Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of Geopolymer Samples with Metals
2.3. Stabilization Efficiency on Iron-Rich Lateritic Soil-Based Geopolymer
2.4. Characterization of Geopolymer Samples
3. Results and Discussion
3.1. Stabilization Efficiency and Mechanism on Iron-Rich Laterite-Based Geopolymer
- Leaching of Cd and Cu from iron-rich lateritic soil-based geopolymer.
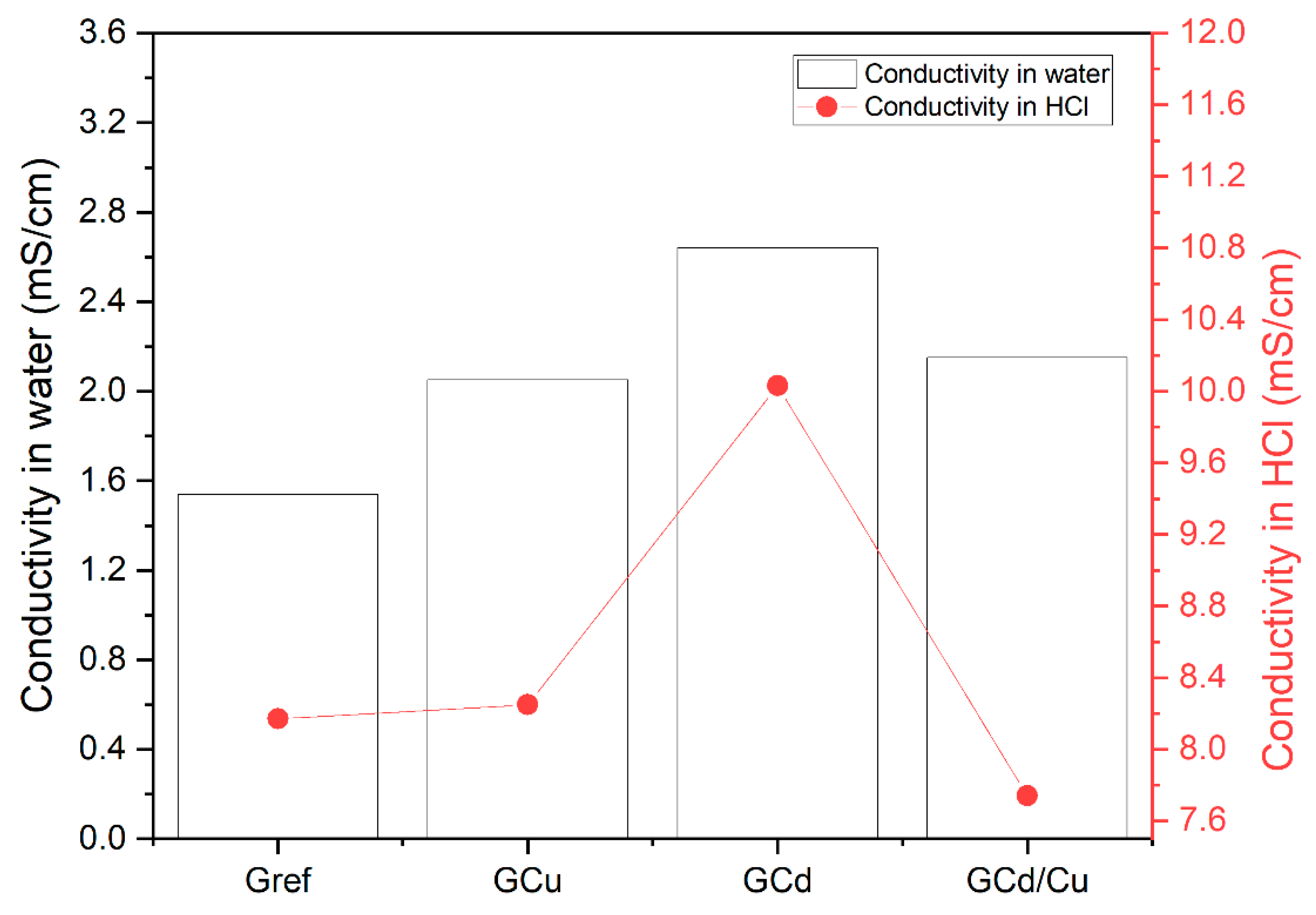
- pH and conductivity of solutions after leaching tests.
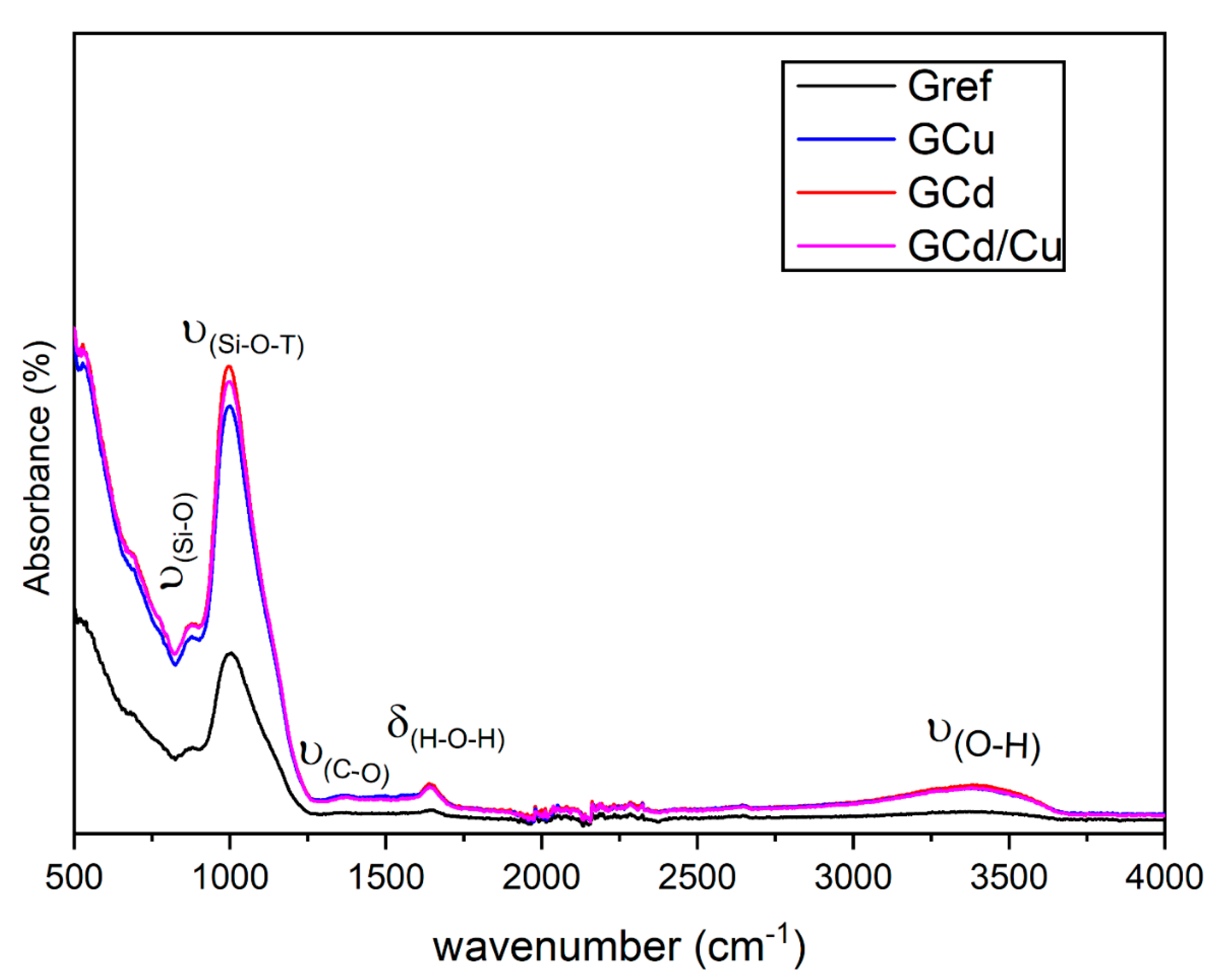
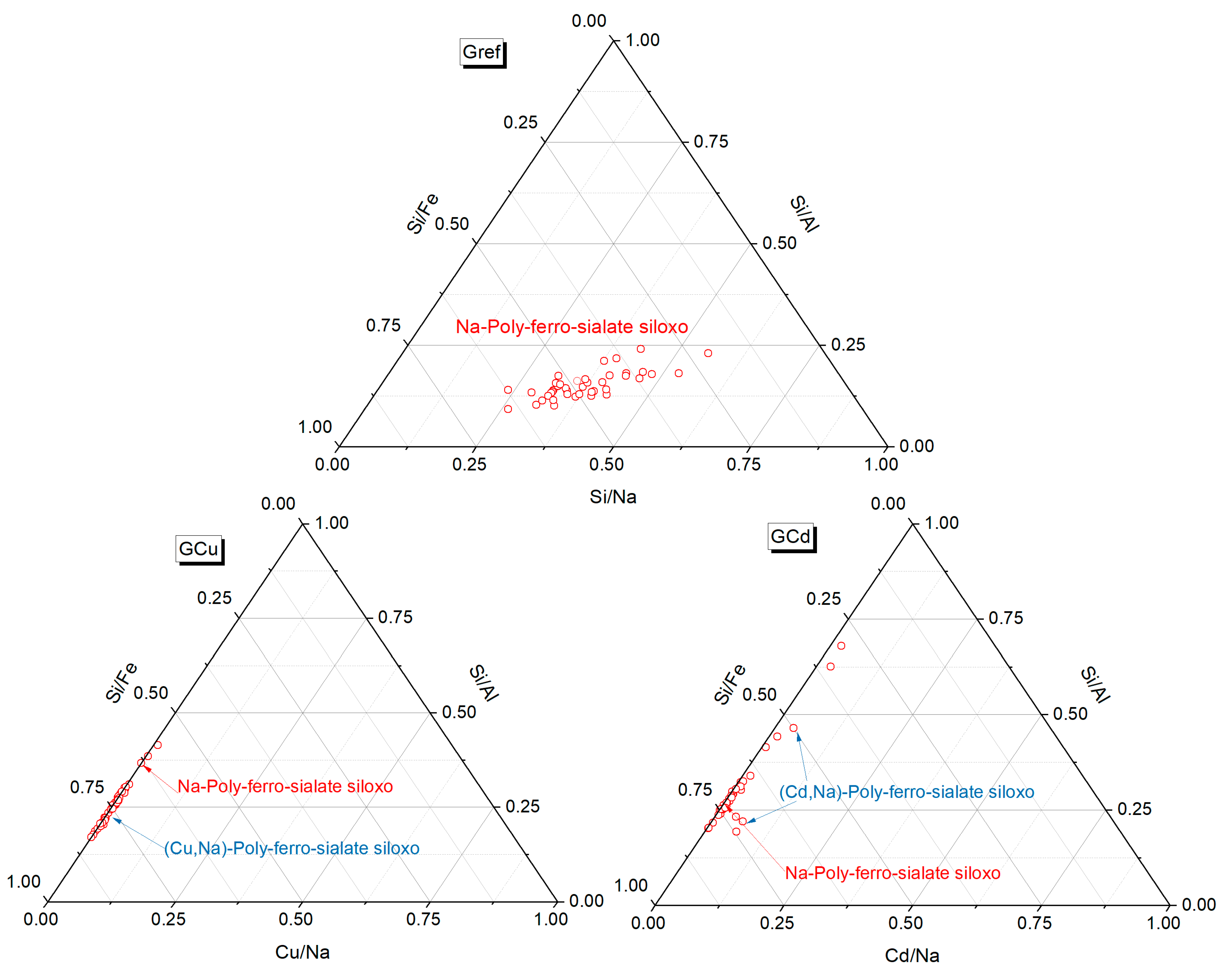
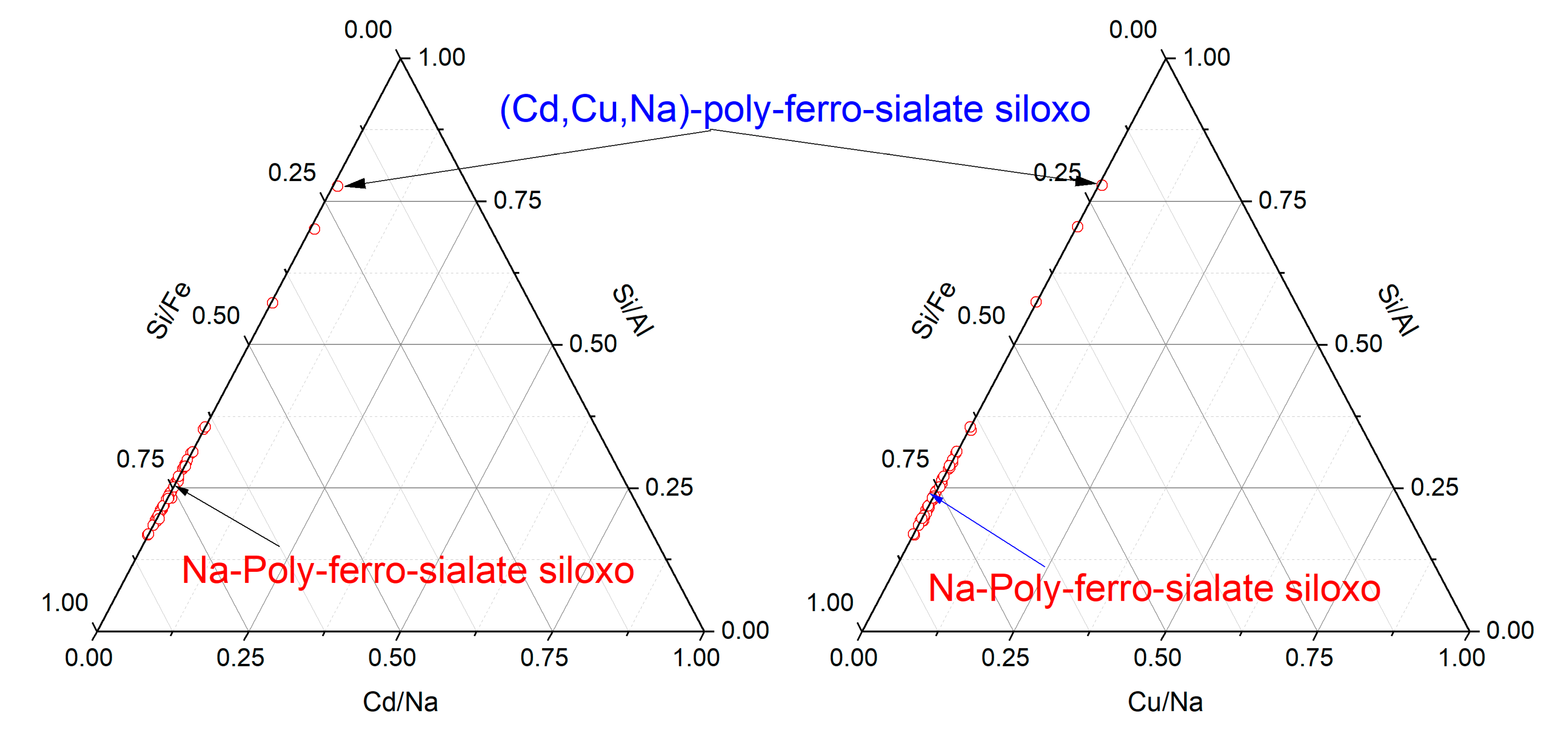
3.2. The Effect of Heavy Metals on the Mineralogy of Geopolymer
3.3. Hardened Characteristics of Contaminated Geopolymers
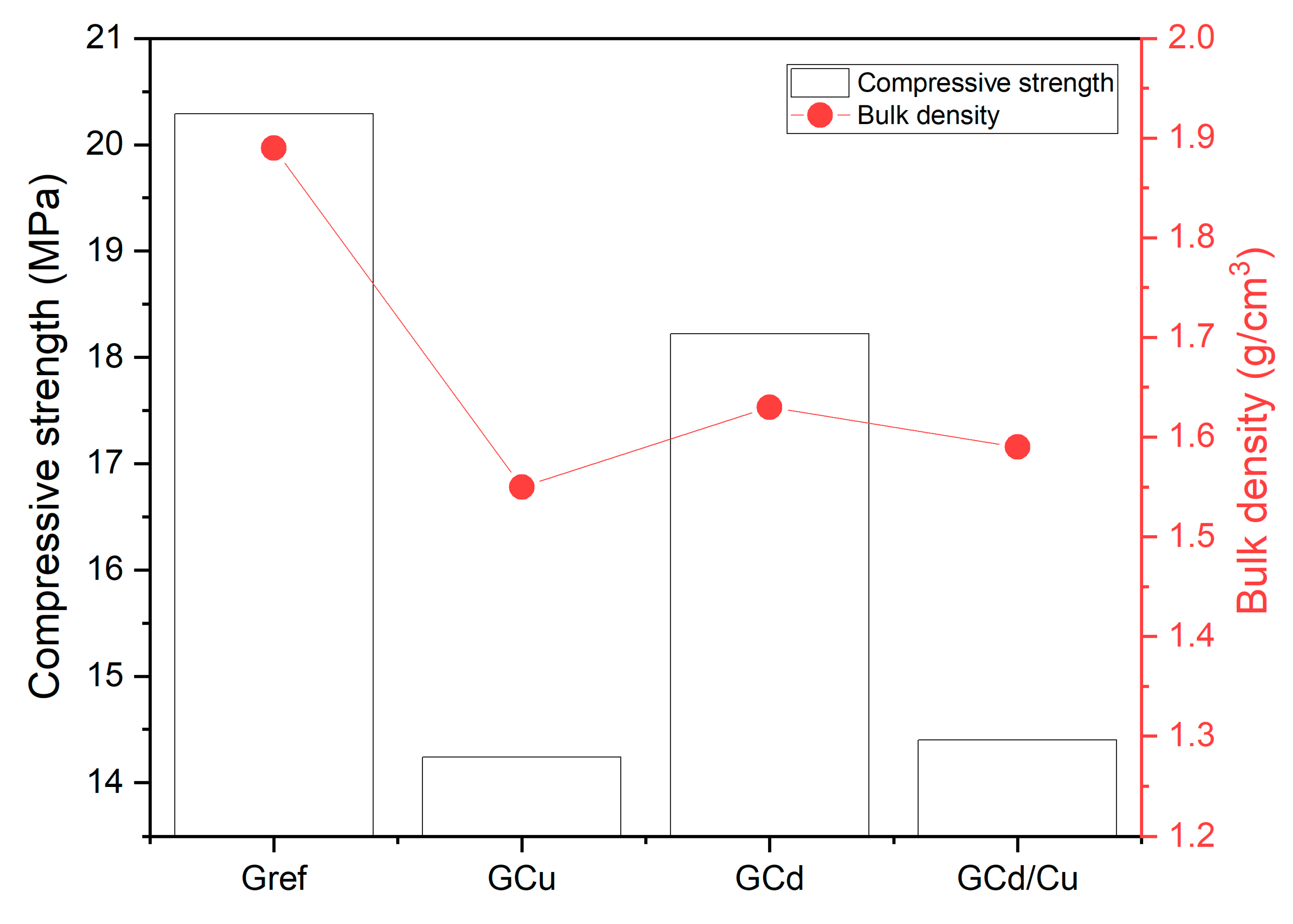
- Compressive strength and physical properties.
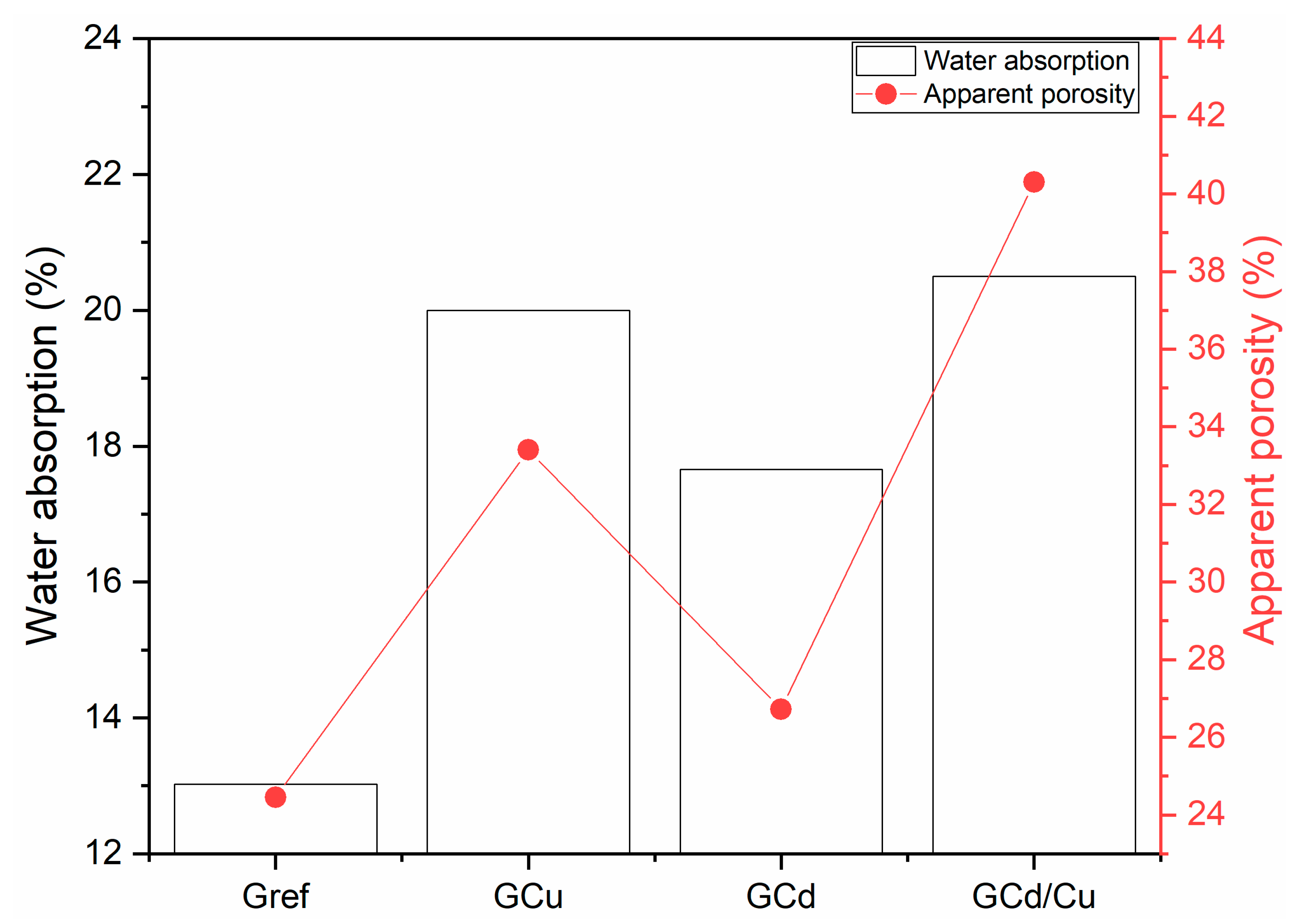
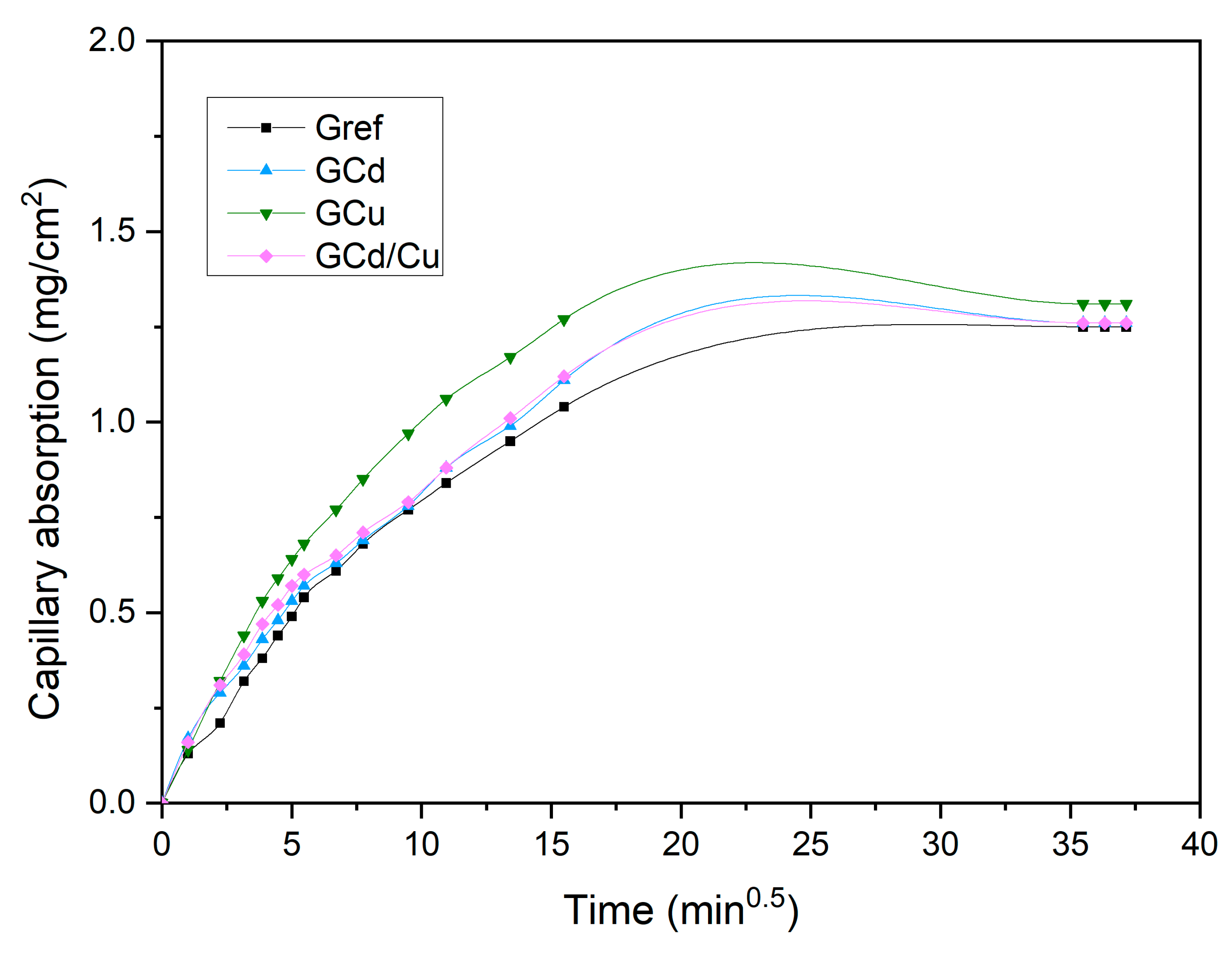
- Capillarity water absorption.
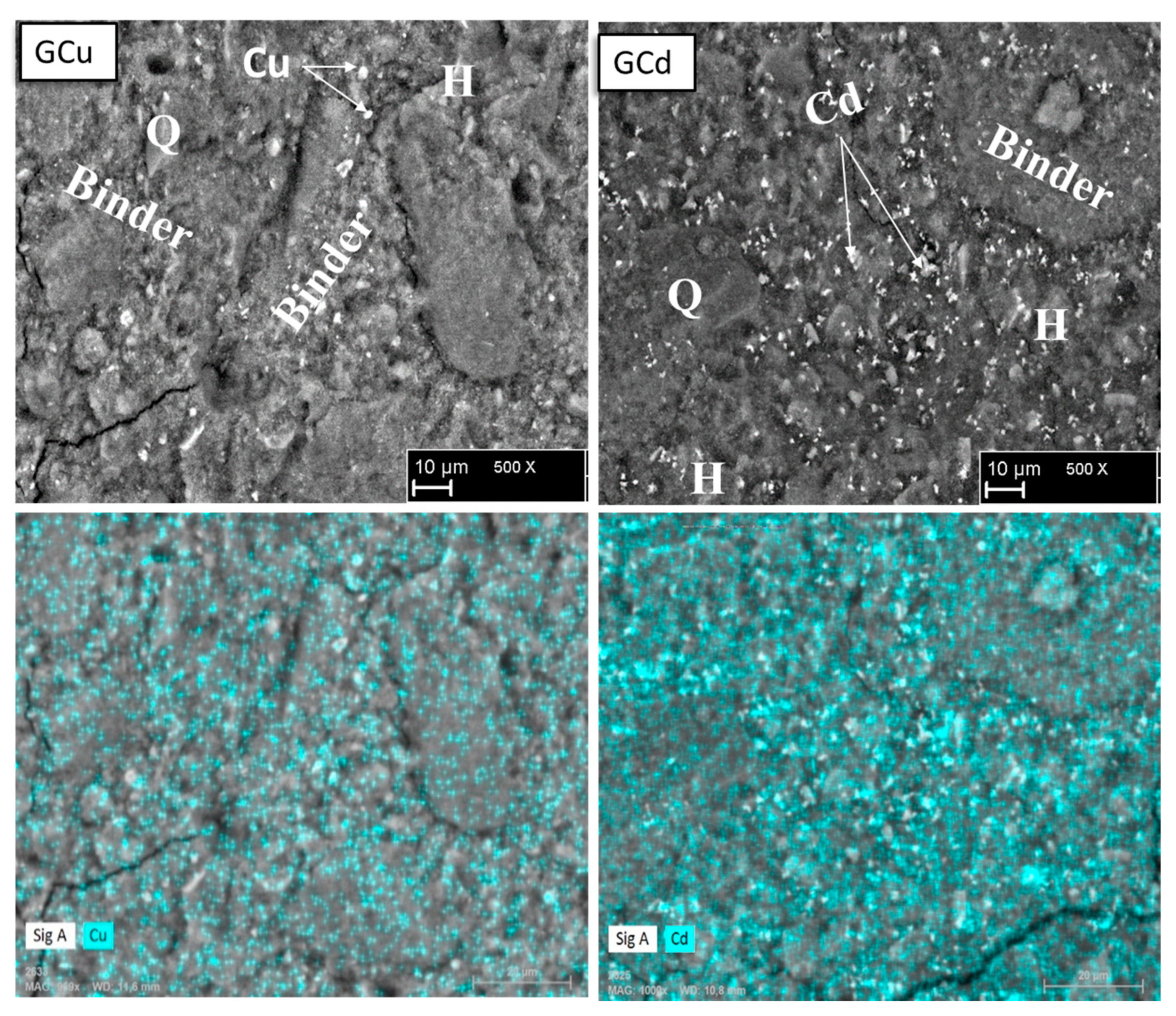
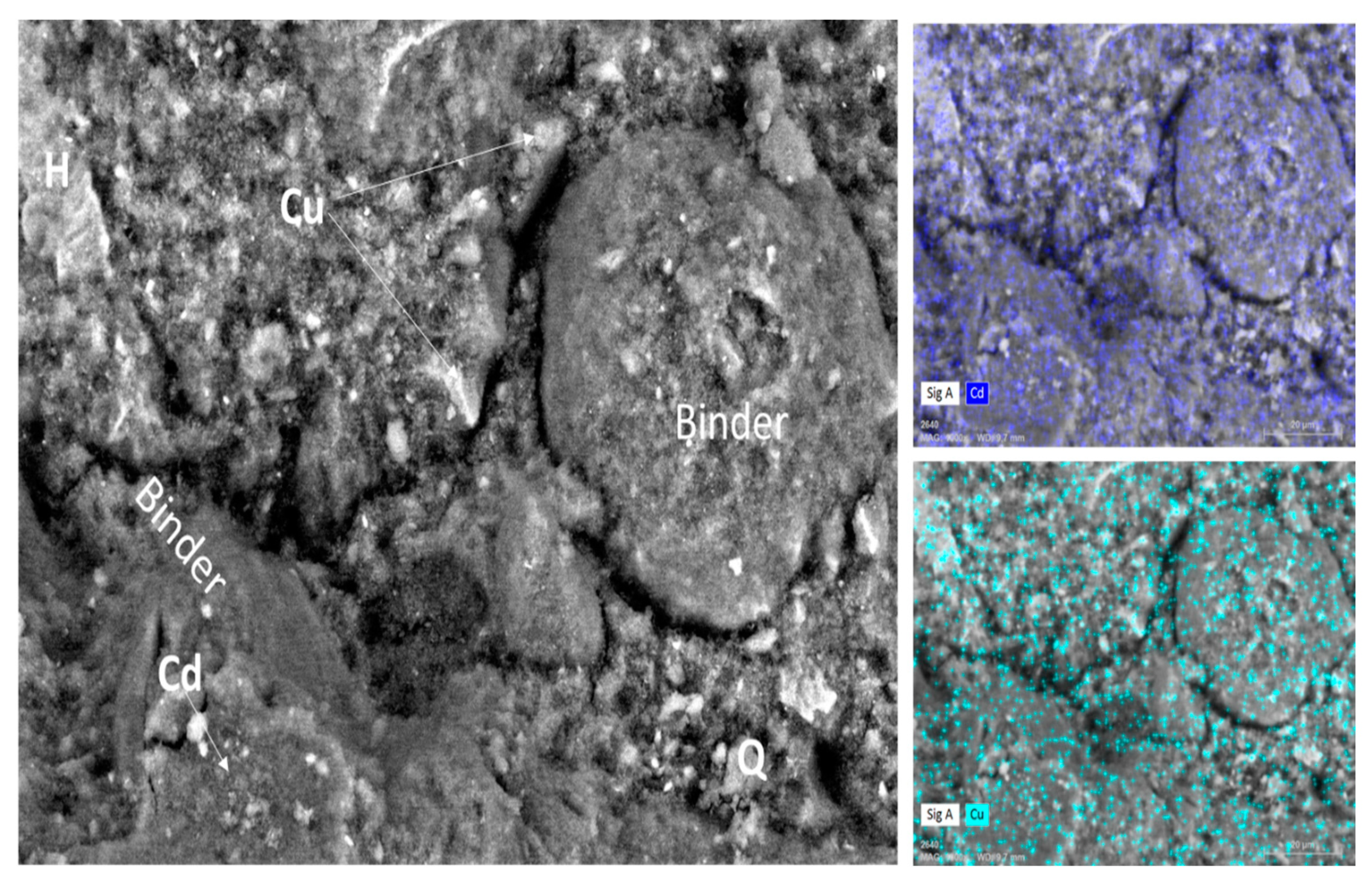
3.4. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Tian, G.; Zong, L.; Zhou, Y.; Kang, Y.; Wang, Q.; Wang, A. From illite/smectite clay to mesoporous silicate adsorbent for efficient removal of chlortetracycline from water. J. Environ. Sci. 2017, 51, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiang, C.; Wu, C.; Gao, W.; Ke, W.; Zeng, J.; Li, W.; Xue, S. Geochemical fractionation and potential release behaviour of heavy metals in lead‒zinc smelting soils. J. Environ. Sci. 2024, 139, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Muhammad, F.; Yu, L.; Xia, M.; Zhao, J.; Jiao, B.; Shiau, Y.; Li, D. Waste solidification/stabilization of lead–zinc slag by utilizing fly ash based geopolymers. RSC Adv. 2018, 8, 32956–32965. [Google Scholar] [CrossRef]
- Fan, J.; Yan, J.; Zhou, M.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Li, W.; Wang, A.; et al. Heavy metals immobilization of ternary geopolymer based on nickel slag, lithium slag and metakaolin. J. Hazard. Mater. 2023, 453, 131380. [Google Scholar] [CrossRef]
- Santa, R.A.A.B.; Soares, C.; Riella, H.G. Geopolymers with a high percentage of bottom ash for solidification/immobilization of different toxic metals. J. Hazard. Mater. 2016, 318, 145–153. [Google Scholar] [CrossRef]
- El-Eswed, B.I.; Aldagag, O.M.; Khalili, F.I. Efficiency and mechanism of stabilization/solidification of Pb(II), Cd(II), Cu(II), Th(IV) and U(VI) in metakaolin based geopolymers. Appl. Clay Sci. 2017, 140, 148–156. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.; Pu, S.; Song, W. Solidification/Stabilization of Pb2+ and Cd2+ Contaminated Soil Using Fly Ash and GGBS Based Geopolymer. Arab. J. Sci. Eng. 2022, 47, 4385–4400. [Google Scholar] [CrossRef]
- Zribi, M.; Baklouti, S. Phosphate-based geopolymers: A critical review. Polym. Bull. 2021, 79, 6827–6855. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef]
- Nikravan, M.; Firdous, R.; Stephan, D. Life cycle assessment of alkali-activated materials: A systematic literature review. Low-Carbon Mater. Green Constr. 2023, 1, 13. [Google Scholar] [CrossRef]
- Majdoubi, H.; Makhlouf, R.; Haddaji, Y.; Nadi, M.; Mansouri, S.; Semllal, N.; Oumam, M.; Manoun, B.; Alami, J.; Hannache, H.; et al. Valorization of phosphogypsum waste through acid geopolymer technology: Synthesis, characterization, and environmental assessment. Constr. Build. Mater. 2023, 371, 130710. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Volcanic ash-based geopolymer cements/concretes: The current state of the art and perspectives. Environ. Sci. Pollut. Res. 2017, 24, 4433–4446. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Madi, A.B.; Kamseu, E.; Melo, U.C.; Delplancke, M.P.; Rahier, H. Influence of the processing temperature on the compressive strength. Constr. Build. Mater. 2014, 65, 60–66. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Romagnoli, M.; Pollastri, S.; Gualtieri, A. Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: Mechanical and microstructural properties. Cem. Concr. Res. 2015, 67, 259–270. [Google Scholar] [CrossRef]
- Abbass, A.M.; Firdous, R.; Djobo, J.N.Y.; Stephan, D.; Elrahman, M.A. The role of chemistry and fineness of metakaolin on the fresh properties and heat resistance of blended fly ash-based geopolymer. SN Appl. Sci. 2023, 5, 896. [Google Scholar] [CrossRef]
- Hardneck, F.; de Villiers, C.; Maree, L. Effect of Copper Sulphate and Cadmium Chloride on Non-Human Primate Sperm Function In Vitro. Int. J. Environ. Res. Public Health 2021, 18, 6200. [Google Scholar] [CrossRef]
- Grba, N.; Grengg, C.; Petronijević, M.; Dietzel, M.; Baldermann, A. Substantial Copper (Cu2+) Uptake by Metakaolin-Based Geopolymer and Its Resistance to Acid Leaching and Ion Exchange. Polymers 2023, 15, 1971. [Google Scholar] [CrossRef]
- Lee, S.; van Riessen, A.; Chon, C.-M.; Kang, N.-H.; Jou, H.-T.; Kim, Y.-J. Impact of activator type on the immobilisation of lead in fly ash-based geopolymer. J. Hazard. Mater. 2016, 305, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, X.; Sun, S.; Yang, W.; Fang, L.; Ma, R.; Lin, C.; Li, H. Lead zinc slag-based geopolymer: Demonstration of heavy metal solidification mechanism from the new perspectives of electronegativity and ion potential. Environ. Pollut. 2022, 293, 118509. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Zhou, F.; Lu, M.; Hou, H.; Li, J.; Liu, D.; Wang, T. Early solidification/stabilization mechanism of heavy metals (Pb, Cr and Zn) in Shell coal gasification fly ash based geopolymer. Sci. Total. Environ. 2022, 802, 149905. [Google Scholar] [CrossRef]
- El-Eswed, B.I. Chemical evaluation of immobilization of wastes containing Pb, Cd, Cu and Zn in alkali-activated materials: A critical review. J. Environ. Chem. Eng. 2020, 8, 104194. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Reactivity of volcanic ash in alkaline medium, microstructural and strength characteristics of resulting geopolymers under different synthesis conditions. J. Mater. Sci. 2016, 51, 10301–10317. [Google Scholar] [CrossRef]
- Nadia, N.F.J.; Gharzouni, A.; Nait-Ali, B.; Ouamara, L.; Ndassa, I.M.; Bebga, G.; Elie, K.; Rossignol, S. Comparative study of laterite and metakaolin/hematite-based geopolymers: Effect of iron source and alkalization. Appl. Clay Sci. 2023, 233, 106824. [Google Scholar] [CrossRef]
- Nkwaju, R.; Djobo, J.; Nouping, J.; Huisken, P.; Deutou, J.; Courard, L. Iron-rich laterite-bagasse fibers based geopolymer composite: Mechanical, durability and insulating properties. Appl. Clay Sci. 2019, 183, 105333. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. Office of Standards, Metrology and Testing: Bratislava, Slovakia, 2016.
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Qiao, W.; Shi, Y.; Liu, X. Immobilization of Cu2+, Zn2+, Pb2+, and Cd2+ during geopolymerization. Front. Environ. Sci. Eng. 2015, 9, 642–648. [Google Scholar] [CrossRef]
- El-Eswed, B.I.; Yousef, R.I.; Alshaaer, M.; Hamadneh, I.; Al-Gharabli, S.I.; Khalili, F. Stabilizationsolidification of heavy metals in kaolin zeolite. Int. J. Miner. Process. 2015, 137, 34–42. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Immobilization efficiency and mechanism of metal cations (Cd2+, Pb2+ and Zn2+) and anions (AsO43− and Cr2O72−) in wastes-based geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef]
- Su, Q.; Xie, Y.; Chen, M.; Xue, X.; Cui, X. Enhance the removal and immobilization of Cd(II) by the synthesis in situ of dithiocarbamate-geopolymer microsphere composite. J. Colloid Interface Sci. 2022, 622, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; van Deventer, J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Mu, J. Solidification/stabilization mechanism of Pb(II), Cd(II), Mn(II) and Cr(III) in fly ash based geopolymers. Constr. Build. Mater. 2018, 160, 818–827. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C. The role of zinc in metakaolin-based geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Geopolymers produced from drinking water treatment residue and bottom ash for the immobilization of heavy metals. Chemosphere 2019, 225, 579–587. [Google Scholar] [CrossRef]



| Sample Code | Calcined Laterite (g) | Ratio Liquid/Solid | Metal Salt Content (g) |
|---|---|---|---|
| Gref | 300 | 0.6 | 0 |
| GCd | 300 | 0.6 | 3 |
| GCu | 300 | 0.6 | 3 |
| GCd/Cu | 300 | 0.6 | 1.5/1.5 |
| Heavy Metals | Leaching in Water | Leaching in HCl | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | ||||||||
| Gref | GCd | GCu | GCd/Cu | Gref | GCd | GCu | GCd/Cu | |
| Al | 2.95 | 1.63 | 2.16 | 2.95 | 491.70 | 28.63 | 361.30 | 382 |
| Fe | 0.36 | 0.17 | 0.17 | 0.16 | 0.78 | 0.55 | 0.48 | 0.50 |
| Na | 222.2 | 367.70 | 247 | 220.90 | 786.30 | 2377 | 1014 | 865.50 |
| Si | 20.44 | 23.10 | 31.09 | 21.60 | 7.12 | 37.66 | 56.18 | 58.85 |
| Cd | 0 | 0.017 | 0 | 0.024 | 0 | 51.68 | 0 | 17.31 |
| Cu | 0 | 0 | 0.03 | 0.03 | 0 | 0 | 19.89 | 7.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkwaju, R.Y.; Nouping, J.N.F.; Bachirou, S.; Abo, T.M.; Deutou, J.G.N.; Djobo, J.N.Y. Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties. Materials 2023, 16, 7605. https://doi.org/10.3390/ma16247605
Nkwaju RY, Nouping JNF, Bachirou S, Abo TM, Deutou JGN, Djobo JNY. Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties. Materials. 2023; 16(24):7605. https://doi.org/10.3390/ma16247605
Chicago/Turabian StyleNkwaju, Rachel Yanou, Joëlle Nadia Fekoua Nouping, Soumayah Bachirou, Tatiane Marina Abo, Juvenal Giogetti Nemaleu Deutou, and Jean Noël Yankwa Djobo. 2023. "Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties" Materials 16, no. 24: 7605. https://doi.org/10.3390/ma16247605
APA StyleNkwaju, R. Y., Nouping, J. N. F., Bachirou, S., Abo, T. M., Deutou, J. G. N., & Djobo, J. N. Y. (2023). Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties. Materials, 16(24), 7605. https://doi.org/10.3390/ma16247605







