Microstructure and Salt Fog Corrosion of Wrought Mg-Al-Zn and Mg-RE Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructural Characterization of Uncorroded Materials
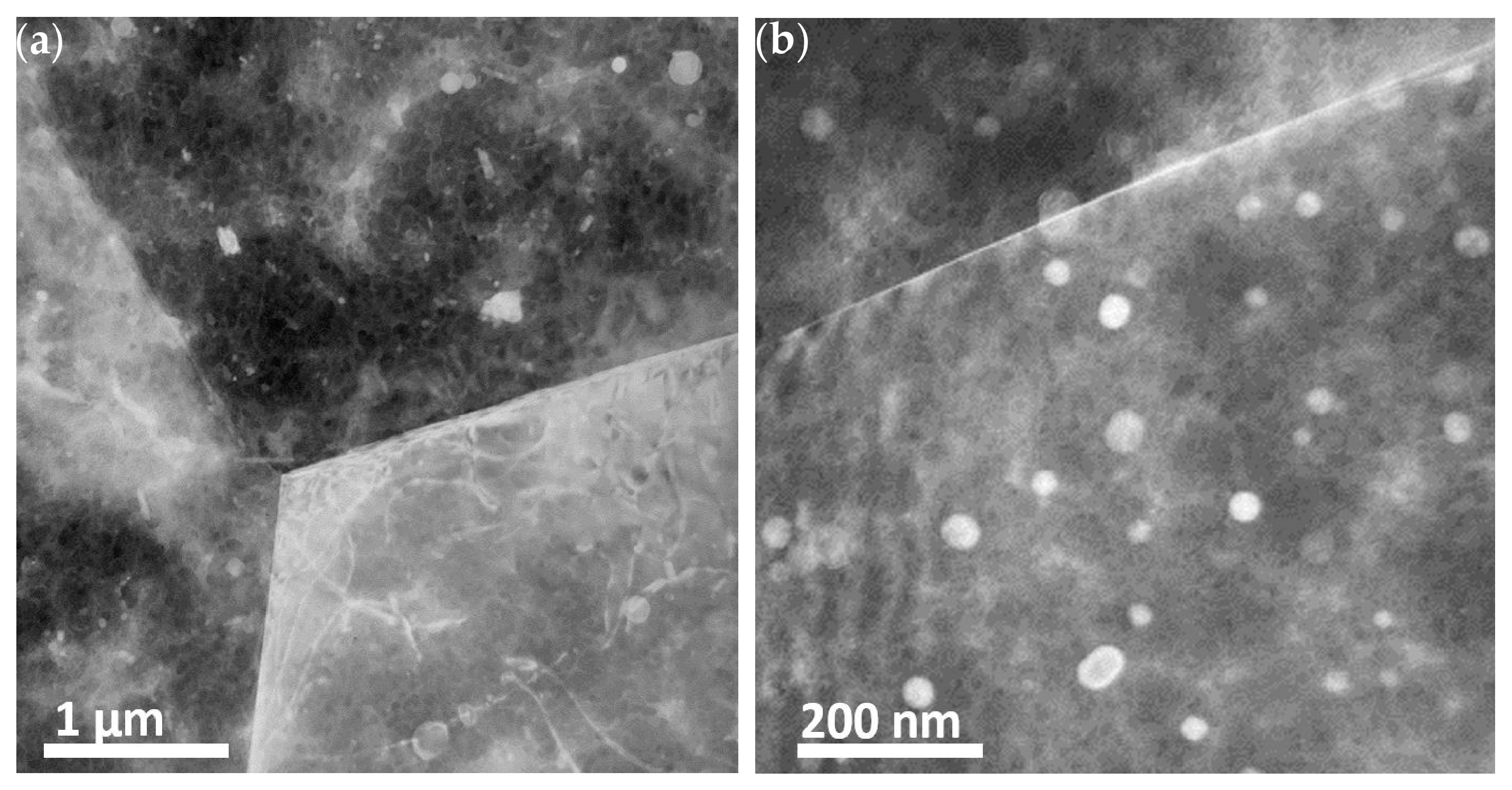
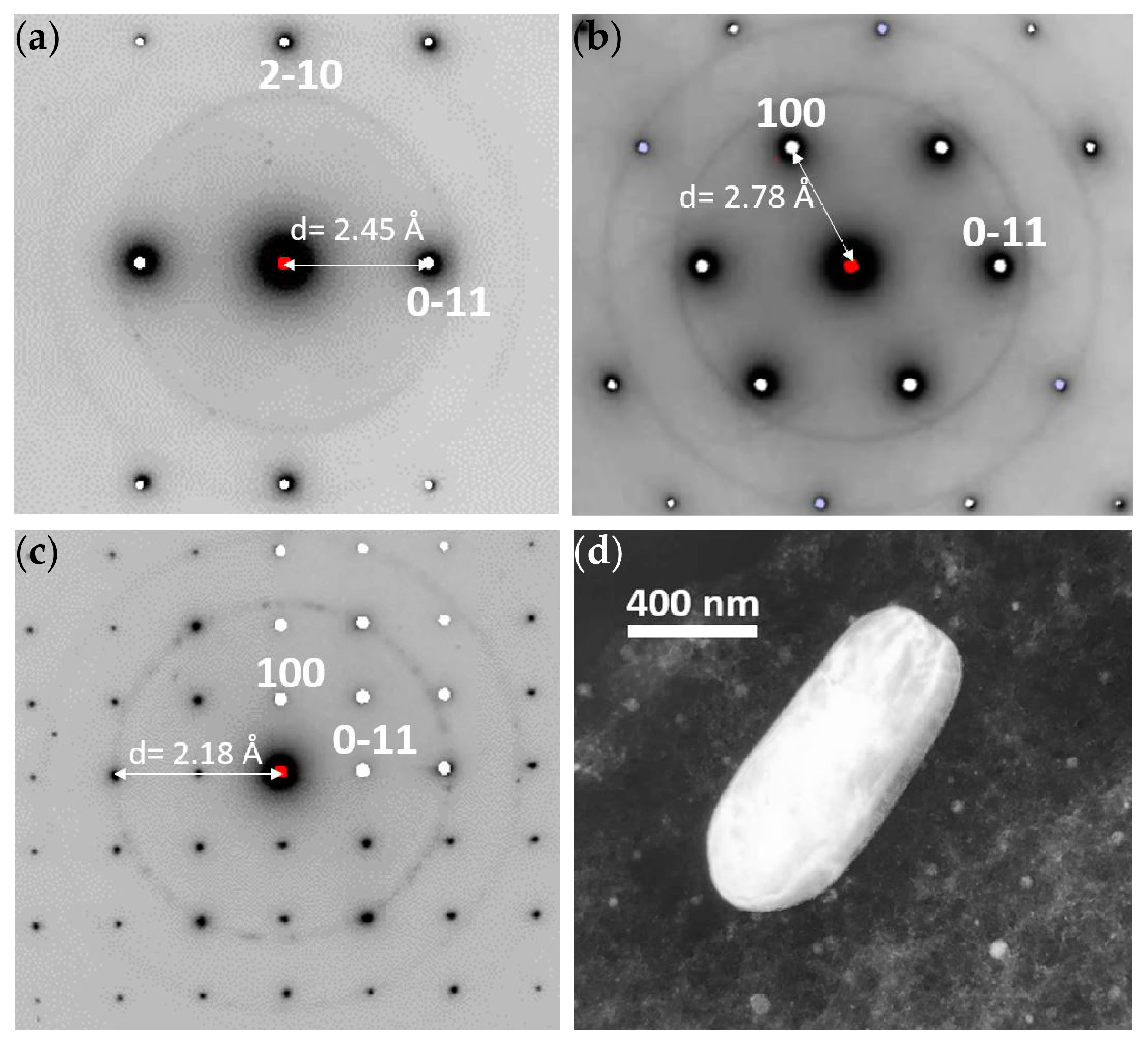
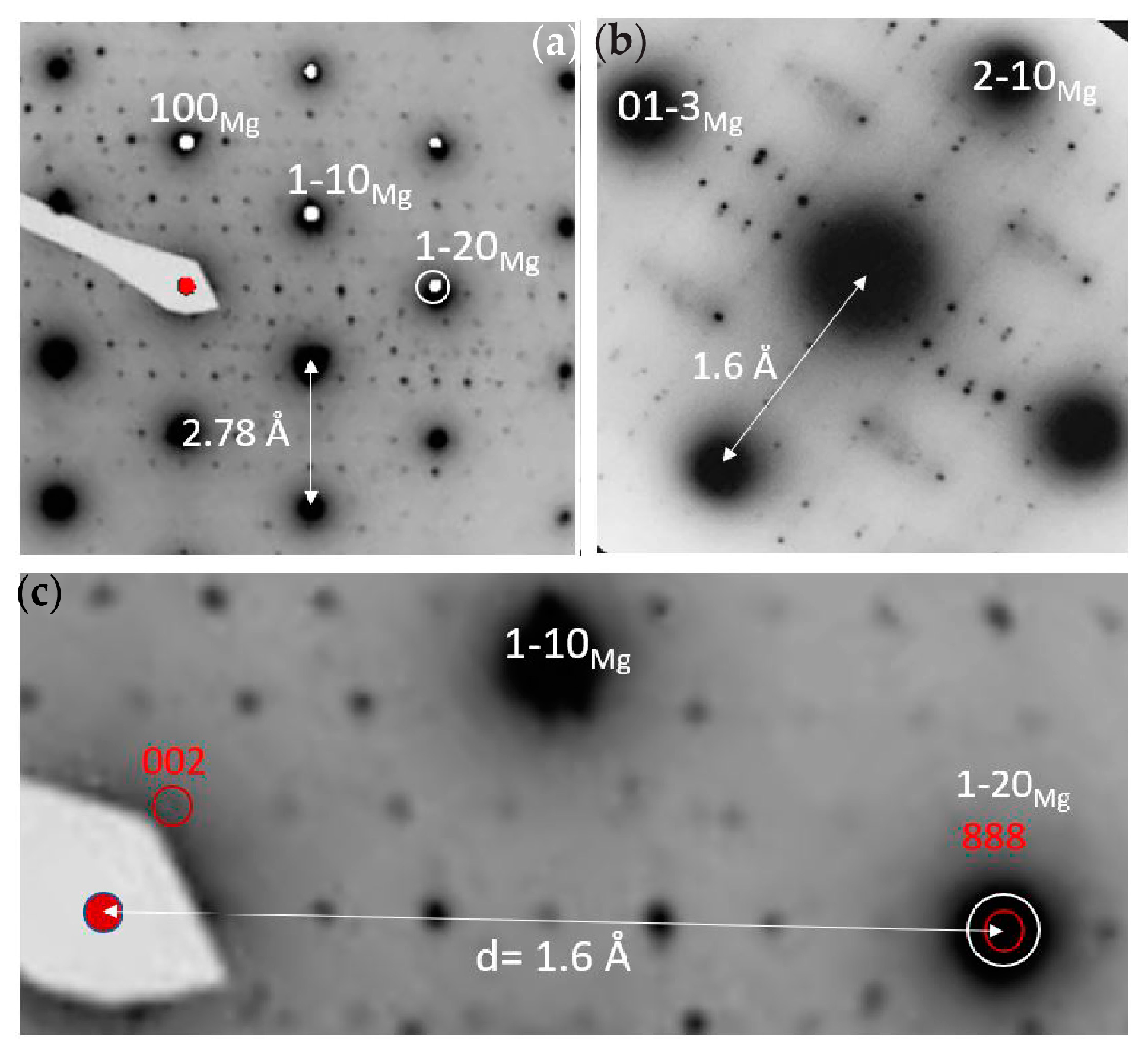
3.1.1. TEM of Alloy AZ31
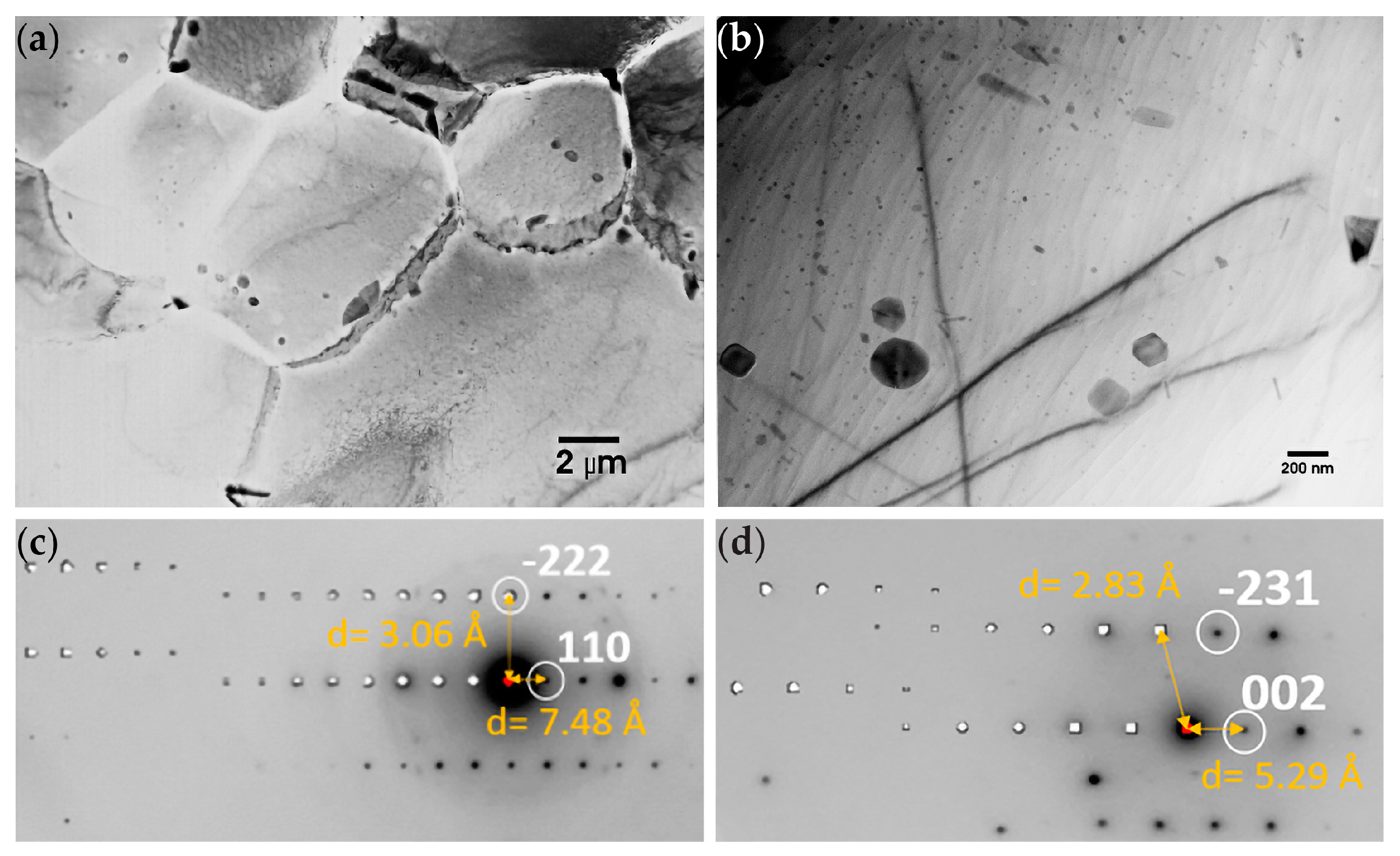
3.1.2. TEM of Alloy AZ61
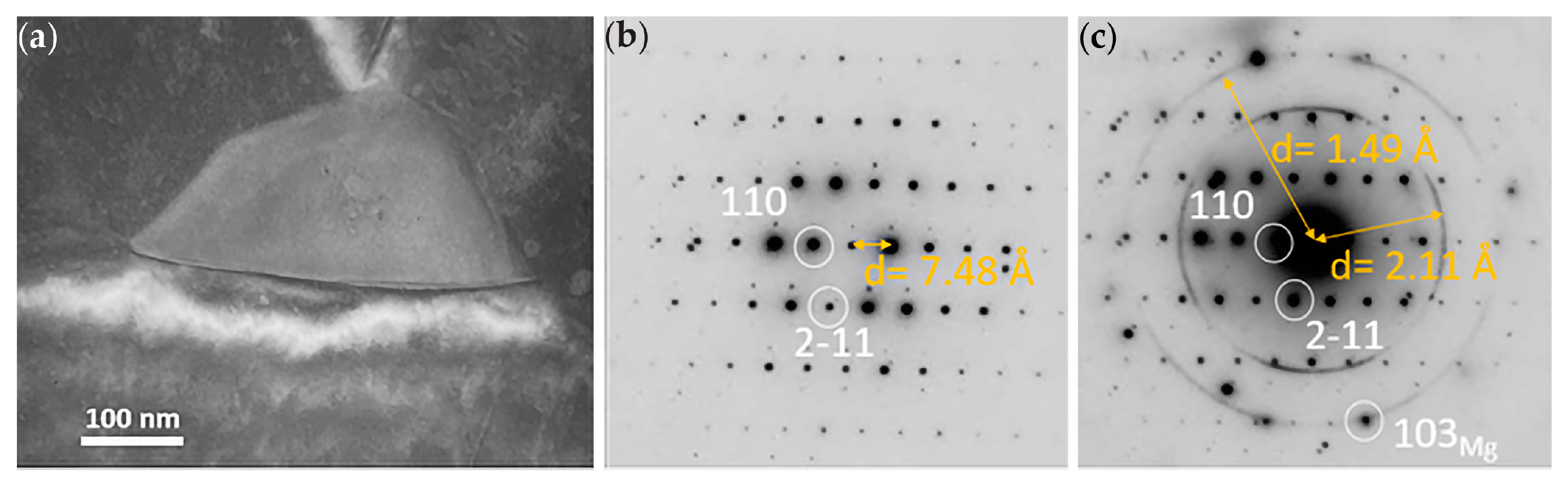
3.1.3. TEM of Alloy WE43-T5
3.2. Microstructural Features after Salt Fog Corrosion
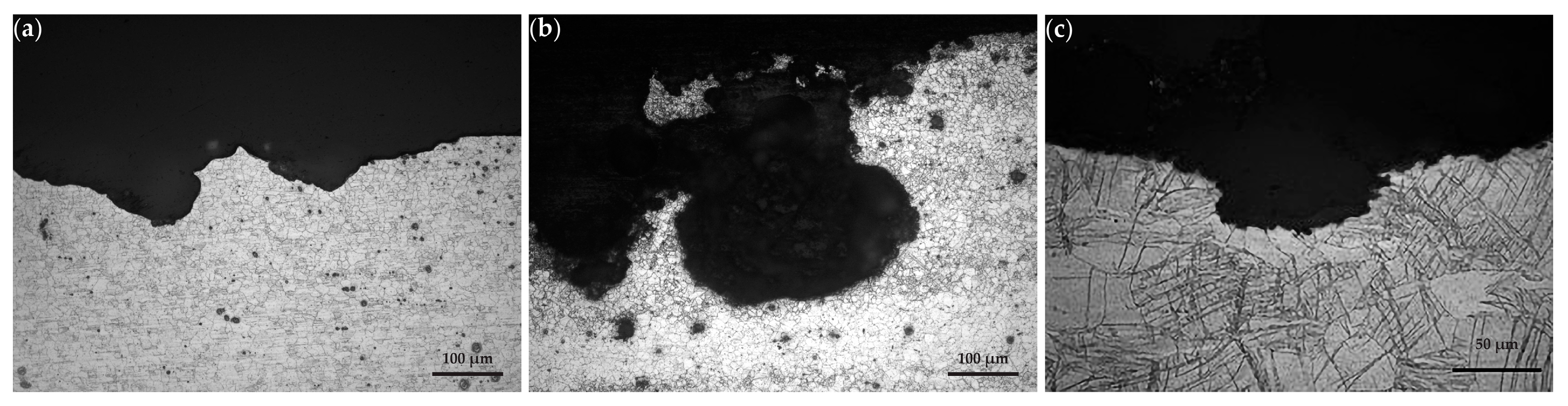
3.2.1. Macro Examination
3.2.2. Metallographic Sections
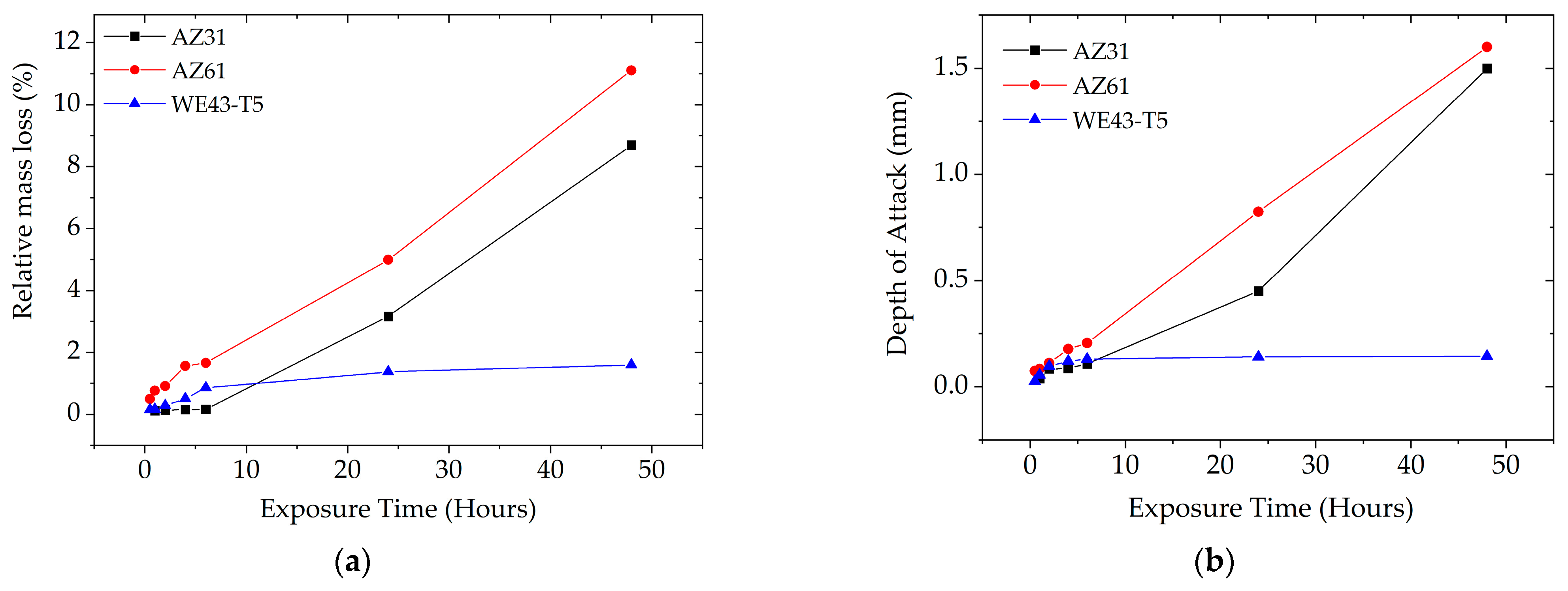
3.2.3. Depth of Attack and Relative Mass Loss
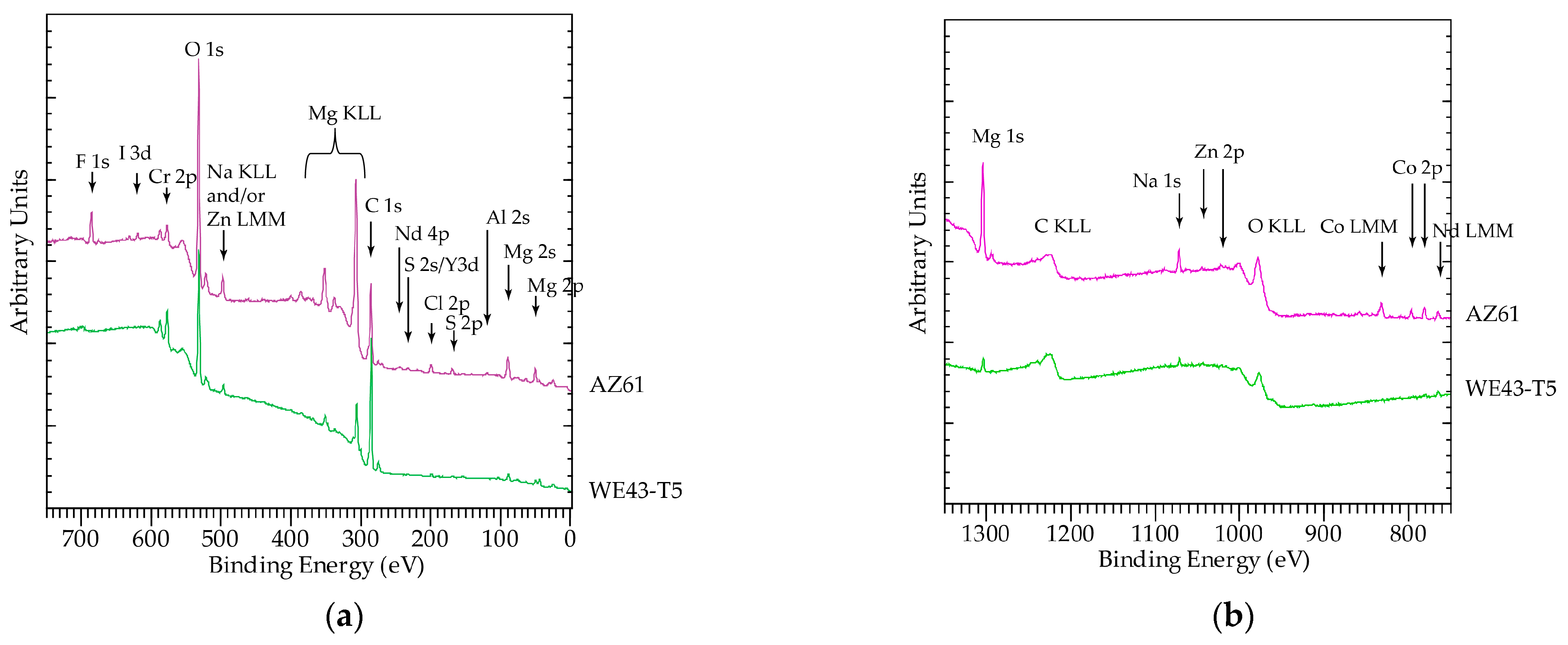
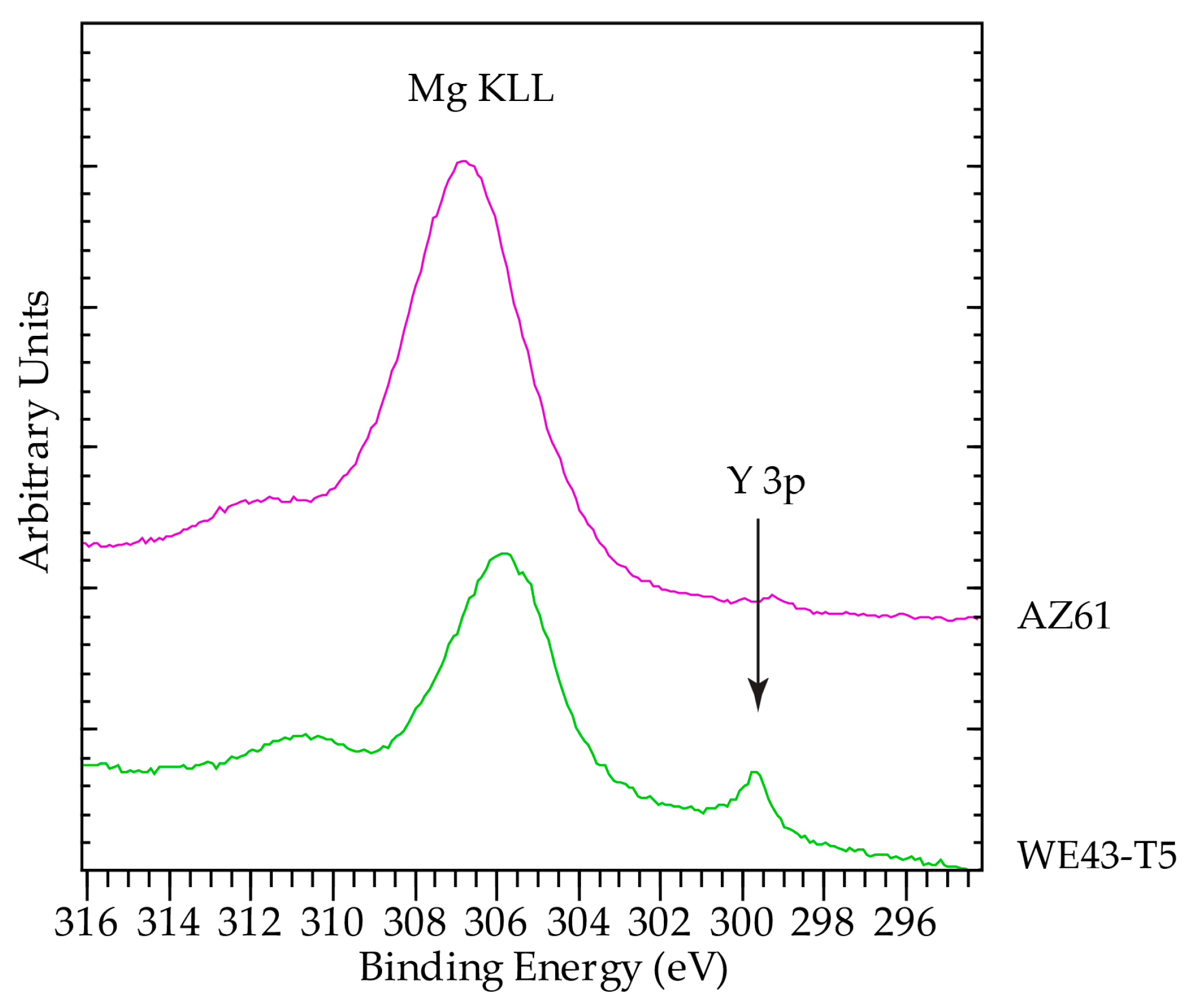
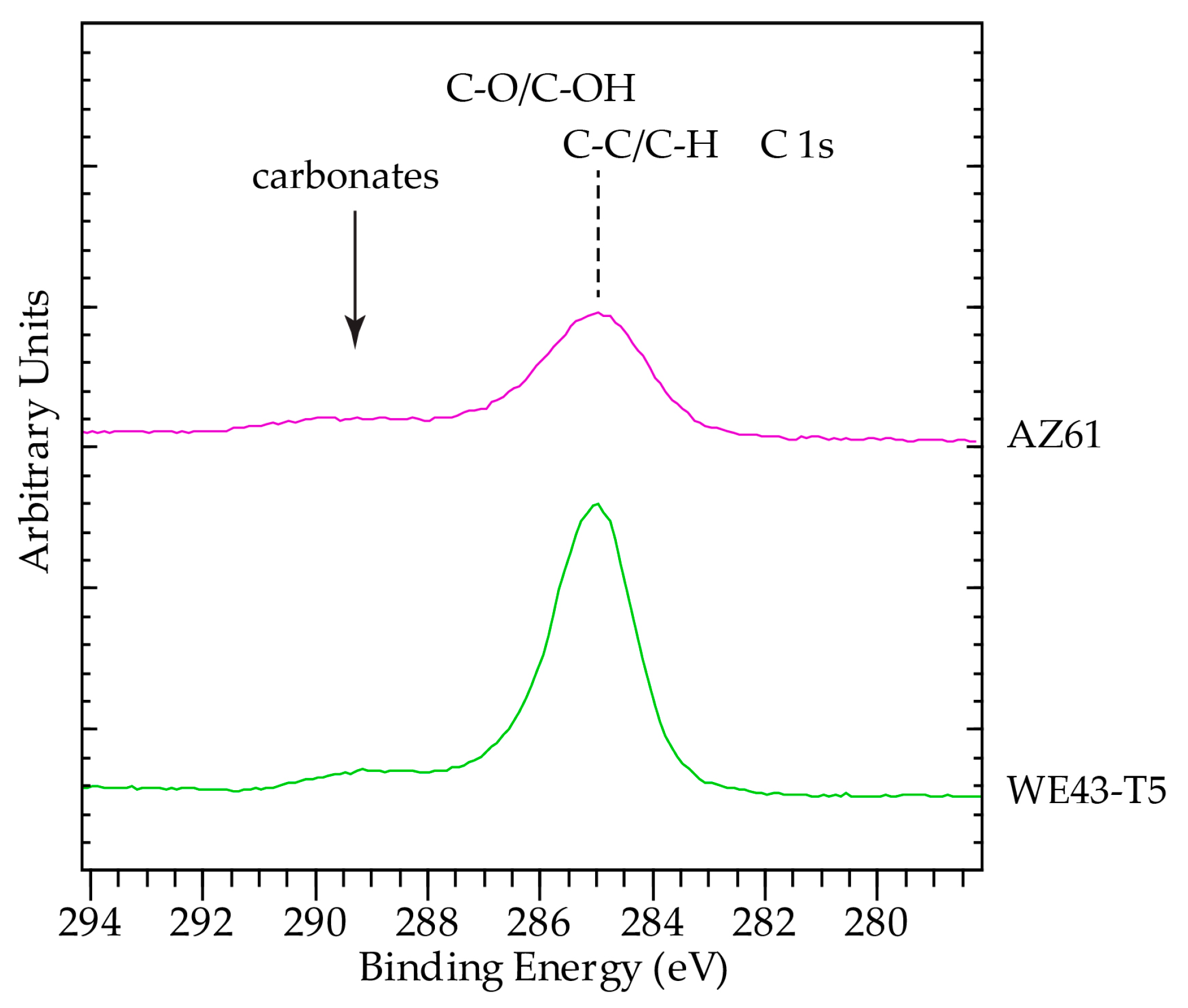
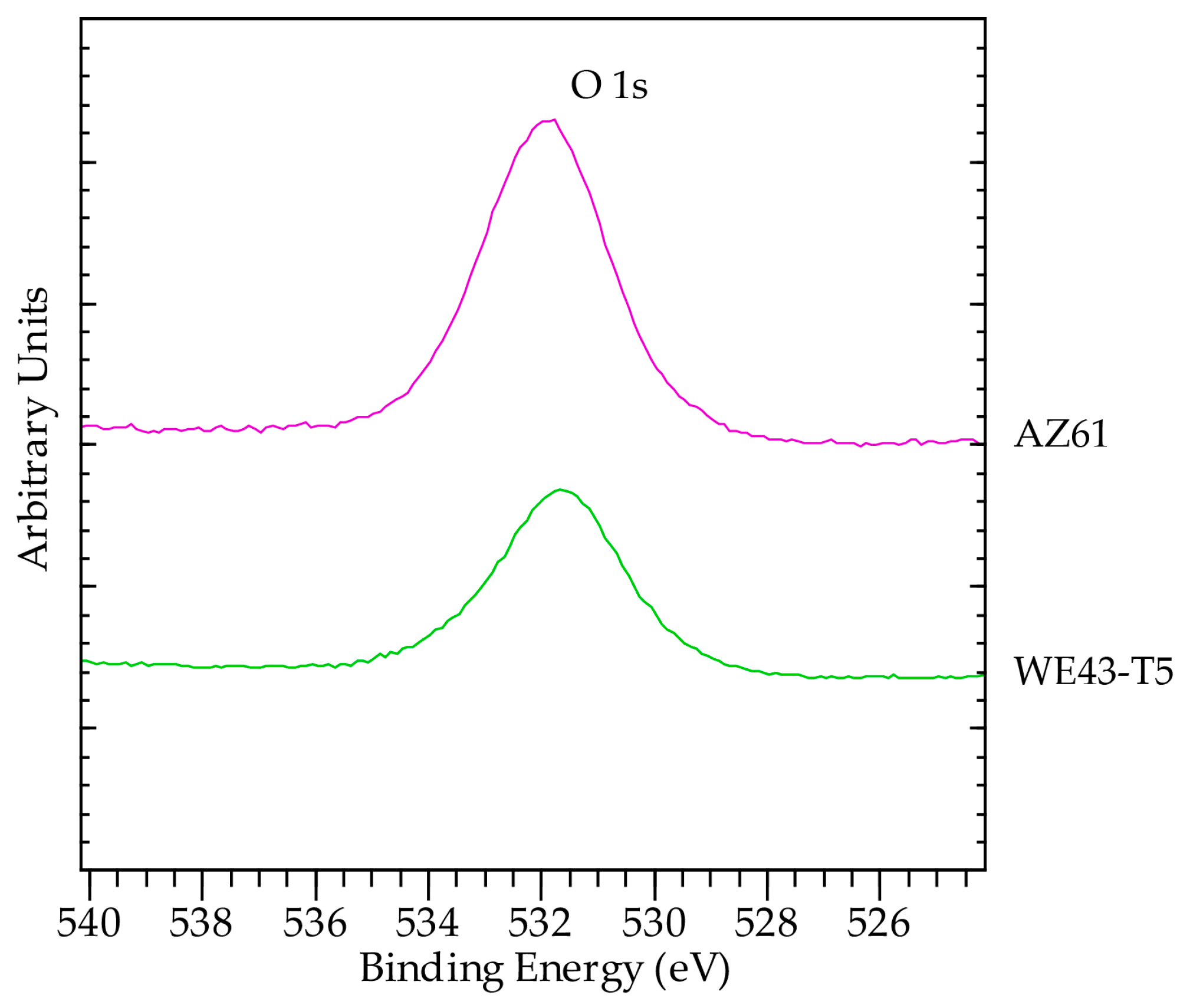
3.3. Analysis of Corrosion Products
4. Discussion
4.1. Corroded AZ31 and AZ61 Alloys
4.2. Corroded WE43-T5 Alloy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avedesian, M.M.; Baker, H.; ASM International (Eds.) Magnesium and Magnesium Alloys; ASM specialty handbook; ASM International: Materials Park, OH, USA, 1999; ISBN 978-0-87170-657-7. [Google Scholar]
- Istrate, B.; Munteanu, C.; Antoniac, I.-V.; Lupescu, Ș.-C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Wang, J.G.; Hsiung, L.M.; Nieh, T.G.; Mabuchi, M. Creep of a Heat Treated Mg–4Y–3RE Alloy. Mater. Sci. Eng. A 2001, 315, 81–88. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.-L.; Liu, M.; Atrens, A. Corrosion Behaviour in Salt Spray and in 3.5% NaCl Solution Saturated with Mg(OH)2 of as-Cast and Solution Heat-Treated Binary Mg–X Alloys: X=Mn, Sn, Ca, Zn, Al, Zr, Si, Sr. Corros. Sci. 2013, 76, 60–97. [Google Scholar] [CrossRef]
- Birbilis, N.; Easton, M.A.; Sudholz, A.D.; Zhu, S.M.; Gibson, M.A. On the Corrosion of Binary Magnesium-Rare Earth Alloys. Corros. Sci. 2009, 51, 683–689. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Abbott, T.; Birbilis, N. The Influence of Zirconium Additions on the Corrosion of Magnesium. Corros. Sci. 2014, 81, 27–35. [Google Scholar] [CrossRef]
- Sudholz, A.D.; Gusieva, K.; Chen, X.B.; Muddle, B.C.; Gibson, M.A.; Birbilis, N. Electrochemical Behaviour and Corrosion of Mg–Y Alloys. Corros. Sci. 2011, 53, 2277–2282. [Google Scholar] [CrossRef]
- Marrow, T.J.; Bin Ahmad, A.; Khan, I.N.; Sim, S.M.A.; Torkamani, S. Environment-Assisted Cracking of Cast WE43-T6 Magnesium. Mater. Sci. Eng. A 2004, 387–389, 419–423. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B.; Mansfeld, F. The Corrosion Protection Afforded by Rare Earth Conversion Coatings Applied to Magnesium. Corros. Sci. 2000, 42, 275–288. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion Resistance of WE43 and AZ91D Magnesium Alloys with Phosphate PEO Coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Ardelean, H.; Seyeux, A.; Zanna, S.; Prima, F.; Frateur, I.; Marcus, P. Corrosion Processes of Mg–Y–Nd–Zr Alloys in Na2SO4 Electrolyte. Corros. Sci. 2013, 73, 196–207. [Google Scholar] [CrossRef]
- Martin, H.J.; Horstemeyer, M.F.; Wang, P.T. Structure–Property Quantification of Corrosion Pitting under Immersion and Salt-Spray Environments on an Extruded AZ61 Magnesium Alloy. Corros. Sci. 2011, 53, 1348–1361. [Google Scholar] [CrossRef]
- Walton, C.A.; Martin, H.J.; Horstemeyer, M.F.; Wang, P.T. Quantification of Corrosion Mechanisms under Immersion and Salt-Spray Environments on an Extruded AZ31 Magnesium Alloy. Corros. Sci. 2012, 56, 194–208. [Google Scholar] [CrossRef]
- Merino, M.C.; Pardo, A.; Arrabal, R.; Merino, S.; Casajús, P.; Mohedano, M. Influence of Chloride Ion Concentration and Temperature on the Corrosion of Mg-Al Alloys in Salt Fog. Corros. Sci. 2010, 52, 1696–1704. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Susceptibility of Rare-Earth-Magnesium Alloys to Micro-Galvanic Corrosion. Corros. Sci. 2010, 52, 3896–3906. [Google Scholar] [CrossRef]
- Argade, G.R.; Kandasamy, K.; Panigrahi, S.K.; Mishra, R.S. Corrosion Behavior of a Friction Stir Processed Rare-Earth Added Magnesium Alloy. Corros. Sci. 2012, 58, 321–326. [Google Scholar] [CrossRef]
- Przondziono, J.; Walke, W.; Hadasik, E.; Szala, J.; Jakub, W. Corrosion Resistance Tests of Magnesium Alloy WE43 after Extrusion. Metalurgija 2013, 52, 243–246. [Google Scholar]
- Chamos, A.N.; Pantelakis, S.G.; Haidemenopoulos, G.N.; Kamoutsi, E. Tensile and Fatigue Behaviour of Wrought Magnesium Alloys AZ31 and AZ61. Fatigue Fract. Eng. Mater. Struct. 2008, 31, 812–821. [Google Scholar] [CrossRef]
- Shaw, B.A. Corrosion Resistance of Magnesium Alloys. In Corrosion: Fundamentals, Testing, and Protection; Cramer, S.D., Covino, B.S., Eds.; ASM International: Materials Park, OH, USA, 2003; pp. 692–696. ISBN 978-1-62708-182-5. [Google Scholar]
- ASTM B 117; “Practice for Operating Salt Spray (Fog) Apparatus,” Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2005. [CrossRef]
- ASTM G 1-03; “Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens,” Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- ASTM G46-94; “Standard Guide for Examination and Evaluation of Pitting Corrosion,” Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2005. [CrossRef]
- Schobinger-Papamantellos, P.; Fischer, P. Neutronenbeugungsuntersuchung der Atomverteilung von Mg17Al12. Naturwissenschaften 1970, 57, 128–129. [Google Scholar] [CrossRef]
- Antion, C.; Donnadieu, P.; Perrard, F.; Deschamps, A.; Tassin, C.; Pisch, A. Hardening Precipitation in a Mg–4Y–3RE Alloy. Acta Mater. 2003, 51, 5335–5348. [Google Scholar] [CrossRef]
- Li, Z.C.; Zhang, H.; Liu, L.; Xu, Y.B. Growth and Morphology of β Phase in an Mg–Y–Nd Alloy. Mater. Lett. 2004, 58, 3021–3024. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Characterisation of Strengthening Precipitate Phases in a Mg–Y–Nd Alloy. Acta Mater. 2000, 48, 1691–1703. [Google Scholar] [CrossRef]
- Thorne, N.A.; Jugnet, Y.; Reboul, M.; Dunlop, H. Comparison of the Natural Oxide Films Formed on Mg and Its Alloys. Surf. Interface Anal. 1990, 16, 315. [Google Scholar] [CrossRef]
- Kruger, J.; Long, G.G.; Tanaka, D. Fundamental Understanding of the Effect of Alloying Elements on the Corrosion Resistance of RS Mg Alloys; Report No. AD-A176/970/2/GAR; The Johns Hopkins University: Baltimore, MD, USA, 1986; p. 26. [Google Scholar]
- Baliga, C.B.; Tsakiropoulos, P.; Watts, J.F. Corrosion Products Formed on a Rapidly Solidified Mg-Al Alloy. Int. J. Rapid Solidif. 1989, 4, 231–250. [Google Scholar]
- Baliga, C.B.; Tsakiropoulos, P. On the Development of Corrosion Resistant Mg-Al Alloys. In Light-Weight Alloys for Aerospace Applications II; Lee, E.W., Kim, N.-J., Minerals, Metals and Materials Society, Eds.; Minerals, Metals & Materials Society: Warrendale, PA, USA, 1991; pp. 439–451. ISBN 978-0-87339-135-1. [Google Scholar]
- Baliga, C.B.; Tsakiropoulos, P. Design of Magnesium Alloys with Improved Corrosion Properties. In Magnesium Alloys and Their Applications; Mordike, B.L., Hehmann, F., Eds.; DGM conference: Garmisch-Partenkirchen, Germany, 1992; pp. 119–126. [Google Scholar]
- Baliga, C.B.; Tsakiropoulos, P. Development of Corrosion-Resistant Magnesium Alloys. 2. Structure of Corrosion Products on Rapidly Solidified Mg-16Al Alloys. Mater. Sci. Technol. 1993, 9, 513–519. [Google Scholar] [CrossRef]
- Lunder, O.; Lein, J.E.; Aune, T.K.; Nisancioglu, K. The Role of Mg 17 Al 12 Phase in the Corrosion of Mg Alloy AZ91. Corrosion 1989, 45, 741–748. [Google Scholar] [CrossRef]
- Nakatsugawa, I.; Kamado, S.; Kojima, Y.; Ninomiya, R.; Kubota, K. Corrosion Behavior of Magnesium Alloys Containing Heavy Rare Earth Elements. In Proceedings of the Third International Magnesium Conference, Manchester, UK, 10–12 April 1996; pp. 687–698. [Google Scholar]
- Nakatsugawa, I.; Kamado, S.; Kojima, Y.; Ninomiya, R.; Kubota, K. Corrosion of Magnesium Alloys Containing Rare Earth Elements. Corros. Rev. 1998, 16, 139–158. [Google Scholar] [CrossRef]
- Kiryuu, M.; Okumura, H.; Kamado, S.; Kojima, Y.; Ninomiya, R.; Nakatsugawa, I. Corrosion Resistance of Heat Resistant Magnesium Alloys Containing Heavy Rare Earth Elements. J. Jpn. Inst. Light Met. 1996, 46, 39–44. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Khobaib, M.; Robertson, E.; Froes, F.H. Corrosion Behavior of Rapidly Solidified MgNd and MgY Alloys. Mater. Sci. Eng. 1988, 99, 507–511. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Weiss, I.; Froes, F.H. Consolidation of Rapidly Solidified Magnesium Alloy Powder. KEM 1988, 29, 135–146. [Google Scholar] [CrossRef]
- Walker, C.G.H.; Morton, S.A.; Beamson, G.; Matthew, J.A.D.; Yousif, F.N. Auger Parameter Studies of Amorphous CuHf Alloys. J. Electron Spectrosc. Relat. Phenom. 1994, 70, 73–81. [Google Scholar] [CrossRef]
- Matthew, J.A.D.; Morton, S.A.; Walker, C.G.H.; Beamson, G. Auger Parameter Studies of Amorphous NbSi. J. Phys. D Appl. Phys. 1995, 28, 1702–1706. [Google Scholar] [CrossRef]




| Al | Zn | Mn | Fe | Ni | Cu | Si | Mg | |
|---|---|---|---|---|---|---|---|---|
| AZ31 | 3.06 | 0.80 | 0.25 | 0.003 | <0.001 | 0.001 | - | bal. |
| AZ61 | 6 | 0.72 | 0.33 | 0.003 | ≤0.001 | ≤0.01 | <0.01 | bal. |
| WE43-T5 | Yttrium | Neodymium | Zirconium | Mg | ||||
| 3.7–4.3 | 2.4–4.4% | 0.4% | bal | |||||
| Temperature (°C) | Time | |
|---|---|---|
| AZ31 | 300 | 30 min |
| AZ61 | 250 | 60 min |
| WE43-T5 | 200 | 16 h |
| Plane | Average Grain Size (μm) | Microhardness (HV) | |
|---|---|---|---|
| AZ31 | LT | 13 | 48 ± 3.5 |
| LS | 12 | 58 ± 3.5 | |
| ST | 18 | 55 ± 4 | |
| AZ61 | LT | 16 | 63 ± 3.2 |
| LS | 13 | 60 ± 3.5 | |
| ST | 18 | 60 ± 4 | |
| WE43-T5 | LT | 50 | 91.5 ± 4.5 |
| LS | 35 | 87.2 ± 3.5 | |
| ST | 45 | 81.8 ± 2.9 |
| Precipitates on a Grain Boundary | Precipitates in the Matrix | |||||||
|---|---|---|---|---|---|---|---|---|
| Element Line | Net Counts | Net Counts Error | Atom % | Atom % Error | Net Counts | Net Counts Error | Atom % | Atom % Error |
| Mg K | 10,319 | +/−143 | 89.10 | +/−1.23 | 24,488 | +/−205 | 94.65 | +/−0.79 |
| Cu K | 1056 | +/−61 | 2.49 | +/−0.14 | 1409 | +/−68 | 1.49 | +/−0.07 |
| Cu L | 4 | +/−38 | --- | --- | 0 | +/−30 | --- | --- |
| Y K | 974 | +/−88 | 3.35 | +/−0.30 | 1216 | +/−129 | 1.87 | +/−0.20 |
| Y L | 1388 | +/−113 | --- | --- | 1807 | +/−117 | --- | --- |
| Y M | 0 | +/−48 | --- | --- | 0 | +/−72 | --- | --- |
| Nd L | 3297 | +/−143 | 5.05 | +/−0.22 | 2903 | +/−141 | 1.99 | +/−0.10 |
| Nd M | 132 | +/−54 | --- | --- | 124 | +/−58 | --- | --- |
| Total | 100.00 | 100.00 | ||||||
| AZ61 | WE43-T5 | Mg (Literature) | MgO (Literature) | Mg(OH)2 (Literature) | |
|---|---|---|---|---|---|
| BE of Mg 2p (eV) | 50.2 ± 0.1 | 49.9 ± 0.1 | 49.4–49.95 | 50.25–51.1 | 49.5 |
| BE of Mg 1s (eV) | 1304.45 ± 0.1 | 1303.9 ± 0.1 | 1303–1303.8 | 1303.9 | 1302.4 |
| KE of Mg KLL (eV) | 1179.8 ± 0.2 | 1180.8 ± 0.1 | 1184.9–1185.9 | 1181.3 | |
| 2p-KLL AP (eV) | 1230 ± 0.22 | 1230.7 ± 0.22 | 1234.7–1235.45 | 1230.8–1231.6 | |
| 1s-KLL AP | 2485.25 ± 0.22 | 2484.7 ± 0.22 | 2488.3–2489.3 | 2485–2485.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoutsi, H.; Haidemenopoulos, G.N.; Gunnaes, A.E.; Diplas, S. Microstructure and Salt Fog Corrosion of Wrought Mg-Al-Zn and Mg-RE Alloys. Materials 2023, 16, 1004. https://doi.org/10.3390/ma16031004
Kamoutsi H, Haidemenopoulos GN, Gunnaes AE, Diplas S. Microstructure and Salt Fog Corrosion of Wrought Mg-Al-Zn and Mg-RE Alloys. Materials. 2023; 16(3):1004. https://doi.org/10.3390/ma16031004
Chicago/Turabian StyleKamoutsi, Helen, Gregory N. Haidemenopoulos, Anette E. Gunnaes, and Spyros Diplas. 2023. "Microstructure and Salt Fog Corrosion of Wrought Mg-Al-Zn and Mg-RE Alloys" Materials 16, no. 3: 1004. https://doi.org/10.3390/ma16031004
APA StyleKamoutsi, H., Haidemenopoulos, G. N., Gunnaes, A. E., & Diplas, S. (2023). Microstructure and Salt Fog Corrosion of Wrought Mg-Al-Zn and Mg-RE Alloys. Materials, 16(3), 1004. https://doi.org/10.3390/ma16031004







