Catalytic Activity of LaFe0.4Ni0.6O3/CeO2 Composites in CO and CH4 Oxidation Depending on Their Preparation Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Catalytic Activity
3. Results and Discussion
3.1. Phase Composition
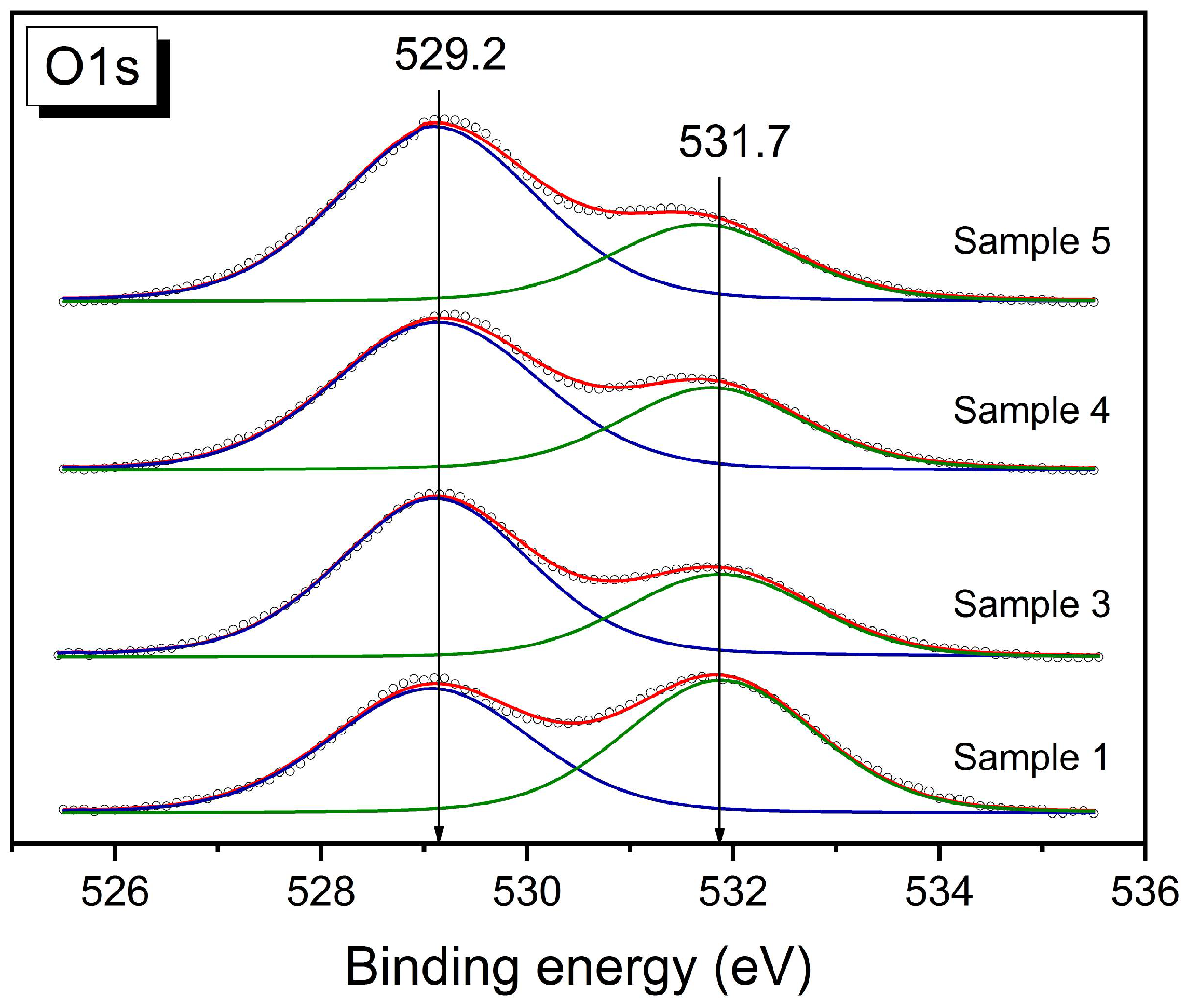
3.2. Microstructure, Bulk, and Surface Particles Composition
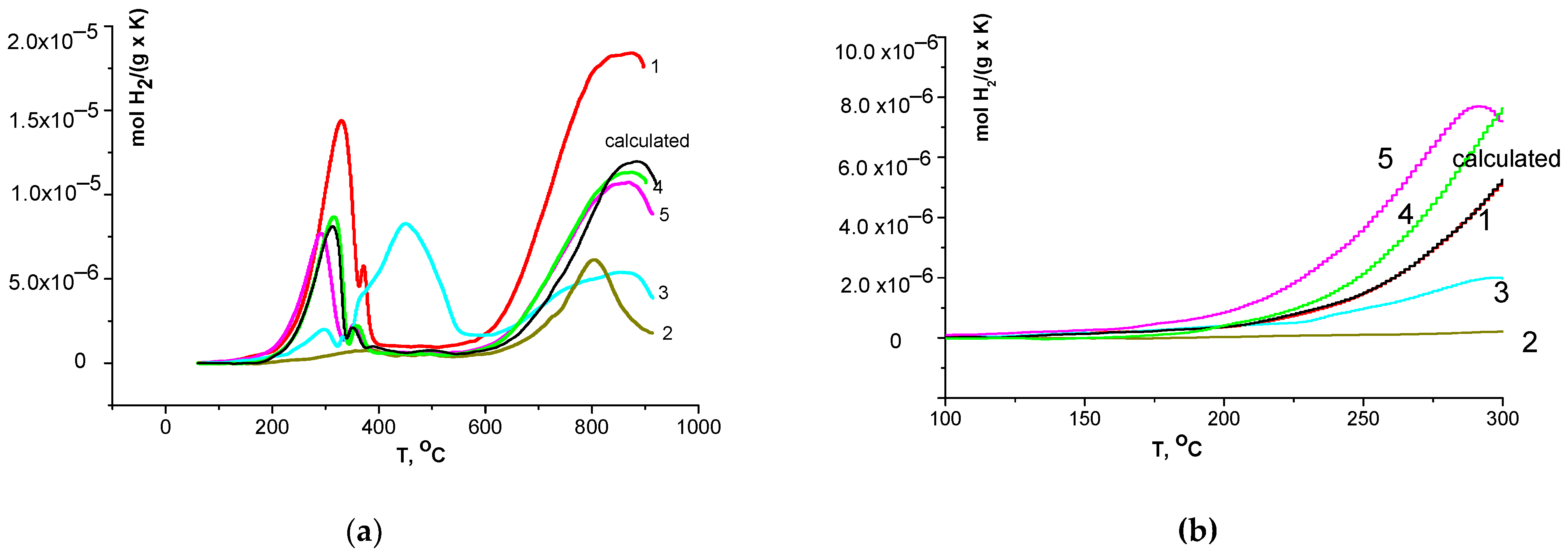
3.3. H2-TPR Data
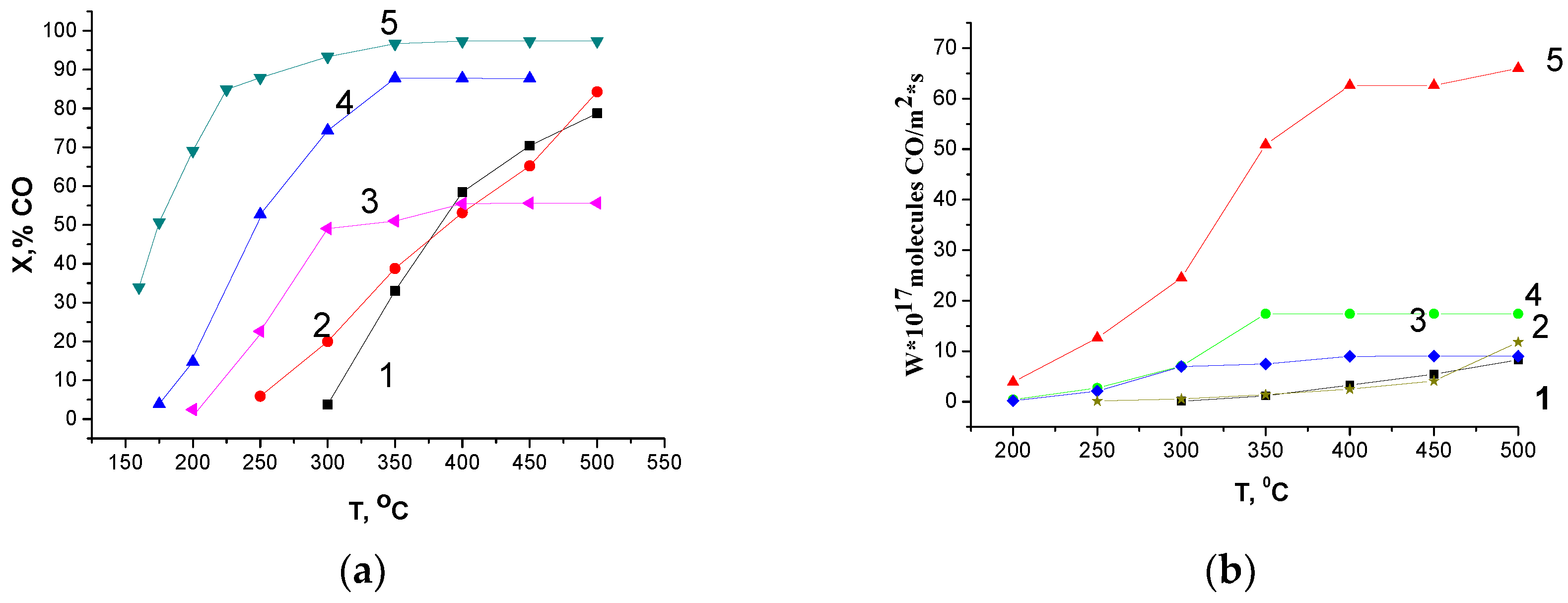
3.4. Catalytic Activity in CO and CH4 Oxidation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Falcon, H.; Carbonio, R.E.; Fierro, J.L.G. Correlation of Oxidation States in LaFexNi1−xO3+δ oxides with catalytic activity for H2O2 decomposition. Catalysis 2001, 203, 264–272. [Google Scholar] [CrossRef]
- Konysheva, E.; Francis, S.M.; Irvine, J.T.S. Crystal structure, oxygen nonstoichiometry, and conductivity of mixed ionic-electronic conducting perovskite composites with CeO2. J. Electrochem. Soc. 2010, 157, B159–B165. [Google Scholar] [CrossRef]
- Konysheva, E.; Irvine, J.T.S. The La0.95Ni0.6Fe0.4O3-CeO2 system: Phase equilibria, crystal structure of components and transport properties. J. Solid State Chem. 2011, 184, 1499–1504. [Google Scholar] [CrossRef]
- Bork, A.H.; Carrillo, A.J.; Hood, Z.D.; Yildiz, B.; Rupp, J.L.M. Oxygen Exchange in Dual-Phase La0.65Sr0.35MnO3–CeO2 Composites for Solar Thermochemical Fuel Production. ACS Appl. Mater. Interfaces 2020, 12, 32622–32632. [Google Scholar] [CrossRef]
- Li, X.; Gao, H. Role of ceria in the improvement of NO removal of lanthanum-based perovskite-type catalysts. RSC Adv. 2018, 8, 11778. [Google Scholar] [CrossRef]
- Kirchnerova, J.; Alifanti, M.; Delmon, B. Evidence of phase cooperation in the LaCoO3–CeO2–Co3O4 catalytic system in relation to activity in methane combustion. Appl. Catal. A Gen. 2002, 231, 65–80. [Google Scholar] [CrossRef]
- Forni, L.; Oliva, C.; Vatti, F.P.; Kandala, M.A.; Ezerets, A.M.; Vishniakov, A.V. La-Ce-Co perovskites as catalysts for exhaust gas depollution. Appl. Catal. B Environ. 1996, 7, 269–284. [Google Scholar] [CrossRef]
- Alifanti, M.; Blangenois, N.; Florea, M.; Delmon, B. Supported Co-based perovskites as catalysts for total oxidation of methane. Appl. Catal. A Gen. 2005, 280, 255–265. [Google Scholar] [CrossRef]
- Gellings, P.J.; Bouwmeester, H.J.M. Ion and mixed conducting oxides as catalysts. Catal. Today 1992, 12, 1–101. [Google Scholar] [CrossRef]
- Stoian, M.; Rogé, V.; Lazar, L.; Maurer, T.; Védrine, J.C.; Marcu, I.-C.; Fechete, I. Total Oxidation of Methane on Oxide and Mixed Oxide Ceria-Containing Catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Isupova, L.A.; Prosvirin, I.P.; Sadovskaya, E.M.; Danilova, I.G.; Ivanov, D.V.; Gerasimov, E.Y. La–Fe–O/CeO2 Based Composites as the Catalysts for High Temperature N2O Decomposition and CH4 Combustion. Catal. Lett. 2013, 143, 1294–1303. [Google Scholar] [CrossRef]
- Provendier, H.; Petit, C.; Estoumes, C.; Kiennemann, A. Dry reforming of methane. Interest of La-Ni-Fe solid solutions compared to LaNiO3 and LaFeO3. Stud. Surf. Sci. Catal. 1998, 119, 741–746. [Google Scholar]
- Provendier, H.; Petit, C.; Kiennemann, A. Steam reforming of methane on LaNi1-xFexO3 (0 ≤ x ≤ 1) perovskites. Reactivity and characterisation after test. C. R. Acad. Sci. Paris Ser. IIc Chem./Chem. 2001, 4, 57–66. [Google Scholar]
- Kumar, R.; Coudhary, R.J.; Khan, M.W.; Srivastava, J.P.; Bao, C.W.; Tsai, H.M.; Chiou, J.W.; Asokan, K.; Pong, W.F. Structural, electrical transport and x-ray absorption spectroscopy studies of LaFe1−xNixO3. J. Appl. Phys. 2005, 97, 093526. [Google Scholar] [CrossRef]
- Sukpirom, N.; Iamsaard, S.; Charojrochkul, S.; Yeyongchaiwat, J. Synthesis and properties of LaNi1-xFexO3-δ as cathode materials in SOFC. J. Mater. Sci. 2011, 45, 6500–6507. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Montini, T.; Tavagnacco, C.; Fonda, E.; Fornasiero, P.; Graziani, M. Preparation, Characterization, and electrochemical properties of pure and composite LaNi0.6F0.4O3-based cathodes for IT-SOFC. Chem. Mater. 2007, 19, 5926–5936. [Google Scholar] [CrossRef]
- Chiba, R.; Komatsu, T.; Orui, H.; Taguchi, H.; Nozawa, K.; Arai, H. An SOFC cathode composed of LaNi0.6F0.4O3 and Ce(Ln)O2 (Ln = Sm, Gd, Pr). J. Korean Ceram. Soc. 2008, 45, 766–771. [Google Scholar] [CrossRef]
- Yaroslavtsev, I.Y.; Bogdanovich, N.M.; Vdovin, G.K.; Dem’yanenko, T.A.; Bronin, D.I.; Isupova, L.A. Cathodes based on rare-earth metal nickelate ferrites prepared from industrial raw materials for solid oxide fuel cells. Russ. J. Electrochem. 2014, 50, 548–553. [Google Scholar] [CrossRef]
- Pavlova, S.; Kharlamova, T.; Sadykov, V.; Krieger, T.; Muzykantov, V.; Bespalko, Y.; Ishenko, A.; Rogov, V.; Belyaev, V.; Okhlupin, Y.; et al. Structural features and transport properties of La(Sr)Fe1-xNixO3-δ–Ce0.9Gd0.1O2-δ nanocomposites-advanced materials for IT SOFC cathodes. Heat Transf. Eng. 2013, 34, 904–916. [Google Scholar] [CrossRef]
- Isupova, L.A.; Tsybulya, S.V.; Kryukova, G.N.; Alikina, G.M.; Boldyreva, N.N.; Yakovleva, I.S.; Ivanov, V.P.; Sadykov, V.A. Real structure and catalytic activity of La1-xCaxMnO3+δ perovskites. J. Solid State Ion. 2001, 141–142, 417–425. [Google Scholar] [CrossRef]
- Dulian, P.; Bąk, W.; Wieczorek-Ciurowa, K.; Kajtoch, C. Controlled mechanochemical synthesis and properties of a selected perovskite-type electroceramics. Mater. Sci. 2013, 31, 462–470. [Google Scholar] [CrossRef]
- Stojanovic, B.D. Mechanochemical synthesis of ceramic powders with perovskite structure. J. Mater. Process. Technol. 2003, 143, 78–81. [Google Scholar] [CrossRef]
- Zyryanov, V.V.; Sadykov, V.A.; Uvarov, N.F.; Alikina, G.M.; Lukashevich, A.I.; Neofitides, S.G.; Criado, J.M. Mechanosynthesis of complex oxides with fluorite and perovskite-related structures and their sintering into nanocomposites with mixed ionic–electronic conductivity. Solid State Ion. 2005, 176, 2813–2818. [Google Scholar] [CrossRef]
- Isupova, L.A.; Obyskalova, E.A.; Rogov, V.A.; Tsybulya, S.V.; Dovlitova, L.S.; Burgina, E.B.; Ischenko, A.V.; Zaikovskii, V.I.; Sadykov, V.A.; Orlovskaya, N. Doped ceria—LaMeO3 (Me = Mn, Fe, Co) nanocomposites: Synthesis via mechanochemical activation route and properties. Mater. Res. Soc. Symp. Proc. 2006, 885E, 0885-A03-04.1. [Google Scholar]
- Gorbunova, V.A.; Sliapniova, L.M.; Gorbunov, A.V. Thermochemical Preparation and Properties of Low-Cost Polylanthanide Manganite Materials of Ln(La, Ce, Nd, Pr)xCayMnO3-Type with Perovskite-Fluorite Structure. Sci. Tech. 2020, 19, 528–535. (In Russian) [Google Scholar]
- Zhao, Z.; Zou, M.; Huang, H.; Wofford, H.; Tong, J. Stable perovskite-fluorite dual-phase composites synthesized by one-pot solid-state reactive sintering for protonic ceramic fuel cells. Ceram. Int. 2021, 47, 32856–32866. [Google Scholar] [CrossRef]
- Ohzeki, T.; Hashimoto, T.; Shozugawa, K.; Matsuo, M. Preparation of LaNi1-xFexO3 Single Phase and Characterization of their Phase Transition Behaviors. Solid State Ion. 2010, 181, 1771–1782. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. US Patent No. 3,330,697, 11 July 1967. [Google Scholar]
- Tsybulya, S.V.; Cherepanova, S.V.; Solovʹeva, L.P. Polycrystal Software Package for IBM/PC. J. Struct. Chem. 1996, 37, 332–334. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- XPSPEAK 4.1. Available online: http://xpspeak.software.informer.com/4.1/ (accessed on 10 January 2023).
- Ren, H.; Wang, Z.; Chen, X.; Jing, Z.; Qu, Z.; Huang, L. Effective mineralization of p-nitrophenol by catalytic ozonation using Ce-substituted La1−xCexFeO3 catalyst. Chemosphere 2021, 285, 131473. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Davies-Jones, J.; Davies, P.R.; Guan, S.; Lee, R.; Morgan, D.J.; Palgrave, R. Advanced XPS characterization: XPS-based multi-technique analyses for comprehensive understanding of functional materials. Mater. Chem. Front. 2021, 5, 7931. [Google Scholar] [CrossRef]
- van der Heide, P.A.W. Systematic x-ray photoelectron spectroscopic study of La1-xSrx-based perovskite-type oxides. Surf. Interface Anal. 2002, 33, 414–425. [Google Scholar] [CrossRef]
- Yakovleva, I.S.; Isupova, L.A.; Rogov, V.A.; Sadykov, V.A. Forms of oxygen in La1−xCaxMnO3+δ (x = 0–1) perovskites and their reactivities in oxidation reactions. Kinet. Catal. 2008, 49, 261–270. [Google Scholar] [CrossRef]
- Liu, W.; Flytzanistephanopoulos, M. Total Oxidation of Carbon-Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts. J. Catal. 1995, 153, 317–332. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Sadovskaya, E.M.; Isupova, L.A. Influence of the mobility of oxygen on the reactivity of La1−xSrxMnO3 perovskites in methane oxidation. Kinet. Catal. 2011, 52, 401–408. [Google Scholar] [CrossRef]
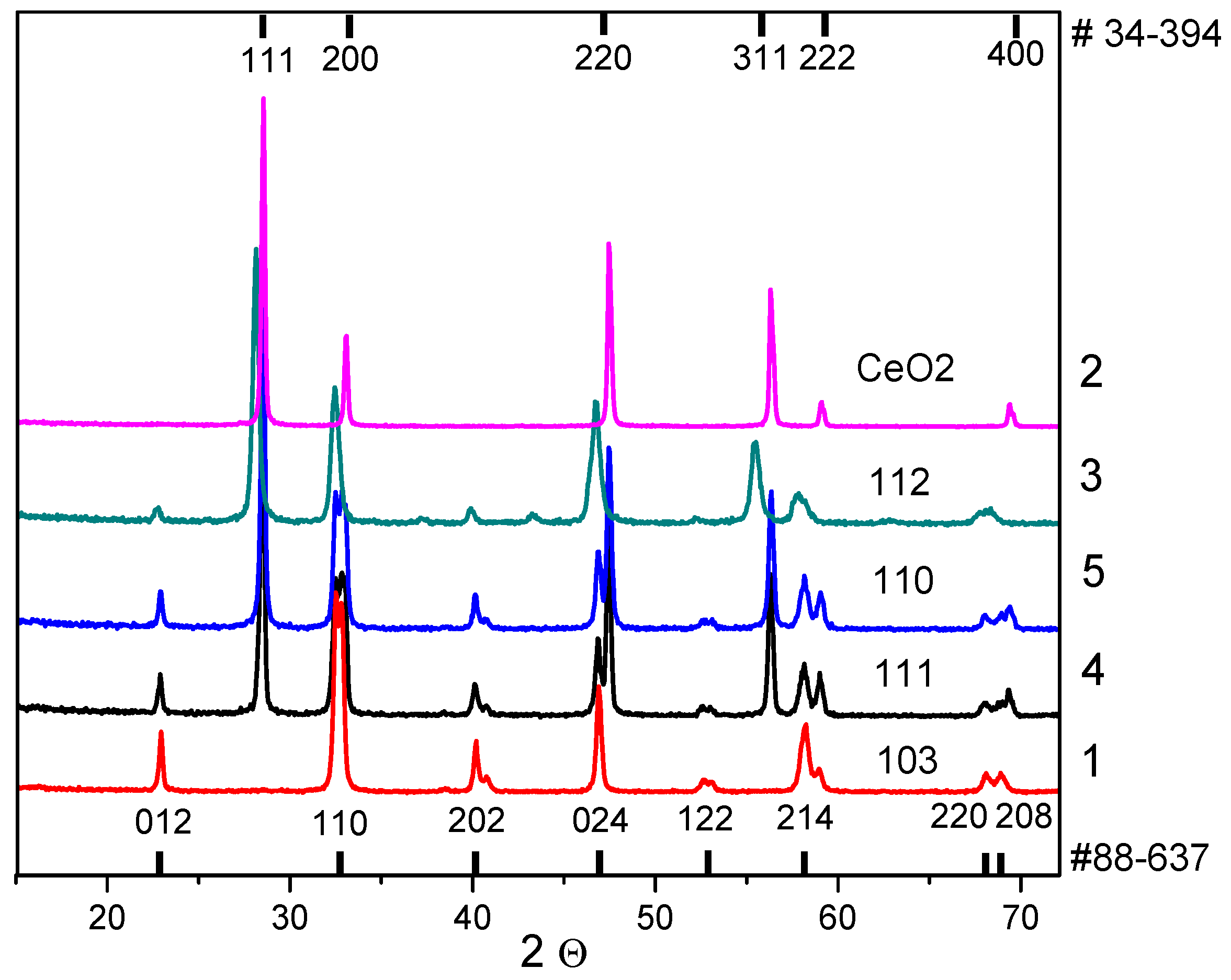
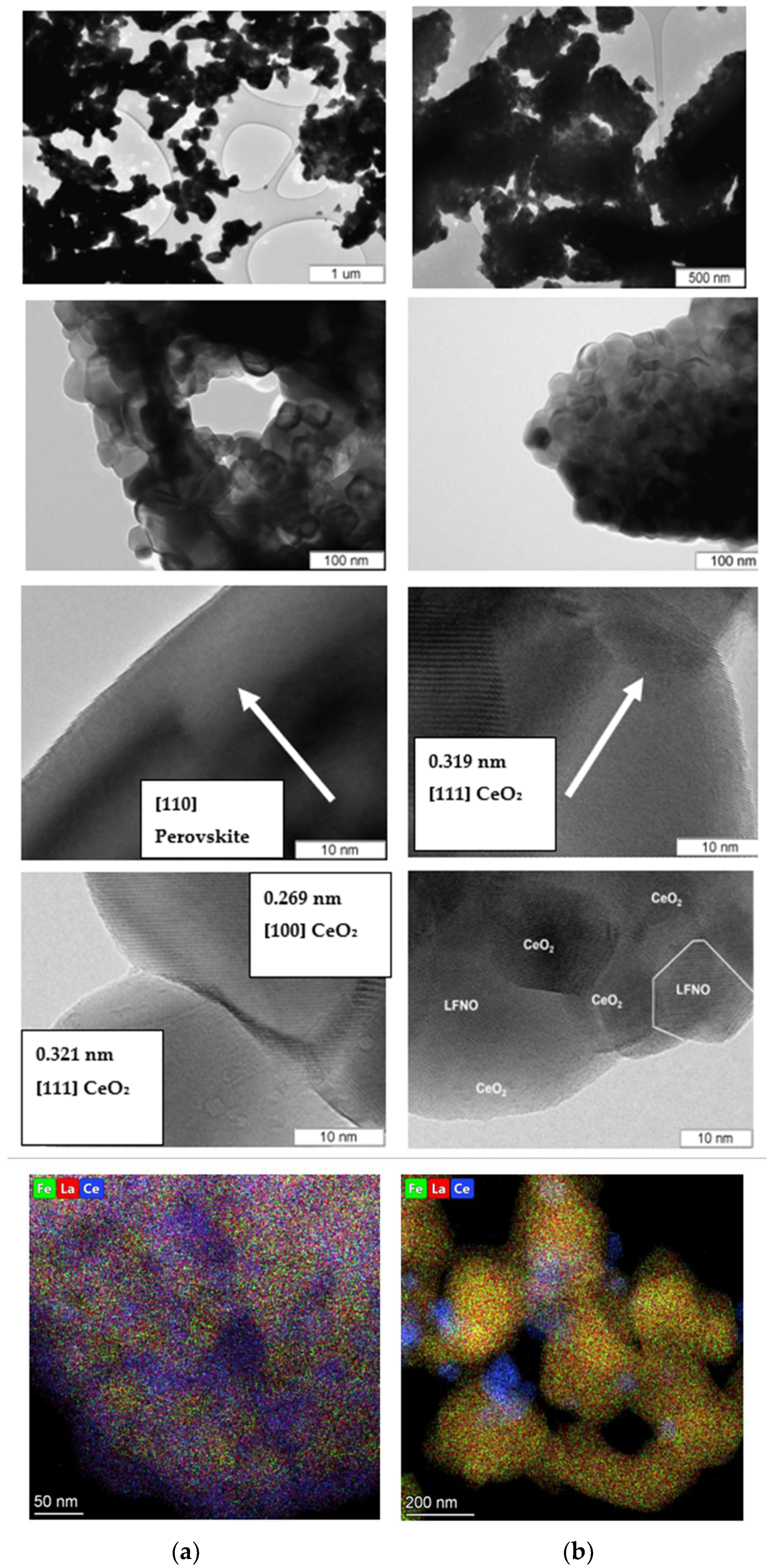
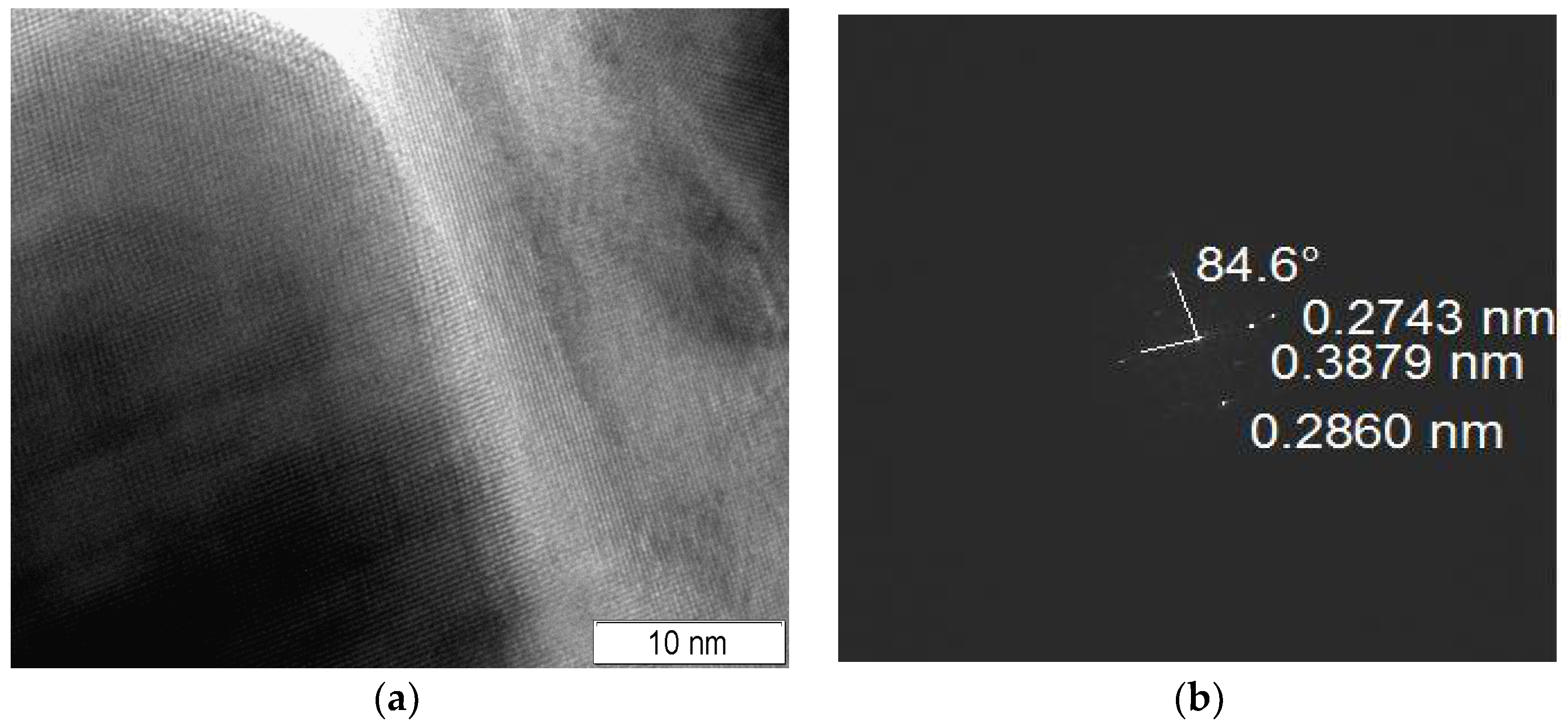

| No. | Reagents for Samples Preparation | La/Ce | Preparation Details | Phase Composition |
|---|---|---|---|---|
| 1 | La, Fe, and Ni nitrate salts taken in a stoichiometric ratio | 1/0 | Prepared via the Pechini route precursor 1: milled and then calcined at 900 °C, 8 h | LaFe0.4Ni0.6O3 |
| 2 | Ce nitrate salt | 0/1 | Prepared via Pechini route precursor 2: milled and then calcined at 900 °C, 8 h | CeO2 |
| 3 | La, Fe, Ni, and Ce nitrate salts taken in a stoichiometric ratio | 1/1 | Prepared via one pot Pechini route precursor 3: milled and then calcined at 900 °C, 8 h | * LaFe0.4Ni0.6O3, * CeO2 |
| 4 | Sample 1 + sample 2 | 1/1 | Sample 1 and sample 2 with a La:Ce ratio = 1:1 were milled and then calcined at 900 °C, 8 h | LaFe0.4Ni0.6O3, CeO2 |
| 5 | Precursor 1 + precursor 2 | 1/1 | Precursor 1 and precursor 2 with a La:Ce ratio = 1:1 were milled and then calcined at 900 °C, 8 h | LaFe0.4Ni0.6O3, CeO2 |
| No | Cell Parameters and X-ray Crystallite Size for Phases | S sp., m2g−1 | |||||
|---|---|---|---|---|---|---|---|
| LaFe0.4Ni0.6O3 | CeO2 | ||||||
| a, Å | b, Å | c, Å | CS, Å | a, Å | CS, Å | ||
| 1 | 5.507(1) | 5.507(1) | 13.304(2) | 500 | 5.5 | ||
| 2 | 5.419(1) | 450 | 6.8 | ||||
| 3 | 5.544(3) | 7.803(5) | 5.453(3) | 250 | 2.0 | ||
| 4 | 5.513(2) | 5.513(2) | 13.308(5) | 400 | 5.423(1) | 400 | 5.2 |
| 5 | 5.511(2) | 5.511(2) | 13.299(4) | 400 | 5.419(1) | 400 | 7.3 |
| N | Sample | La/Ce | (Fe + Ni)/(Ce + La) | C, % | O, % | Fe, % | La, % | Ce, % | Ni, % | O(529)/O(531) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LaFe0.4Ni0.6O3 | - | 0.48 | 59.7 | 33.2 | 0.7 | 4.8 | 0 | 1.6 | 0.98 |
| 3 | LaFe0.4Ni0.6O3/CeO2 | 1.06 | 0.24 | 40.5 | 45.4 | 1.2 | 5.8 | 5.5 | 1.6 | 2.07 |
| 4 | LaFe0.4Ni0.6O3/CeO2 | 1.36 | 0.35 | 41.0 | 44.4 | 1.5 | 6.2 | 4.6 | 2.3 | 1.92 |
| 5 | LaFe0.4Ni0.6O3/CeO2 | 0.83 | 0.29 | 40.4 | 45.2 | 1.1 | 5.1 | 6.1 | 2.1 | 2.47 |
| Sample | Total Consumption Up to 900 °C, mol H2 g−1 | Consumption in the First Peak, mol H2 g−1 | First Peak Temperature Range, °C |
|---|---|---|---|
| 1 | 6.14 × 10−3 | 1.25 × 10−3 | 60–400 |
| 2 | 1.17 × 10−3 | 0.15 × 10−3 | 60–550 |
| 3 | 2.81 × 10−3 | 1.38 × 10−3 | 60–600 |
| 4 | 3.14 × 10−3 | 0.64 × 10−3 | 60–400 |
| 5 | 3.17 × 10−3 | 0.65 × 10−3 | 60–400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isupova, L.; Gerasimov, E.; Prosvirin, I.; Rogov, V. Catalytic Activity of LaFe0.4Ni0.6O3/CeO2 Composites in CO and CH4 Oxidation Depending on Their Preparation Conditions. Materials 2023, 16, 1142. https://doi.org/10.3390/ma16031142
Isupova L, Gerasimov E, Prosvirin I, Rogov V. Catalytic Activity of LaFe0.4Ni0.6O3/CeO2 Composites in CO and CH4 Oxidation Depending on Their Preparation Conditions. Materials. 2023; 16(3):1142. https://doi.org/10.3390/ma16031142
Chicago/Turabian StyleIsupova, Lyubov, Evgeny Gerasimov, Igor Prosvirin, and Vladimir Rogov. 2023. "Catalytic Activity of LaFe0.4Ni0.6O3/CeO2 Composites in CO and CH4 Oxidation Depending on Their Preparation Conditions" Materials 16, no. 3: 1142. https://doi.org/10.3390/ma16031142
APA StyleIsupova, L., Gerasimov, E., Prosvirin, I., & Rogov, V. (2023). Catalytic Activity of LaFe0.4Ni0.6O3/CeO2 Composites in CO and CH4 Oxidation Depending on Their Preparation Conditions. Materials, 16(3), 1142. https://doi.org/10.3390/ma16031142







