Comparison of Coupled Electrochemical and Thermal Modelling Strategies of 18650 Li-Ion Batteries in Finite Element Analysis—A Review
Abstract
:1. Introduction
2. Scope of the Paper
3. Materials and Methods
3.1. Governing Electrochemical Equations Used in FEA
3.2. Governing Thermal Equations Used in FEA
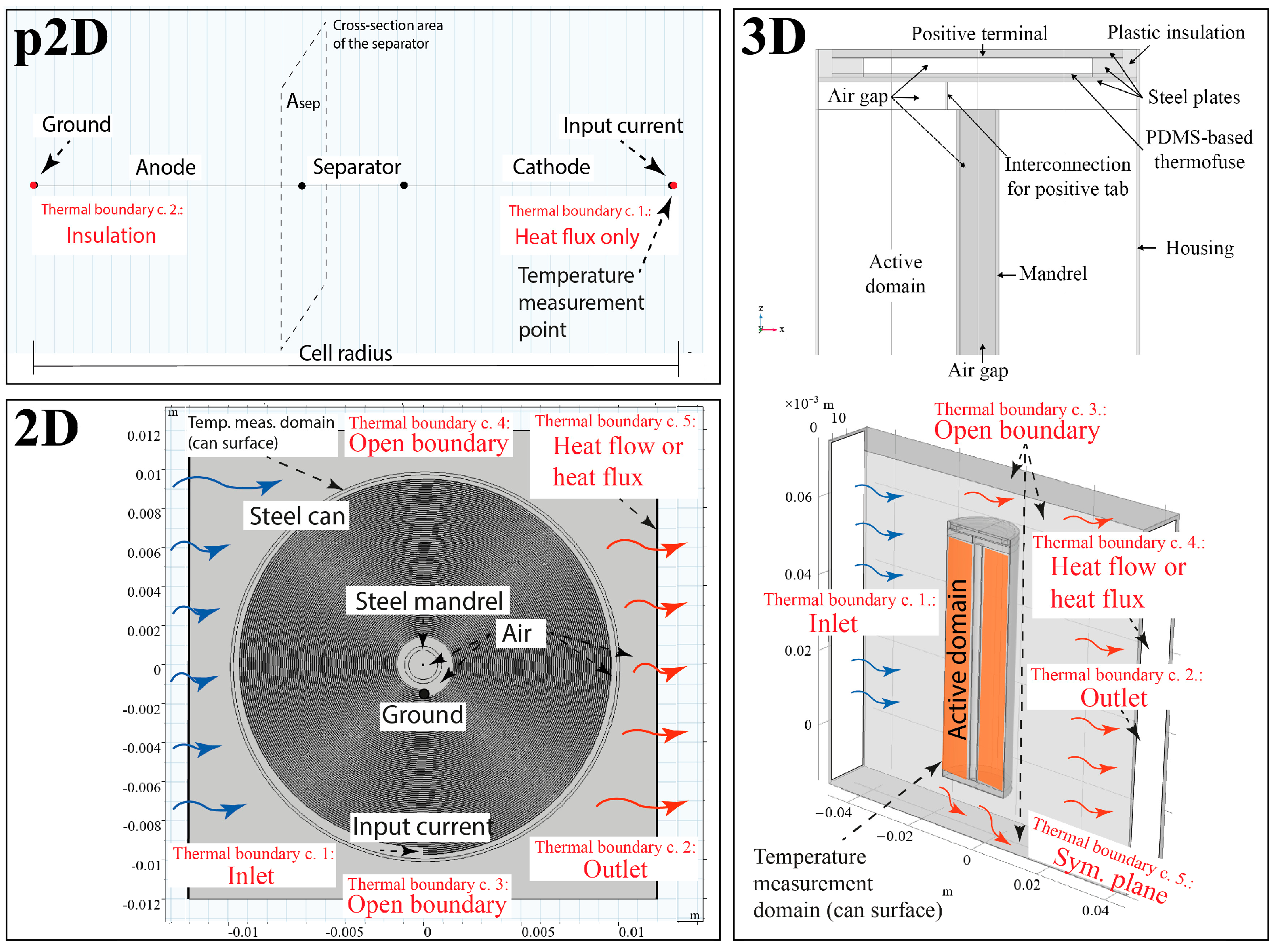
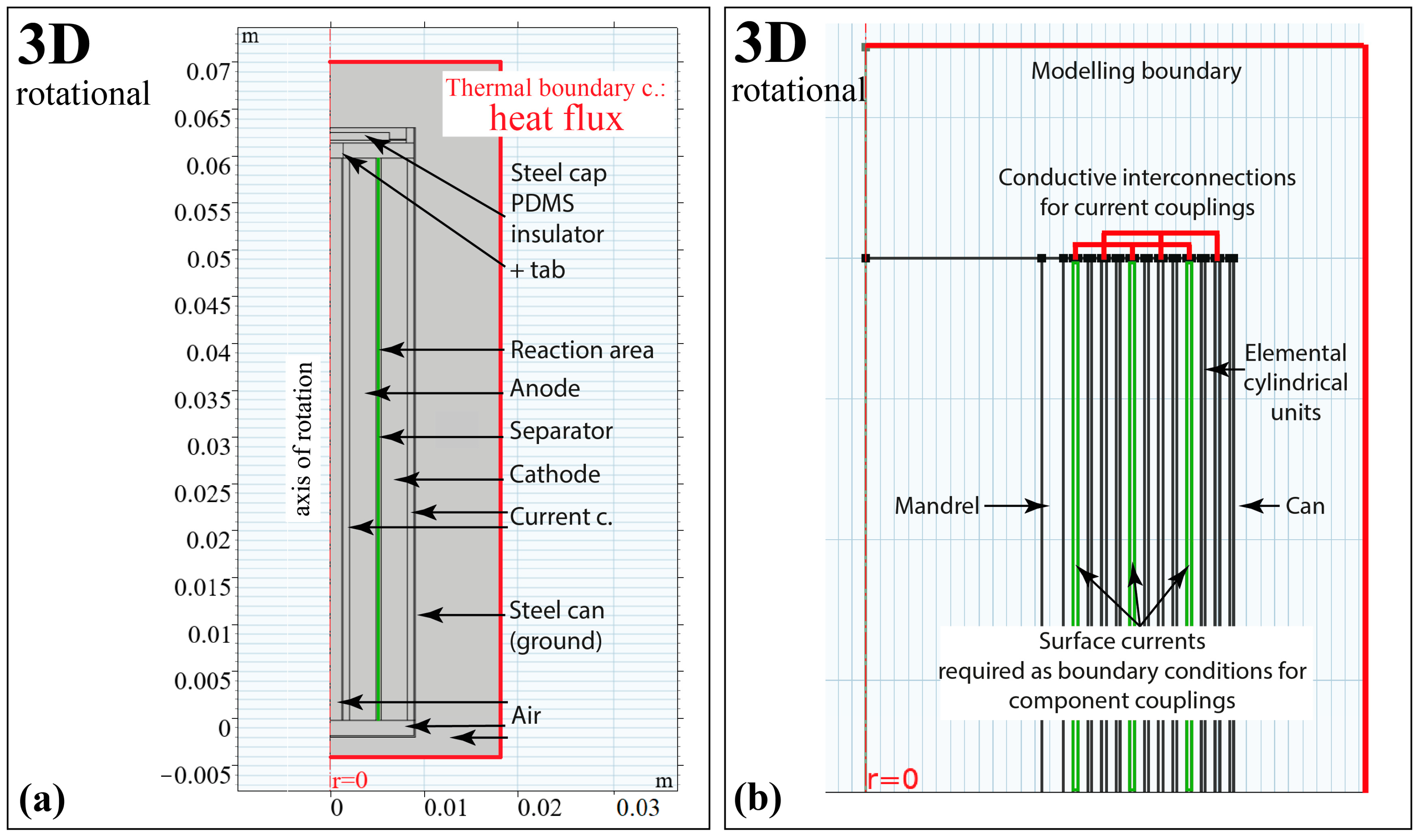
3.3. Consideration of Modelling Domains
3.4. Pros and Cons of the Models
4. Experimental
5. Results and Discussion
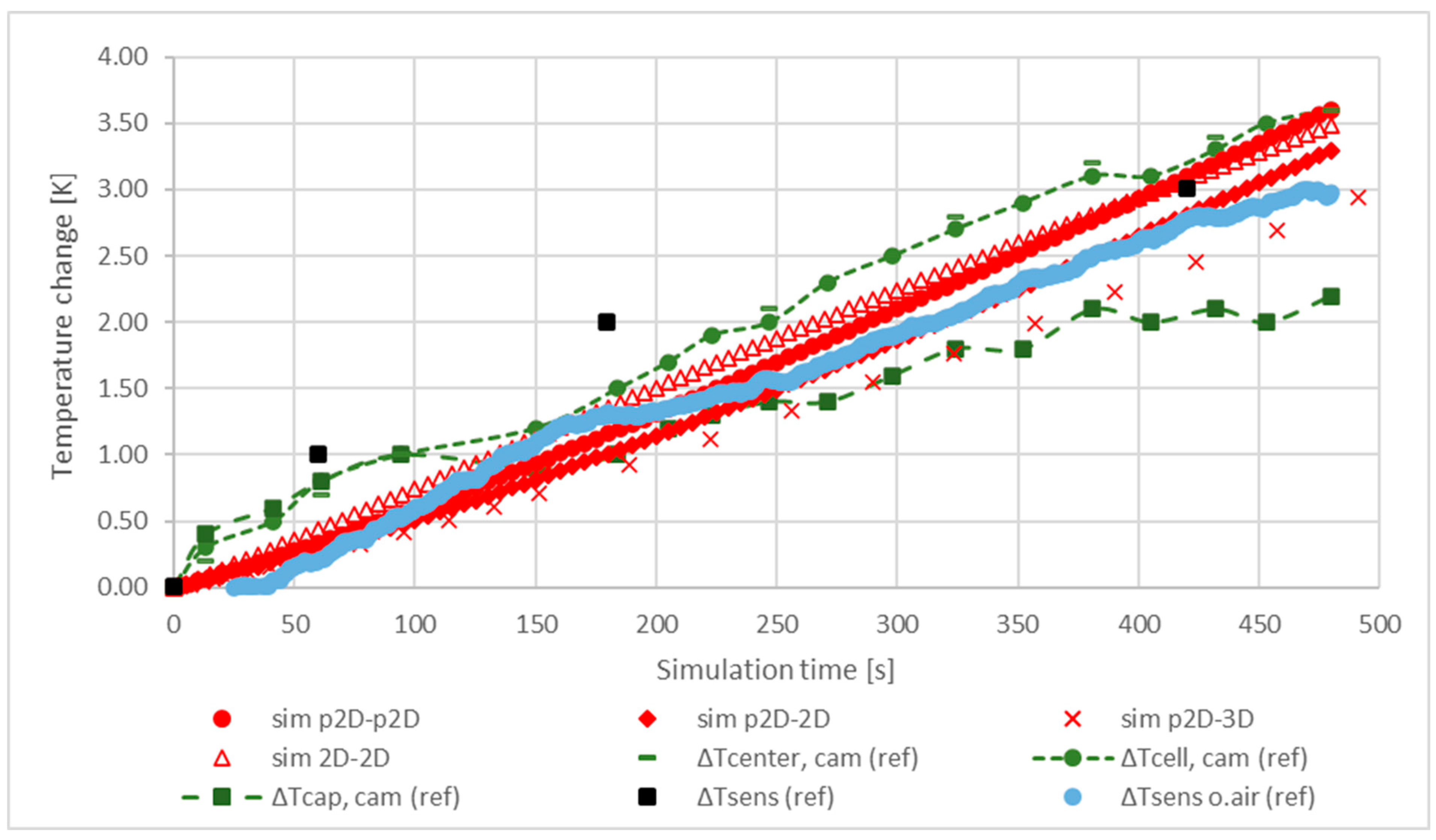
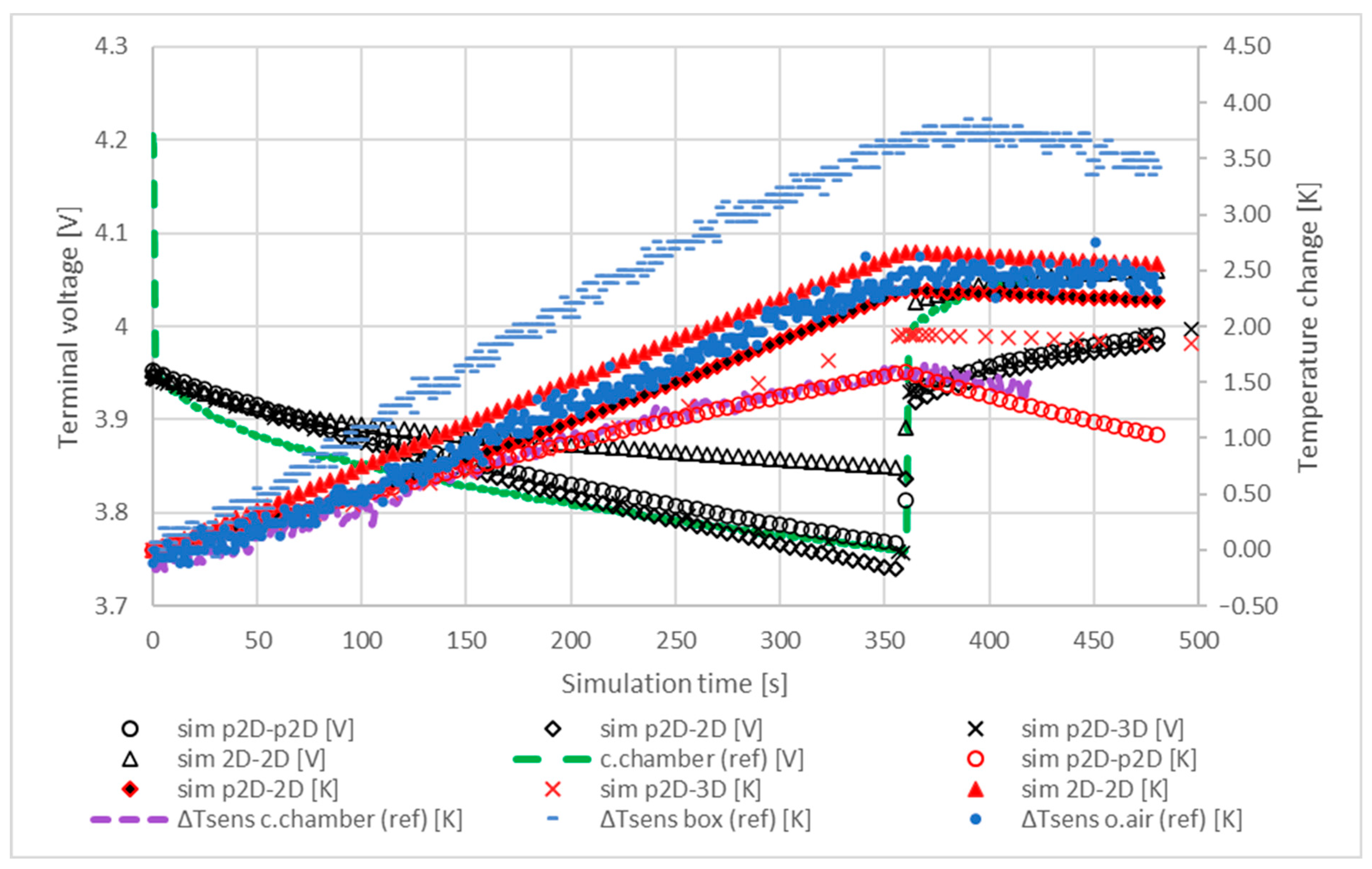
5.1. Temperature and Terminal Voltage Characteristics
5.2. Sensitivity Analysis of the Model Parameters
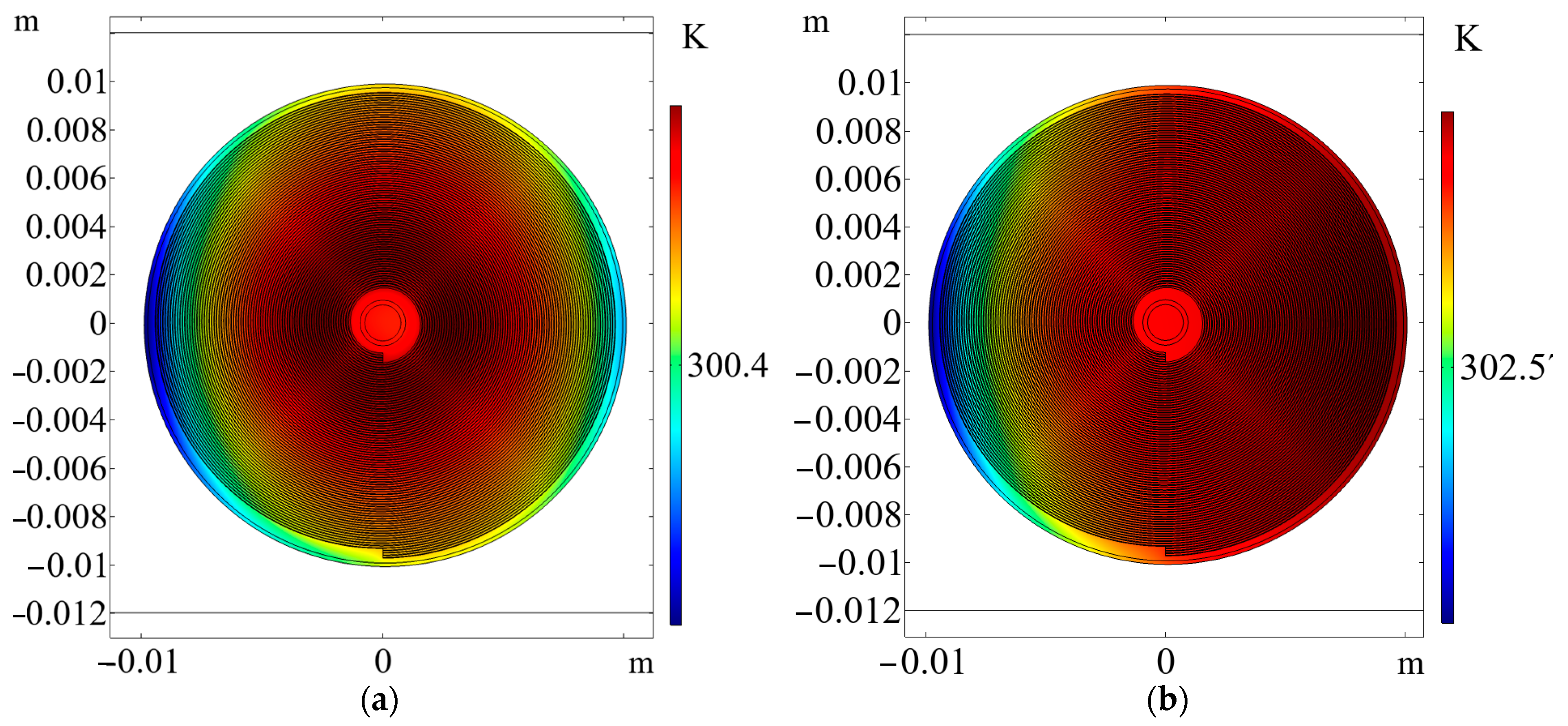
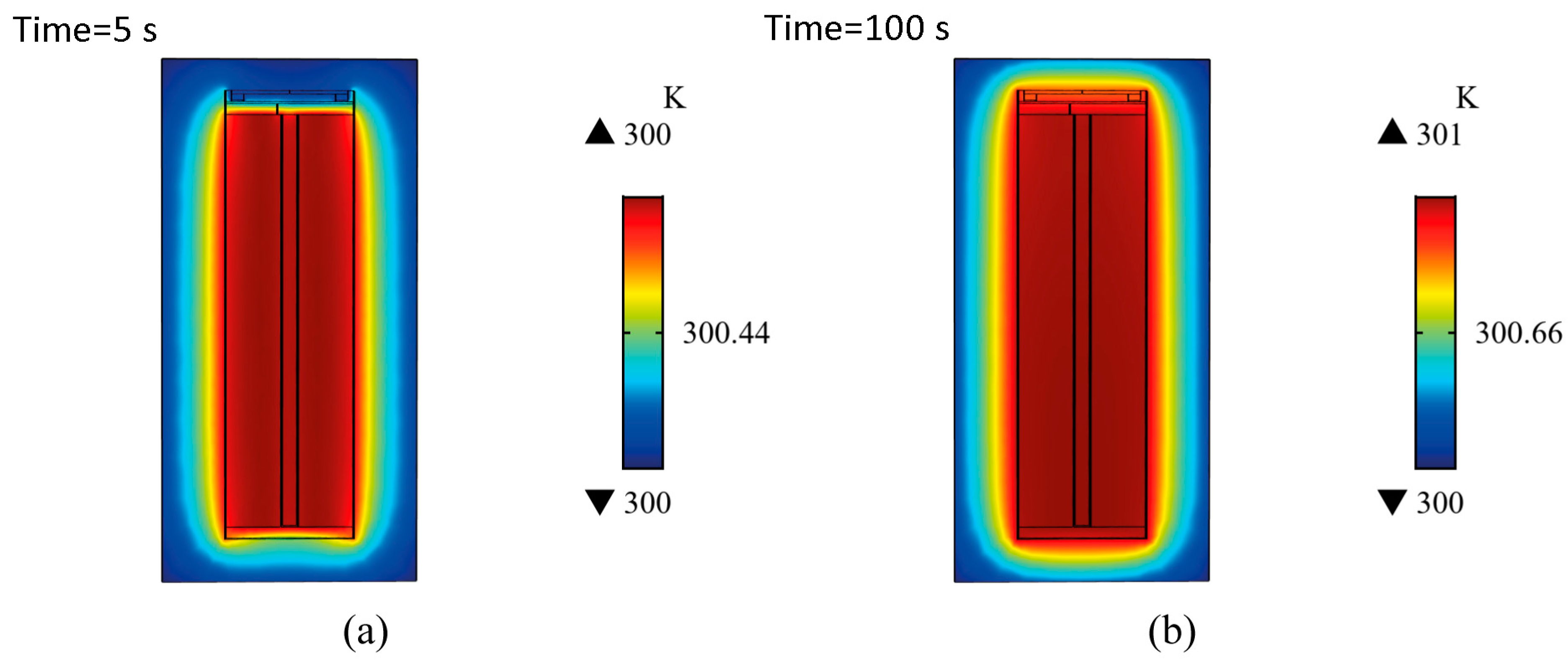
5.3. Characteristic Temperature Distributions in the Internal Structure
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Jayaprabakar, J.; Kumar, J.A.; Parthipan, J.; Karthikeyan, A.; Anish, M.; Joy, N. Review on hybrid electro chemical energy storage techniques for electrical vehicles: Technical insights on design, performance, energy management, operating issues & challenges. J. Energy Storage 2023, 72, 108689. [Google Scholar] [CrossRef]
- Lin, J.; Liu, X.; Li, S.; Zhang, C.; Yang, S. A review on recent progress, challenges and perspective of battery thermal management system. Int. J. Heat Mass Transf. 2021, 167, 120834. [Google Scholar] [CrossRef]
- Ruan, H.; Sun, B.; Zhang, W.; Su, X.; He, X. Quantitative Analysis of Performance Decrease and Fast-Charging Limitation for Lithium-Ion Batteries at Low Temperature Based on the Electrochemical Model. IEEE Trans. Intell. Transp. Syst. 2021, 22, 640–650. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, L.; Ge, S.; Wang, C.-Y.; Shaffer, C.E.; Rahn, C.D. In Situ Measurement of Radial Temperature Distributions in Cylindrical Li-Ion Cells. J. Electrochem. Soc. 2014, 161, A1499–A1507. [Google Scholar] [CrossRef]
- See, K.W.; Wang, G.; Zhang, Y.; Wang, Y.; Meng, L.; Gu, X.; Zhang, N.; Lim, K.C.; Zhao, L.; Xie, B. Critical review and functional safety of a battery management system for large-scale lithium-ion battery pack technologies. Int. J. Coal Sci. Technol. 2022, 9, 36. [Google Scholar] [CrossRef]
- Kumaresan, K.; Sikha, G.; White, R.E. Thermal Model for a Li-Ion Cell. J. Electrochem. Soc. 2008, 155, A164–A171. [Google Scholar] [CrossRef]
- Wang, R.; Liang, Z.; Souri, M.; Esfahani, M.N.; Jabbari, M. Numerical analysis of lithium-ion battery thermal management system using phase change material assisted by liquid cooling method. Int. J. Heat Mass Transf. 2022, 183, 122095. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries. Energy Convers. Manag. X 2022, 16, 100310. [Google Scholar] [CrossRef]
- Szürke, S.K.; Kovács, G.; Sysyn, M.; Liu, J.; Fischer, S. Numerical Optimization of Battery Heat Management of Electric Vehicles. J. Appl. Comput. Mech. 2023, 9, 1076–1092. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Z. Effects of a Separator on the Electrochemical and Thermal Performances of Lithium-Ion Batteries: A Numerical Study. Energy Fuels 2020, 34, 14915–14923. [Google Scholar] [CrossRef]
- Pang, H.; Guo, L.; Wu, L.; Jin, J.; Zhang, F.; Liu, K. A novel extended Kalman filter-based battery internal and surface temperature estimation based on an improved electro-thermal model. J. Energy Storage 2021, 41, 102854. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Wang, L.; Lu, L.; Ren, D.; Santhanagopalan, S. A Coupled Electrochemical-Thermal Failure Model for Predicting the Thermal Runaway Behavior of Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3748–A3765. [Google Scholar] [CrossRef]
- Wu, B.; Yufit, V.; Marinescu, M.; Offer, G.J.; Martinez-Botas, R.F.; Brandon, N.P. Coupled thermal–electrochemical modelling of uneven heat generation in lithium-ion battery packs. J. Power Sources 2013, 243, 544–554. [Google Scholar] [CrossRef]
- Wang, C.Y.; Gu, W.B.; Liaw, B.Y. Micro-Macroscopic Coupled Modeling of Batteries and Fuel Cells: I. Model Development. J. Electrochem. Soc. 1998, 145, 3407–3417. [Google Scholar] [CrossRef]
- Gu, W.B.; Wang, C.Y.; Liaw, B.Y. Micro—Macroscopic Coupled Modeling of Batteries and Fuel Cells: II. Application to Nickel—Cadmium and Nickel—Metal Hydride Cells Micro-Macroscopic Coupled Modeling of Batteries and Fuel Cells. J. Electrochem. Soc. 1998, 145, 3418. [Google Scholar] [CrossRef]
- Gu, W.B.; Wang, C.Y. Thermal-Electrochemical Modeling of Battery Systems. J. Electrochem. Soc. 2000, 147, 2910. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.J.; Yin, S.; Hu, X. Development of a one-dimensional thermal-electrochemical model of lithium ion battery. In Proceedings of the IECON 2013—39th Annual Conference of the IEEE Industrial Electronics Society, Vienna, Austria, 10–13 November 2013; pp. 6709–6714. [Google Scholar] [CrossRef]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Electrochemical-thermal analysis of 18650 Lithium Iron Phosphate cell. Energy Convers. Manag. 2013, 75, 162–174. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Lyu, C.; Zheng, J.; Li, F. Multi-physics modeling of lithium-ion batteries and charging optimization. In Proceedings of the 2014 Prognostics and System Health Management Conference (PHM-2014 Hunan), Zhangjiajie, China, 24–27 August 2014; pp. 391–400. [Google Scholar] [CrossRef]
- Baba, N.; Yoshida, H.; Nagaoka, M.; Okuda, C.; Kawauchi, S. Numerical simulation of thermal behavior of lithium-ion secondary batteries using the enhanced single particle model. J. Power Sources 2014, 252, 214–228. [Google Scholar] [CrossRef]
- Jokar, A.; Rajabloo, B.; Désilets, M.; Lacroix, M. Review of simplified Pseudo-two-Dimensional models of lithium-ion batteries. J. Power Sources 2016, 327, 44–55. [Google Scholar] [CrossRef]
- Sambegoro, P.L.; Budiman, B.A.; Philander, E.; Aziz, M. Dimensional and Parametric Study on Thermal Behaviour of Li-ion Batteries. In Proceedings of the 2018 5th International Conference on Electric Vehicular Technology (ICEVT), Surakarta, Indonesia, 30–31 October 2018; pp. 123–127. [Google Scholar] [CrossRef]
- Tran, N.T.; Vilathgamuwa, M.; Farrell, T.; Choi, S.S.; Li, Y.; Teague, J. A Computationally-Efficient Electrochemical-Thermal Model for Small-Format Cylindrical Lithium Ion Batteries. In Proceedings of the 2018 IEEE 4th Southern Power Electronics Conference, Singapore, 10–13 December 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Raihan, G.A.; Basir, A.; Al Rafi, A.; Haque, R. Comparative Characteristics Analysis of Thermal and Electro-Chemical Behavior of Lithium Ion Battery Using Finite Element Method. In Proceedings of the 2018 International Conference on Computer, Communication, Chemical, Material and Electronic Engineering, Rajshahi, Bangladesh, 8–9 February 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Chiew, J.; Chin, C.S.; Toh, W.D.; Gao, Z.; Jia, J.; Zhang, C. A pseudo three-dimensional electrochemical-thermal model of a cylindrical LiFePO4/graphite battery. Appl. Therm. Eng. 2019, 147, 450–463. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Zhang, X.; Dong, T.; Zhu, C. A novel pseudo two-dimensional model for NCM Liion battery based on electrochemical-thermal coupling analysis. In Proceedings of the 2020 3rd International Conference on Electron Device and Mechanical Engineering, Suzhou, China, 1–3 May 2020; pp. 110–116. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Z.; Wang, X.; Jia, L.; Yang, L. Two-dimensional electrochemical-thermal coupled modeling of cylindrical LiFePO4 batteries. J. Power Sources 2014, 256, 233–243. [Google Scholar] [CrossRef]
- Ye, Y.; Shi, Y.; Huat, L.; Tay, A.A.O. An electro-thermal model and its application on a spiral-wound lithium ion battery with porous current collectors. Electrochim. Acta 2014, 121, 143–153. [Google Scholar] [CrossRef]
- Yao, X.Y.; Pecht, M.G. Tab design and failures in cylindrical li-ion batteries. IEEE Access 2019, 7, 24082–24095. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Sahraei, E.; Wierzbicki, T. Deformation and failure mechanisms of 18650 battery cells under axial compression. J. Power Sources 2016, 336, 332–340. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Genieser, R.; Worwood, D.; Barai, A.; Marco, J.; Jennings, P. A systematic approach for electrochemical-thermal modelling of a large format lithium-ion battery for electric vehicle application. J. Power Sources 2018, 382, 77–94. [Google Scholar] [CrossRef]
- Liebig, G.; Gupta, G.; Kirstein, U.; Schuldt, F.; Agert, C. Parameterization and Validation of an Electrochemical Thermal Model of a Lithium-Ion Battery. Batteries 2019, 5, 62. [Google Scholar] [CrossRef]
- Khan, M.R.; Kaer, S.K. Three Dimensional Thermal Modeling of Li-Ion Battery Pack Based on Multiphysics and Calorimetric Measurement. In Proceedings of the 2016 IEEE Vehicle Power and Propulsion Conference VPPC, Hangzhou, China, 17–20 October 2016. [Google Scholar] [CrossRef]
- Lin, C.; Cui, C.; Xu, X. Lithium-ion battery electro-thermal model and its application in the numerical simulation of short circuit experiment. World Electr. Veh. J. 2013, 6, 603–610. [Google Scholar] [CrossRef]
- Tong, W.; Somasundaram, K.; Birgersson, E.; Mujumdar, A.S.; Yap, C. Thermo-electrochemical model for forced convection air cooling of a lithium-ion battery module. Appl. Therm. Eng. 2016, 99, 672–682. [Google Scholar] [CrossRef]
- Robinson, J.B.; Darr, J.A.; Eastwood, D.S.; Hinds, G.; Lee, P.D.; Shearing, P.R.; Taiwo, O.O.; Brett, D.J.L. Non-uniform temperature distribution in Li-ion batteries during discharge—A combined thermal imaging, X-ray micro-tomography and electrochemical impedance approach. J. Power Sources 2014, 252, 51–57. [Google Scholar] [CrossRef]
- Bolsinger, C.; Birke, K.P. Effect of different cooling configurations on thermal gradients inside cylindrical battery cells. J. Energy Storage 2019, 21, 222–230. [Google Scholar] [CrossRef]
- Jindal, P.; Kumar, B.S.; Bhattacharya, J. Coupled electrochemical-abuse-heat-transfer model to predict thermal runaway propagation and mitigation strategy for an EV battery module. J. Energy Storage 2021, 39, 102619. [Google Scholar] [CrossRef]
- Yin, L.; Björneklett, A.; Söderlund, E.; Brandell, D. Analyzing and mitigating battery ageing by self-heating through a coupled thermal-electrochemical model of cylindrical Li-ion cells. J. Energy Storage 2021, 39, 102648. [Google Scholar] [CrossRef]
- Li, H.; Saini, A.; Liu, C.; Yang, J.; Wang, Y.; Yang, T.; Pan, C.; Chen, L.; Jiang, H. Electrochemical and thermal characteristics of prismatic lithium-ion battery based on a three-dimensional electrochemical-thermal coupled model. J. Energy Storage 2021, 42, 102976. [Google Scholar] [CrossRef]
- Veth, C.; Dragicevic, D.; Pfister, R.; Arakkan, S.; Merten, C. 3D Electro-Thermal Model Approach for the Prediction of Internal State Values in Large-Format Lithium Ion Cells and Its Validation. J. Electrochem. Soc. 2014, 161, 1943–1952. [Google Scholar] [CrossRef]
- Gerver, R.E.; Meyers, J.P. Three-Dimensional Modeling of Electrochemical Performance and Heat Generation of Lithium-Ion Batteries in Tabbed Planar Configurations. J. Electrochem. Soc. 2011, 158, 835–843. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, G.; Wang, C. Effect of tab design on large-format Li-ion cell performance. J. Power Sources 2014, 257, 70–79. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. 1-Modeling of Galvanostatic Charge and Discharge. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Subramanian, V.R.; Ritter, J.A.; White, R.E. Approximate Solutions for Galvanostatic Discharge of Spherical Particles I. Constant Diffusion Coefficient. J. Electrochem. Soc. 2001, 148, E444–E449. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X. Electrode thickness correlated parameters estimation for a Li-ion NMC battery electrochemical model. ECS Trans. 2017, 77, 491–507. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890–1903. [Google Scholar] [CrossRef]
- Somerville, L.; Ferrari, S.; Lain, M.J.; McGordon, A.; Jennings, P.; Bhagat, R. An in-situ reference electrode insertion method for commercial 18650-type cells. Batteries 2018, 4, 18. [Google Scholar] [CrossRef]
- Carelli, S.; Quarti, M.; Yagci, M.C.; Bessler, W.G. Modeling and experimental validation of a high-power lithium-ion pouch cell with LCO/NCA blend cathode. J. Electrochem. Soc. 2019, 166, A2990–A3003. [Google Scholar] [CrossRef]
- Tiedemann, W.; Newman, J. Maximum Effective Capacity in an Ohmically Limited Porous Electrode. J. Electrochem. Soc. 1975, 122, 1482–1485. [Google Scholar] [CrossRef]
- Valøen, L.O.; Reimers, J.N. Transport Properties of LiPF6-Based Li-Ion Battery Electrolytes. J. Electrochem. Soc. 2005, 152, A882. [Google Scholar] [CrossRef]
- Carelli, S.; Bessler, W.G. Prediction of Reversible Lithium Plating with a Pseudo-3D Lithium-Ion Battery Model. J. Electrochem. Soc. 2020, 167, 100515. [Google Scholar] [CrossRef]
- Maleki, H.; Al Hallaj, S.; Selman, J.R.; Dinwiddie, R.B.; Wang, H. Thermal Properties of Lithium-Ion Battery and Components. J. Electrochem. Soc. 2019, 146, 947–954. [Google Scholar] [CrossRef]
- Spinner, N.S.; Mazurick, R.; Brandon, A.; Rose-Pehrsson, S.L.; Tuttle, S.G. Analytical, Numerical and Experimental Determination of Thermophysical Properties of Commercial 18650 LiCoO2 Lithium-Ion Battery. J. Electrochem. Soc. 2015, 162, A2789–A2795. [Google Scholar] [CrossRef]
- Ghisalberti, L.; Kondjoyan, A. Convective heat transfer coefficients between air flow and a short cylinder. Effect of air velocity and turbulence. Effect of body shape, dimensions and position in the flow. J. Food Eng. 1999, 42, 33–44. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Waldmann, T.; Bisle, G.; Hogg, B.-I.; Stumpp, S.; Danzer, M.A.; Kasper, M.; Axmann, P.; Wohlfahrt-Mehrens, M. Influence of Cell Design on Temperatures and Temperature Gradients in Lithium-Ion Cells: An In Operando Study. J. Electrochem. Soc. 2015, 162, A921–A927. [Google Scholar] [CrossRef]
- Christensen, J.; Cook, D.; Albertus, P. An Efficient Parallelizable 3D Thermoelectrochemical Model of a Li-Ion Cell. J. Electrochem. Soc. 2013, 160, A2258–A2267. [Google Scholar] [CrossRef]




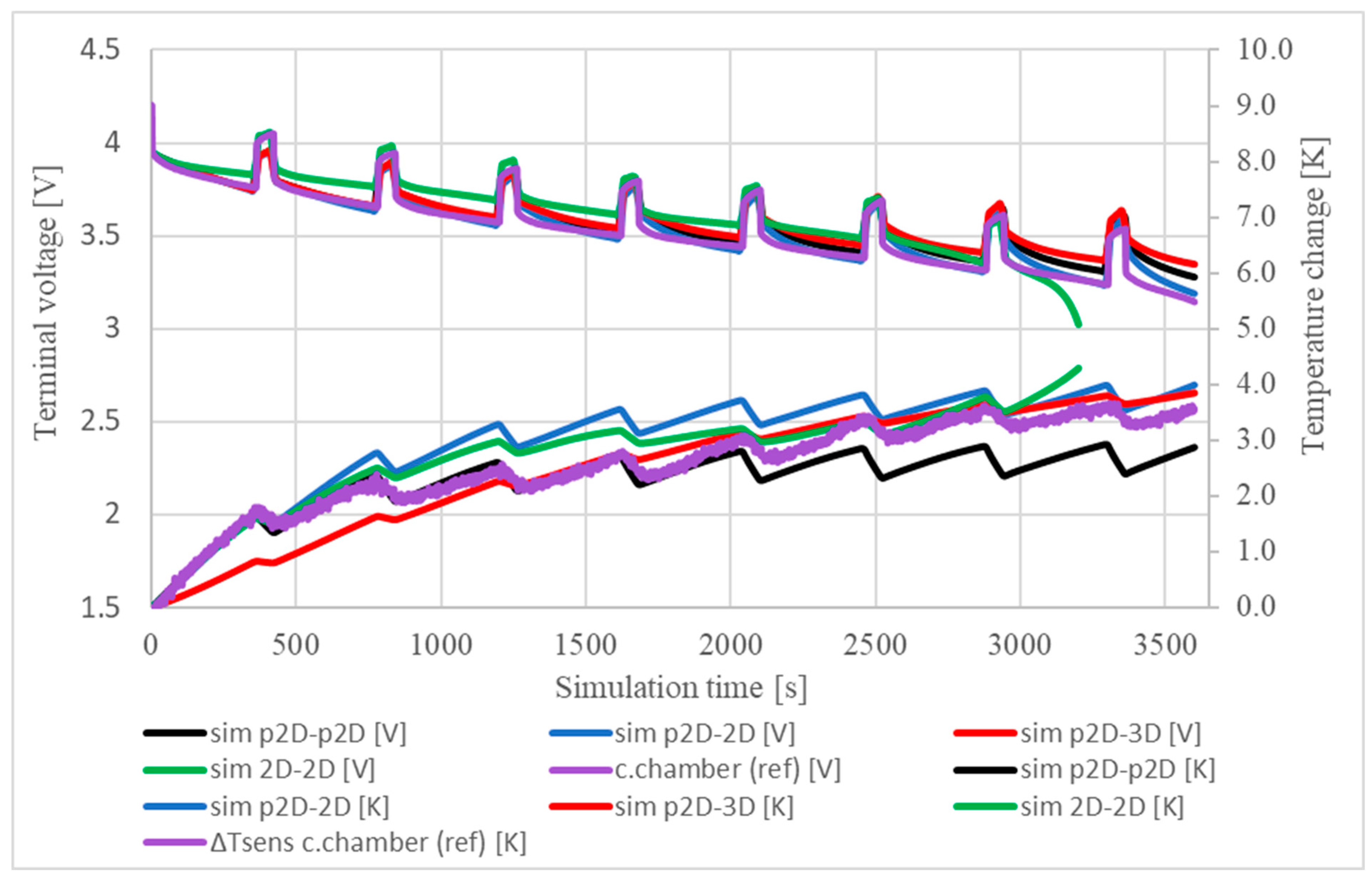
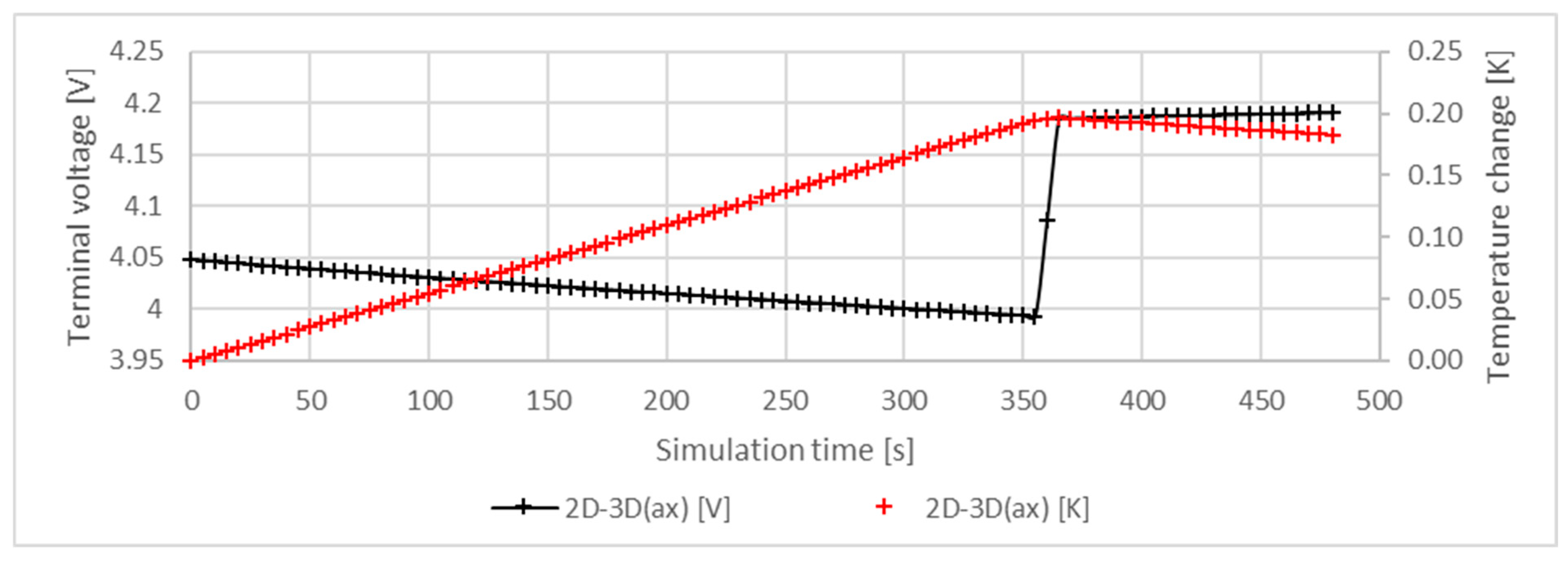
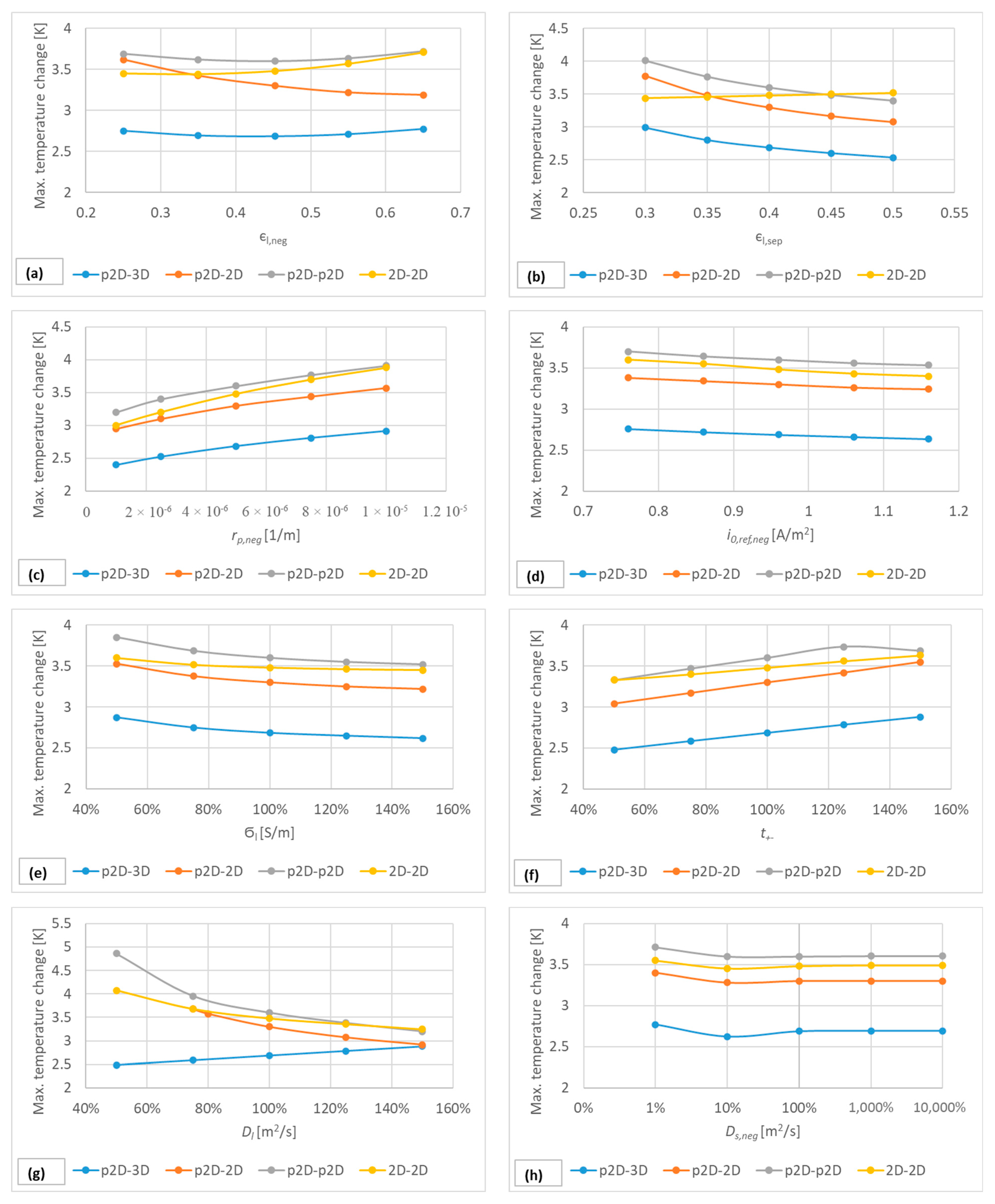
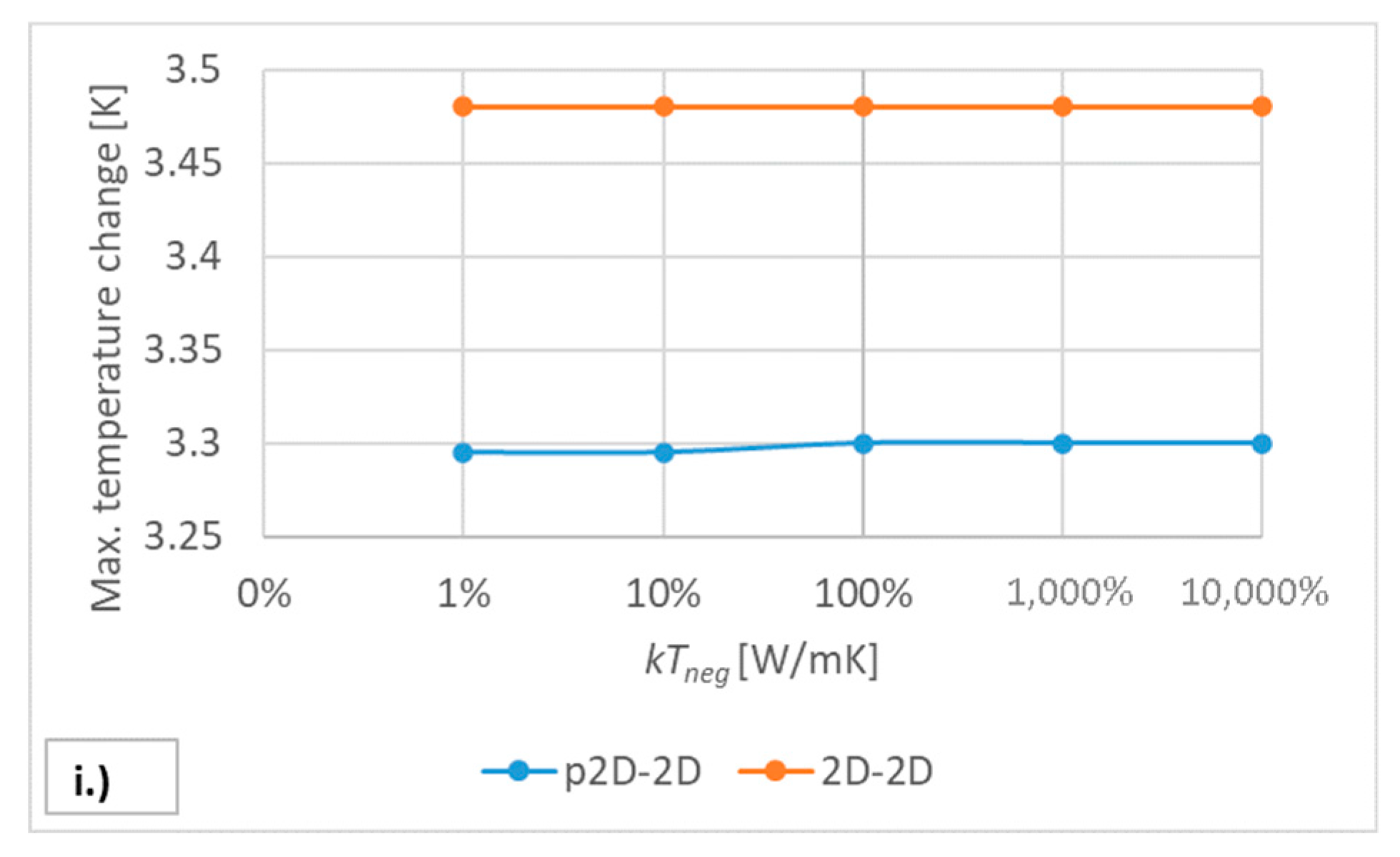
| Region | Equation No. | Governing Equation |
|---|---|---|
| Charge conservation in solid | (1) | |
| Charge balance in electrolyte | (2) | |
| Mass conservation in solid | (3) | |
| Mass conservation in electrolyte | (4) | |
| Butler–Volmer kinetics | (5) | |
| k = n, p, where n and p represent the anode and cathode, respectively. |
| Region | Equation No. | Governing Equation |
|---|---|---|
| Ohmic losses | (7) | |
| Reversible heat losses | (8) | |
| Irreversible heat losses | (9) | |
| Total heat generated | (10) | , |
| where and . | ||
| k = n, p, where n and p represent the anode and cathode, respectively. |
| Dimension | Identifying Feature | Advantages | Disadvantages | |
|---|---|---|---|---|
| DFN Model | Thermal Model | |||
| p2D | p2D | Flat geometry model | The best compromise between computational speed and model details. | Missing temperature details in the axial direction and in the fittings. |
| 2D | Lumped parameter model | The temperature distribution in a radial direction is more realistic. | Missing temperature details in the axial direction and in the cap. | |
| 3D | Lumped parameter model | Temperature distribution in the fittings and each spatial direction is covered. A realistic cooling scenario is easy to implement. | Missing the effect of the layered structure on the heat transport inside the cell. | |
| 2D | 2D | Spiral geometry model | The most detailed model: the effect of porous electrodes and separator, the voltage drop in the current collectors and the cell capacity defined by the spiral turns are considered. | The high computational demand and meshing is challenging because of the differences in size of several orders of magnitude. |
| 2D | 2D-3D (ax) | A 3D model based on axial symmetry | Reduced computational demand due to symmetricity. | The merged electrode structure and the resulting reduced reaction cross-section results in an unrealistic model. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csomós, B.; Kocsis Szürke, S.; Fodor, D. Comparison of Coupled Electrochemical and Thermal Modelling Strategies of 18650 Li-Ion Batteries in Finite Element Analysis—A Review. Materials 2023, 16, 7613. https://doi.org/10.3390/ma16247613
Csomós B, Kocsis Szürke S, Fodor D. Comparison of Coupled Electrochemical and Thermal Modelling Strategies of 18650 Li-Ion Batteries in Finite Element Analysis—A Review. Materials. 2023; 16(24):7613. https://doi.org/10.3390/ma16247613
Chicago/Turabian StyleCsomós, Bence, Szabolcs Kocsis Szürke, and Dénes Fodor. 2023. "Comparison of Coupled Electrochemical and Thermal Modelling Strategies of 18650 Li-Ion Batteries in Finite Element Analysis—A Review" Materials 16, no. 24: 7613. https://doi.org/10.3390/ma16247613
APA StyleCsomós, B., Kocsis Szürke, S., & Fodor, D. (2023). Comparison of Coupled Electrochemical and Thermal Modelling Strategies of 18650 Li-Ion Batteries in Finite Element Analysis—A Review. Materials, 16(24), 7613. https://doi.org/10.3390/ma16247613






