0001 | |
| CD | 97.71 | 1 | 97.71 | 23.08 | 0.0003 | |
| A2 | 293.37 | 1 | 293.37 | 69.31 | <0.0001 | |
| B2 | 786.79 | 1 | 786.79 | 185.87 | <0.0001 | |
| C2 | 1129.88 | 1 | 1129.88 | 266.93 | <0.0001 | |
| Residual | 59.26 | 14 | 4.23 | |||
| Lack of Fit | 54.63 | 12 | 4.55 | 1.97 | 0.3862 | not significant |
| Pure Error | 4.63 | 2 | 2.32 | |||
| Cor Total | 15,023.32 | 25 | ||||
| C.V. % = 4.42 | R2 = 0.9961 | Adeq Precision = 61.803 | ||||
| R2Adj = 0.9930 | R2Pred = 0.9844 | |||||
| Alcohol-to-Oil Molar Ratio, mol/mol | Temperature, °C | Amount of Catalyst, % (from Oil Mass) | Duration, h | Transesterification Degree, % | |
|---|---|---|---|---|---|
| Predicted | Actual | ||||
| 6.00 | 45.00 | 11.00 | 4.00 | 88.45 | 87.23 ± 0.03 |
| Properties | Unit | EN 14214 | ASTM 6751 | EN 590 | Produced Biodiesel |
|---|---|---|---|---|---|
| Ester content | % (m/m) | Min 96.5 | 96.5 ± 2.05 | ||
| Density at 15 °C | kg/m3 | 860–900 | 860–900 | 820–845 | 880.67 ± 0.23 |
| Viscosity at 40 °C | mm2/s | 3.5–5 | 1.9–6 | 2.0–4.5 | 4.93 ± 0.014 |
| Acid value | mg KOH/g | 0.5 max | 0.5 max | 0.41 ± 0.12 | |
| Iodine value | g L2/100 g | 120 max | 98.04 ± 1.02 | ||
| Free glycerol | % (m/m) | 0.02 max | 0.02 max | 0.004 ± 0.001 | |
| Monoglyceride content | % (m/m) | 0.8 max | 0.36 ± 0.02 | ||
| Diglyceride content | % (m/m) | 0.2 max | 0.02 ± 0.001 | ||
| Triglyceride content | % (m/m) | 0.2 max | 0.02 ± 0.001 | ||
| Total glycerol | % (m/m) | 0.25 max | 0.24 max | 0.011 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumbytė, M.; Makareviciene, V.; Sendzikiene, E. Enzymatic Transesterification of Atlantic Salmon (Salmo salar) Oil with Isoamyl Alcohol. Materials 2023, 16, 1185. https://doi.org/10.3390/ma16031185
Gumbytė M, Makareviciene V, Sendzikiene E. Enzymatic Transesterification of Atlantic Salmon (Salmo salar) Oil with Isoamyl Alcohol. Materials. 2023; 16(3):1185. https://doi.org/10.3390/ma16031185
Chicago/Turabian StyleGumbytė, Milda, Violeta Makareviciene, and Egle Sendzikiene. 2023. "Enzymatic Transesterification of Atlantic Salmon (Salmo salar) Oil with Isoamyl Alcohol" Materials 16, no. 3: 1185. https://doi.org/10.3390/ma16031185
APA StyleGumbytė, M., Makareviciene, V., & Sendzikiene, E. (2023). Enzymatic Transesterification of Atlantic Salmon (Salmo salar) Oil with Isoamyl Alcohol. Materials, 16(3), 1185. https://doi.org/10.3390/ma16031185








