The Effect of Copper on the Microstructure, Wear and Corrosion Resistance of CoCrCuFeNi High-Entropy Alloys Manufactured by Powder Metallurgy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Preparing Powder Mixtures of CoCrCuFeNi HEAs
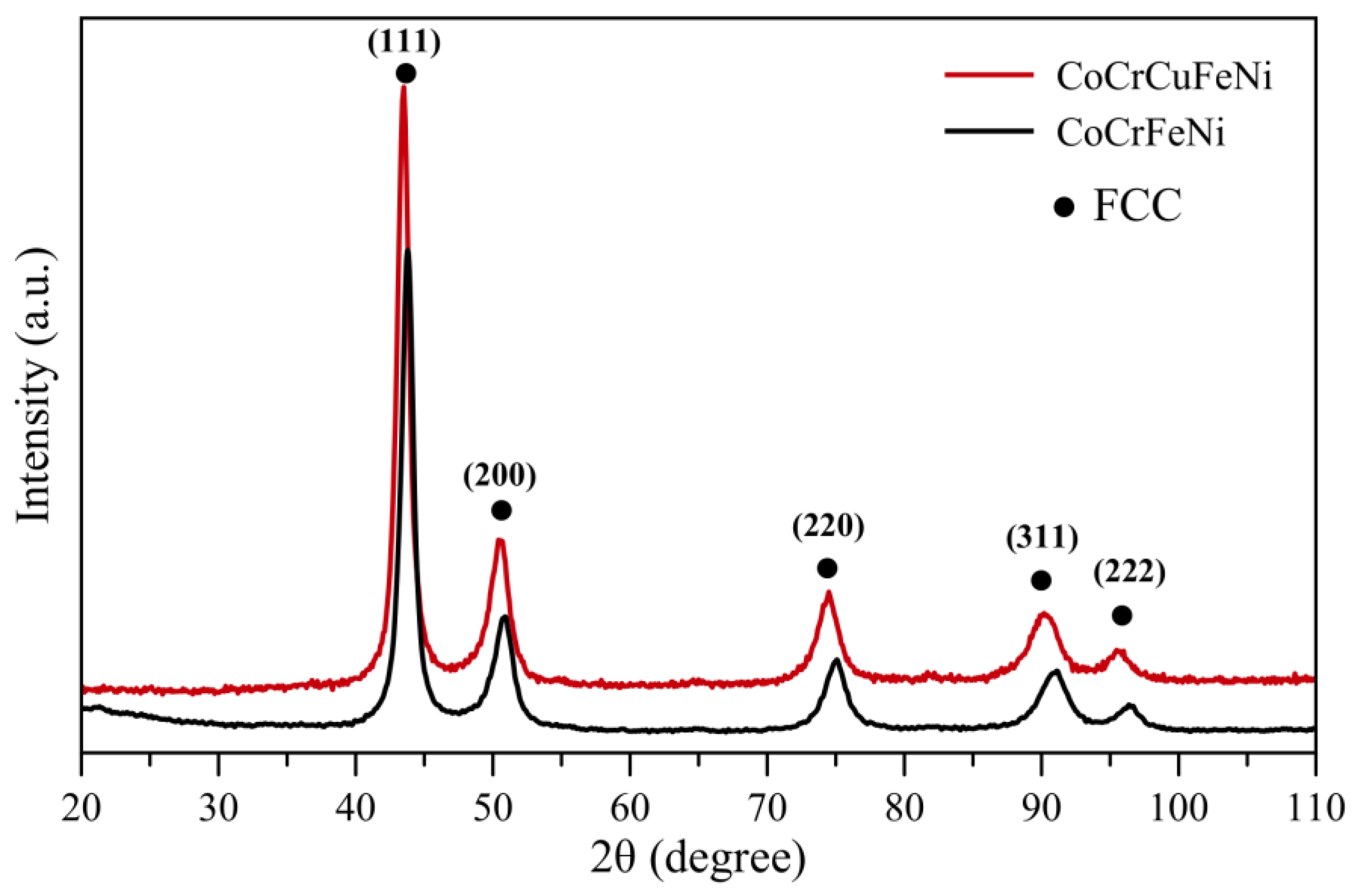
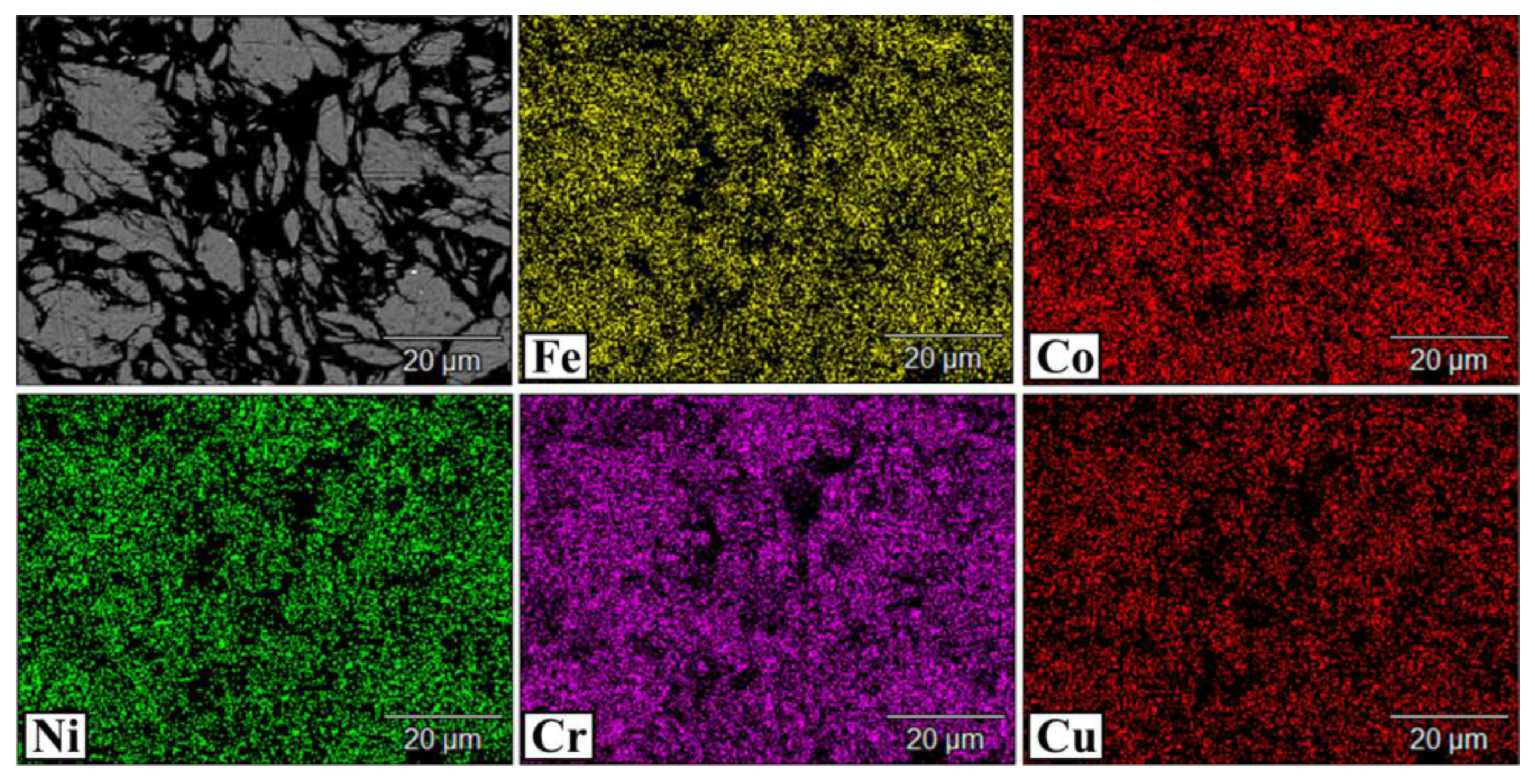
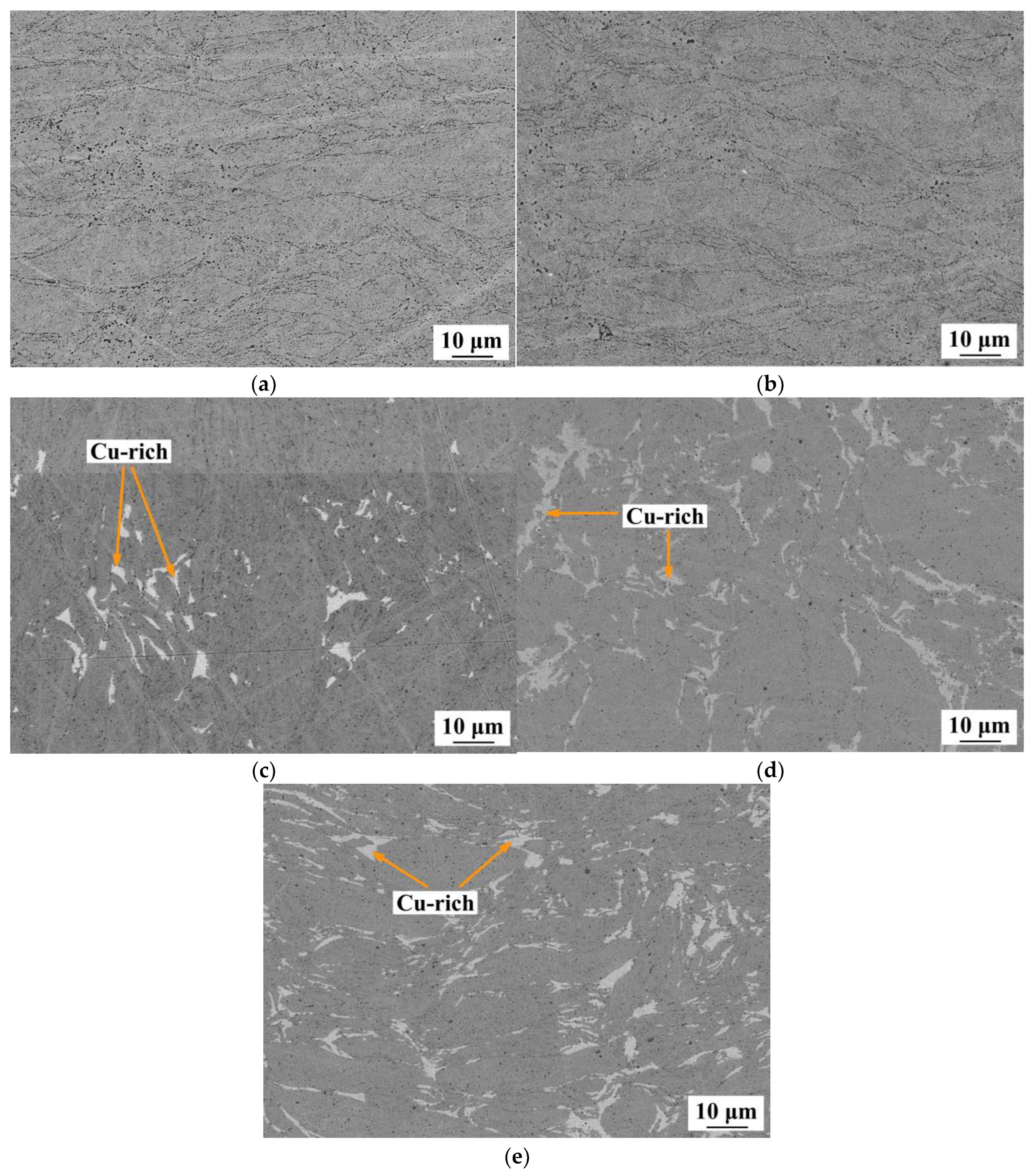
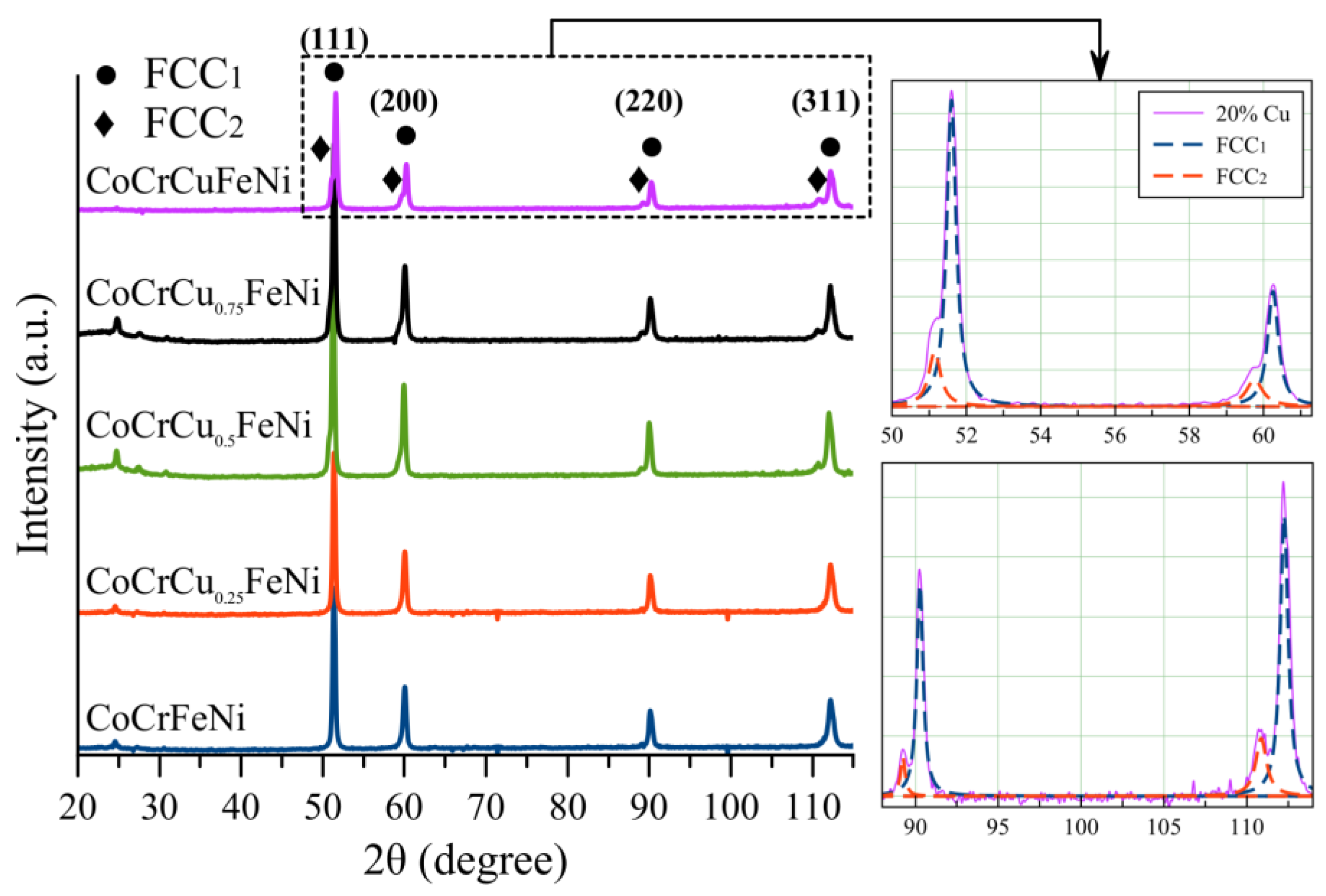
3.2. The Structure of CoCrCuFeNi Compact Samples
3.3. Tribological and Physicomechanical Properties
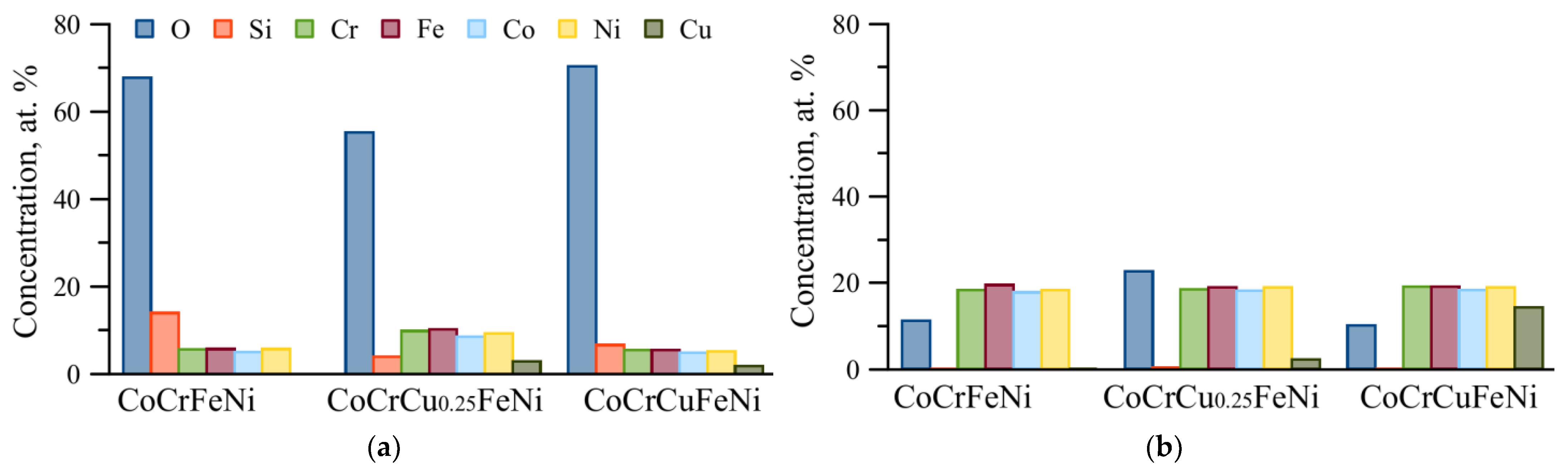
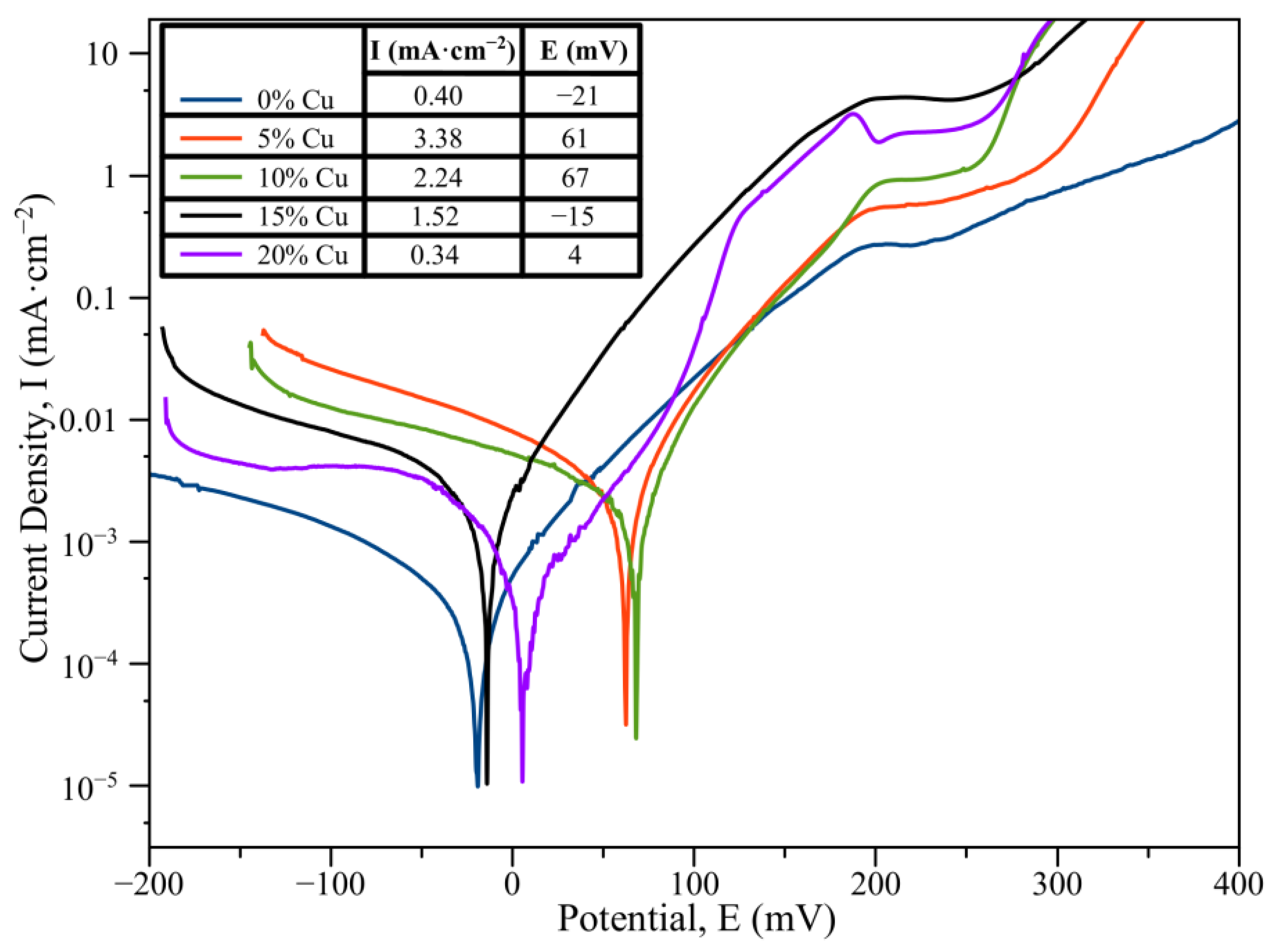
3.4. Corrosion Resistance of the HEA (Corrosion Analysis)
4. Conclusions
- Compact samples of CoCrCuxFeNi HEA with copper content ranging from 0 to 20 at. % were fabricated using the powder metallurgy technology that combined high-energy mechanical alloying and hot pressing. A single-phase solid solution was formed at copper concentrations up to 5 at. %, while at copper concentrations of 10–20 at. %, the structure was dual-phase. The excess phase having a fcc lattice precipitated during hot pressing consisted of 90 at. % copper and caused disordering of the HEA.
- The CoCrCu0.25FeNi alloy exhibited the maximum wear resistance upon friction in a couple with a counterbody made of sintered Si3N4. Doping with copper reduced the specific wear by 23% (from 6.68 to 5.17 10−5·mm3/N·m). The increase in wear resistance was attributed to solid solution hardening caused by distortion of the crystal lattice of the (Fe)Co,Ni,Cr solid solution. Wear resistance declined with increasing copper concentration due to segregation of the excess FCC2 phase and decreasing hardness.
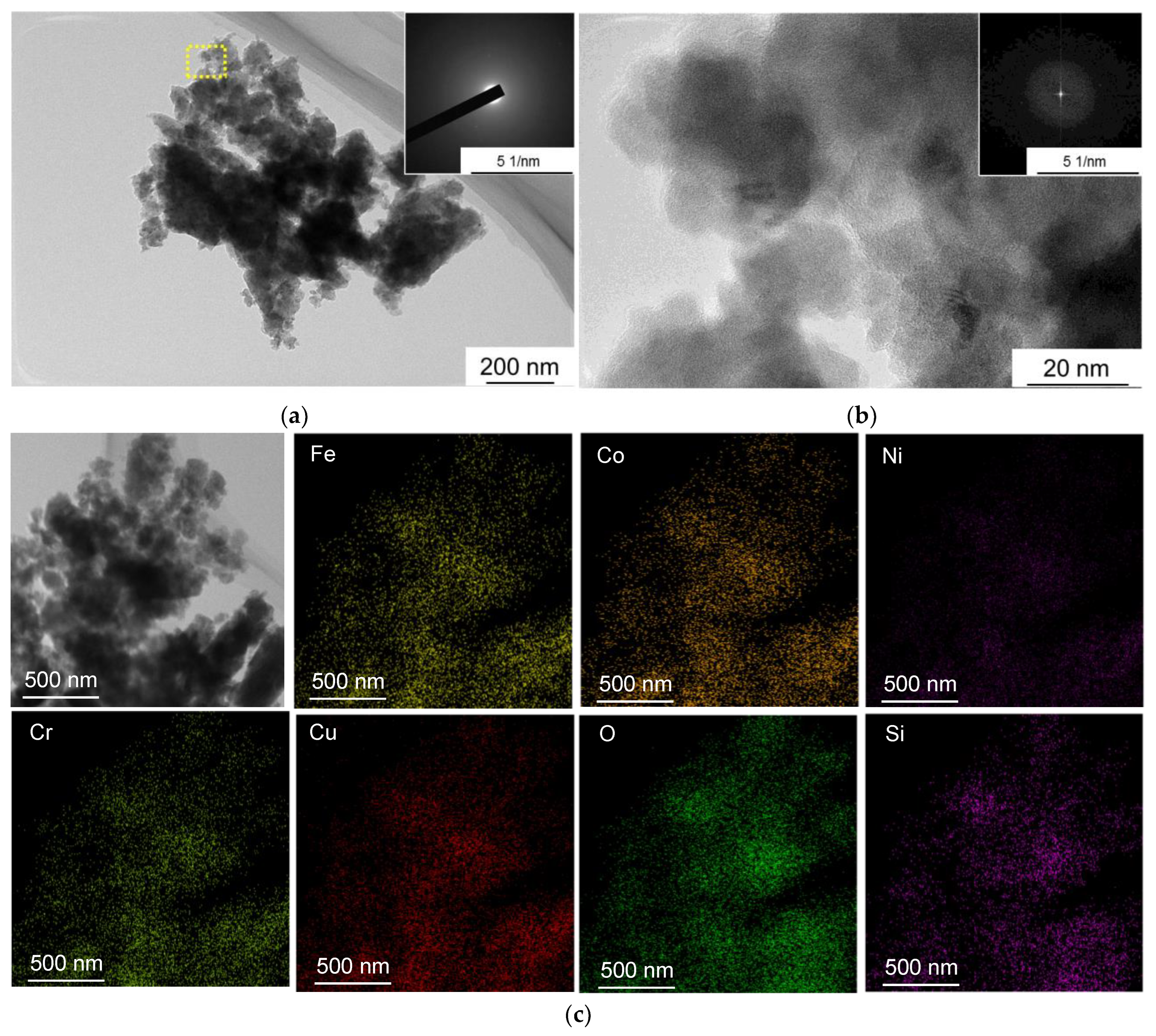
- Wear of the resulting CoCrCuxFeNi HEAs occurred via the abrasive-oxidation mechanism. The wear products were amorphous particles consisting of the alloy components with uniformly distributed oxygen and silicon elements.
- The tests of the CoCrCuxFeNi HEAs in 3.5% NaCl solution revealed that formation of the duplex structure FCC1 + FCC2 had a beneficial effect on corrosion resistance compared to the single-phase HEA with composition CoCrCu0.25FeNi.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 5, 299–303. [Google Scholar] [CrossRef]
- Rogachev, A.S. Structure, stability, and properties of high-entropy alloys. Phys. Met. Metallogr. 2020, 121, 733–764. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High Entropy Alloys; Butterworth-Heinemann: Oxford, UK, 2014; p. 218. [Google Scholar] [CrossRef]
- Gali, A.; George, E.P. Tensile properties of high- and medium-entropy alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Antonyuk, M.N.; Sheveyko, A.N.; Bondarev, A.V.; Ignatov, S.G.; Slukin, P.V.; Dwivedi, P.; Fraile, A.; Polcar, T.; Shtansky, D.V. High-entropy Fe-Cr-Ni-Co-(Cu) coatings produced by vacuum electro-spark deposition for marine and coastal applications. Surf. Coat. Technol. 2023, 453, 129136. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Soto, A.O.; Salgado, A.S.; Niño, E.B. Thermodynamic analysis of high entropy alloys and their mechanical behavior in high and low-temperature conditions with a microstructural approach–A review. Intermetallics 2020, 124, 106850. [Google Scholar] [CrossRef]
- Ali, N.; Zhang, L.; Liu, D.; Zhou, H.; Sanaullah, K.; Zhang, C.; Chu, J.; Nian, Y.; Cheng, J. Strengthening mechanisms in high entropy alloys: A review. Mater. Today Commun. 2022, 33, 104686. [Google Scholar] [CrossRef]
- Tung, C.-C.; Yehv, J.-W.; Shun, T.-T.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. On the elemental effect of AlCoCrCuFeNi high-entropy alloy system. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; He, F.; Qiao, Z.; Wang, Z.; Liu, W.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Effects of AL addition on microstructure and mechanical properties of AlxCoCrFeNi High-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. Mater. Sci. Eng. B 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Tsai, C.W.; Tsai, M.H.; Yeh, J.W.; Yang, C.C. Effect of temperature on mechanical properties of Al0.5CoCrCuFeNi wrought alloy. J. Alloys Compd. 2010, 490, 160–165. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Niu, M.; Jiang, Z.; Chen, H.; Sun, Y. High-temperature tribological behavior of CoCrFeNiV high-entropy alloys: A parallel comparison with CoCrFeNiMn high-entropy alloys. Tribol. Int. 2022, 174, 107736. [Google Scholar] [CrossRef]
- Verma, A.; Tarate, P.; Abhyankar, A.C.; Mohape, M.R.; Gowtam, D.S.; Deshmukh, V.P.; Shanmugasundaram, T. High temperature wear in CoCrFeNiCux high entropy alloys: The role of Cu. Scr. Mater. 2019, 161, 28–31. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Huang, B.; Zhao, X.; Chen, H.; Wang, C. Effect of Cu segregation on the phase transformation and properties of AlCrFeNiTiCux high-entropy alloys. Intermetallics 2022, 14, 107397. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Luo, Z.; Gao, F.; Li, L. Manufacturing of FeCoCrNiCux medium-entropy alloy coating using laser cladding technology. Mater. Des. 2017, 133, 91–108. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Liu, J.; An, X.; Zhang, J.; Kong, Q.; Li, Q.; Wang, H.; Yao, W.; Wang, Q. Microstructure, mechanical and corrosion properties of Co- and Cu-free AlxCrFeNi2.5Mo1-x high entropy alloys. Intermetallics 2023, 153, 107775. [Google Scholar] [CrossRef]
- Churyumov, A.Y.; Pozdniakov, A.V.; Bazlov, A.I.; Mao, H.; Polkin, V.I.; Louzguine-Luzgin, D.V. Effect of Nb Addition on Microstructure and Thermal and Mechanical Properties of Fe-Co-Ni-Cu-Cr Multiprincipal-Element (High-Entropy) Alloys in As-Cast and Heat-Treated State. JOM 2019, 71, 3481–3489. [Google Scholar] [CrossRef]
- Ye, X.C.; Wang, T.; Xu, Z.Y.; Liu, C.; Wu, H.H.; Zhao, G.W.; Fang, D. Effect of Ti content on microstructure and mechanical properties of CuCoFeNi high-entropy alloys. Int. J. Miner. Metall. Mater. 2020, 27, 1326. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Liu, X.; Wang, Q.; Chen, X.; Hui, X. High strength NiMnFeCrAlCu multi-principal-element alloys with marine application perspective. Scr. Mater. 2021, 202, 113992. [Google Scholar] [CrossRef]
- Shu, C.; Yao, Z.; Li, X.; Du, W.; Tao, X.; Yang, H. Microstructure and wear mechanism of CoCrCuFeNiVx high entropy alloy by sintering and electron beam remelting. Phys. B Condens. Matter 2022, 638, 413834. [Google Scholar] [CrossRef]
- Loginov, P.A.; Zhassay, U.A.; Bychkova, M.Y.; Petrzhik, M.I.; Mukanov, S.K.; Sidorenko, D.A.; Orekhov, A.S.; Rupasov, S.I.; Levashov, E.A. Chromium-doped Fe-Co-Ni binders for diamond cutting tools: The features of the structure, mechanical properties, and adhesion to diamond. Int. J. Refract. Met. Hard Mater. 2020, 92, 105289. [Google Scholar] [CrossRef]
- Loginov, P.A.; Levashov, E.A.; Kurbatkina, V.V.; Zaitsev, A.A.; Sidorenko, D.A. Evolution of the microstructure of Cu–Fe–Co–Ni powder mixtures upon mechanical alloying. Powder Technol. 2015, 276, 166–174. [Google Scholar] [CrossRef]
- Shkodich, N.F.; Kovalev, I.D.; Kuskov, K.V.; Kovalev, D.Y.; Vergunova, Y.S.; Scheck, Y.B.; Vadchenko, S.G.; Politano, O.; Baras, F.; Rogachev, A.S. Fast mechanical synthesis, structure evolution, and thermal stability of nanostructured CoCrFeNiCu high entropy alloy. J. Alloys Compd. 2022, 893, 161839. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Ma, K.H.; Wang, Q.; Shek, H. Compositional dependence of phase formation and mechanical properties in three CoCrFeNi-(Mn/Al/Cu) high entropy alloys. Intermetallics 2016, 79, 1–11. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462–463, 203493. [Google Scholar] [CrossRef]
- Rogal, Ł. Semi-solid processing of the CoCrCuFeNi high entropy alloy. Mater. Des. 2017, 119, 406–416. [Google Scholar] [CrossRef]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNi-x (x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Park, N.; Watanabe, I.; Terada, D.; Yokoyama, Y.; Liaw, P.K.; Tsuji, N. Recrystallization Behavior of CoCrCuFeNi High-Entropy Alloy. Metall. Mater. Trans. A. 2015, 46, 1481–1487. [Google Scholar] [CrossRef]
- Lim, S.C. The relevance of wear-mechanism maps to mild-oxidational wear. Tribol. Int. 2002, 35, 717–723. [Google Scholar] [CrossRef]
- Zaveri, N.; Mahapatra, M.; Deceuster, A.; Peng, Y.; Li, L.; Zhou, A. Corrosion resistance of pulsed laser-treated Ti–6Al–4V implant in simulated biofluids. Electrochim. Acta 2008, 53, 5022–5032. [Google Scholar] [CrossRef]
- Öztürk, S.; Alptekin, F.; Önal, S.; Sünbül, S.E.; Şahin, Ö.; İçin, K. Effect of titanium addition on the corrosion behavior of CoCuFeNiMn high entropy alloy. J. Alloys Compd. 2022, 903, 163867. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.; Zhu, S.; Qiao, Z.; Zhang, J.; Yang, J.; Liu, W. A novel Cu-doped high entropy alloy with excellent comprehensive performances for marine application. J. Mater. Sci. Technol. 2021, 69, 48–59. [Google Scholar] [CrossRef]
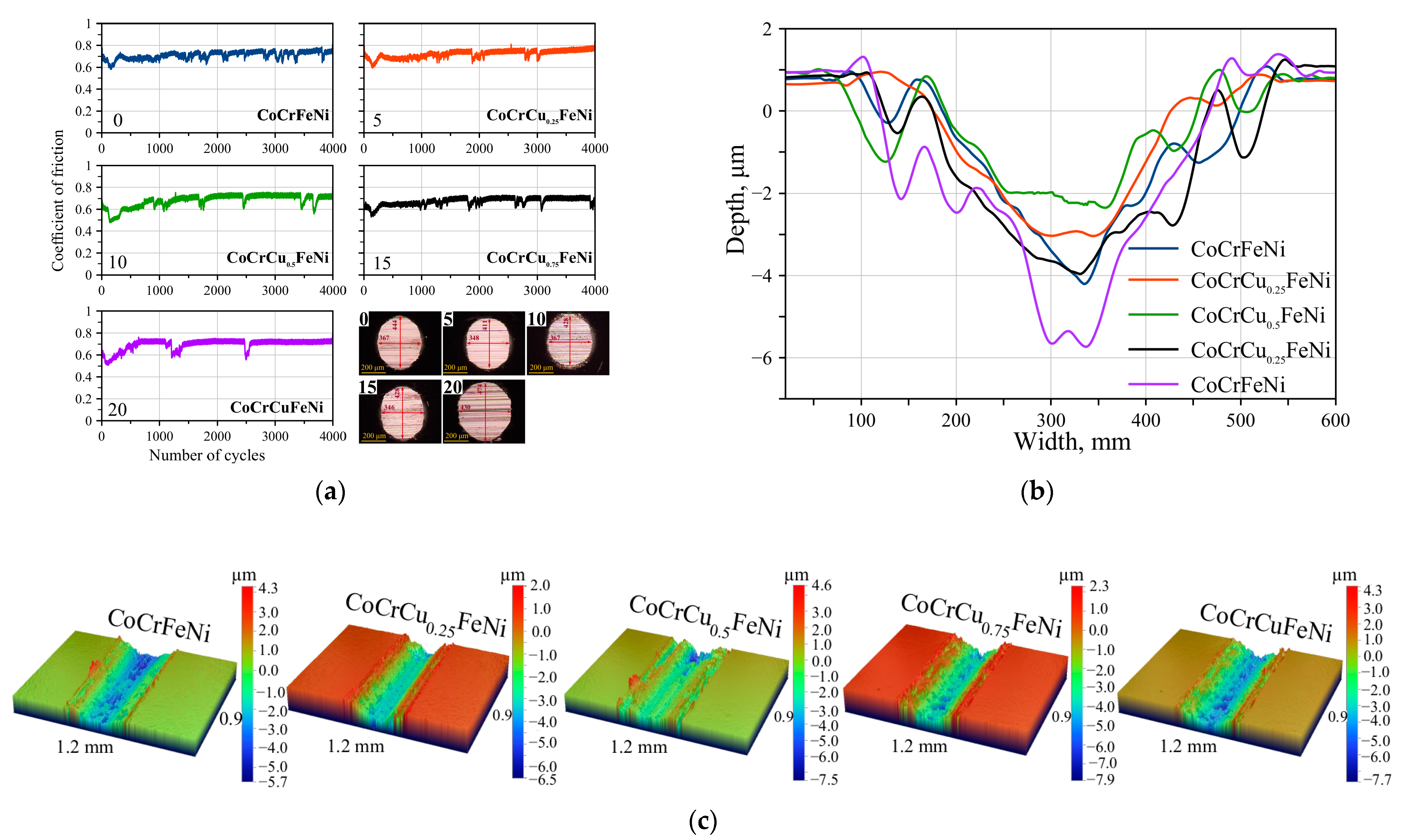


| Sample | Reduced Wear, 10−5·mm3/N·m | Coefficient of Friction (COF) | |||
|---|---|---|---|---|---|
| Sample | Counterbody | Initial | Maximum | Average | |
| CoCrFeNi | 6.68 | 0.91 | 0.09 | 0.81 | 0.67 |
| CoCrCu0.25FeNi | 5.17 | 0.70 | 0.12 | 0.83 | 0.68 |
| CoCrCu0.5FeNi | 5.58 | 0.85 | 0.16 | 0.85 | 0.69 |
| CoCrCu0.75FeNi | 8.88 | 0.79 | 0.29 | 0.81 | 0.68 |
| CoCrCuFeNi | 10.54 | 1.43 | 0.41 | 0.85 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukanov, S.; Loginov, P.; Fedotov, A.; Bychkova, M.; Antonyuk, M.; Levashov, E. The Effect of Copper on the Microstructure, Wear and Corrosion Resistance of CoCrCuFeNi High-Entropy Alloys Manufactured by Powder Metallurgy. Materials 2023, 16, 1178. https://doi.org/10.3390/ma16031178
Mukanov S, Loginov P, Fedotov A, Bychkova M, Antonyuk M, Levashov E. The Effect of Copper on the Microstructure, Wear and Corrosion Resistance of CoCrCuFeNi High-Entropy Alloys Manufactured by Powder Metallurgy. Materials. 2023; 16(3):1178. https://doi.org/10.3390/ma16031178
Chicago/Turabian StyleMukanov, Samat, Pavel Loginov, Alexander Fedotov, Marina Bychkova, Maria Antonyuk, and Evgeny Levashov. 2023. "The Effect of Copper on the Microstructure, Wear and Corrosion Resistance of CoCrCuFeNi High-Entropy Alloys Manufactured by Powder Metallurgy" Materials 16, no. 3: 1178. https://doi.org/10.3390/ma16031178
APA StyleMukanov, S., Loginov, P., Fedotov, A., Bychkova, M., Antonyuk, M., & Levashov, E. (2023). The Effect of Copper on the Microstructure, Wear and Corrosion Resistance of CoCrCuFeNi High-Entropy Alloys Manufactured by Powder Metallurgy. Materials, 16(3), 1178. https://doi.org/10.3390/ma16031178







