Microstructure Optimization for Design of Porous Tantalum Scaffolds Based on Mechanical Properties and Permeability
Abstract
:1. Introduction
2. Materials and Methods
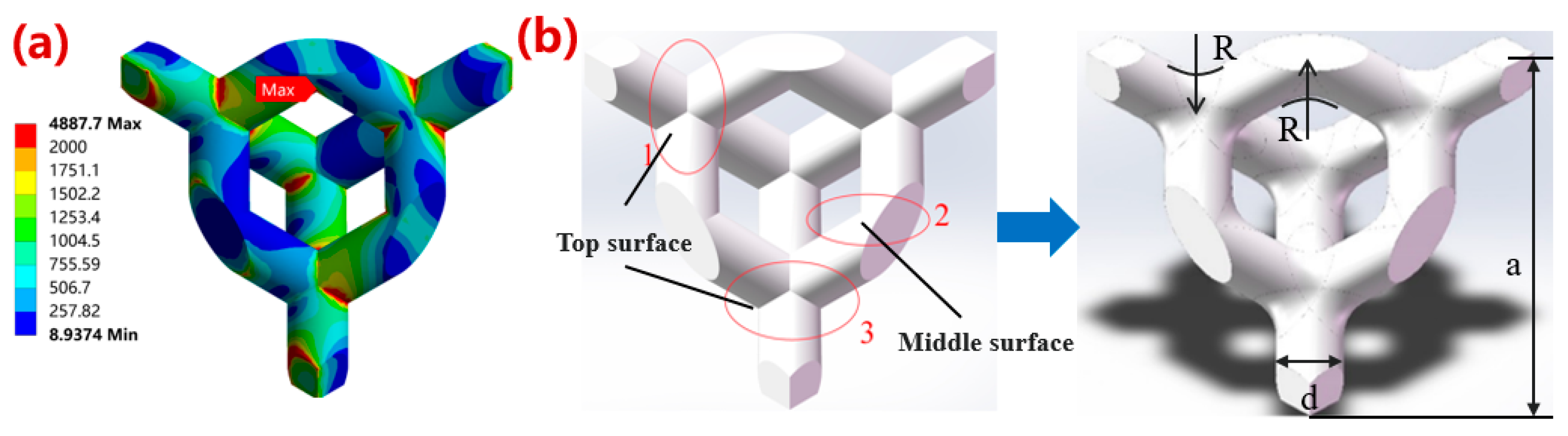
2.1. Optimal Design Strategy of Porous Ta Microstructure
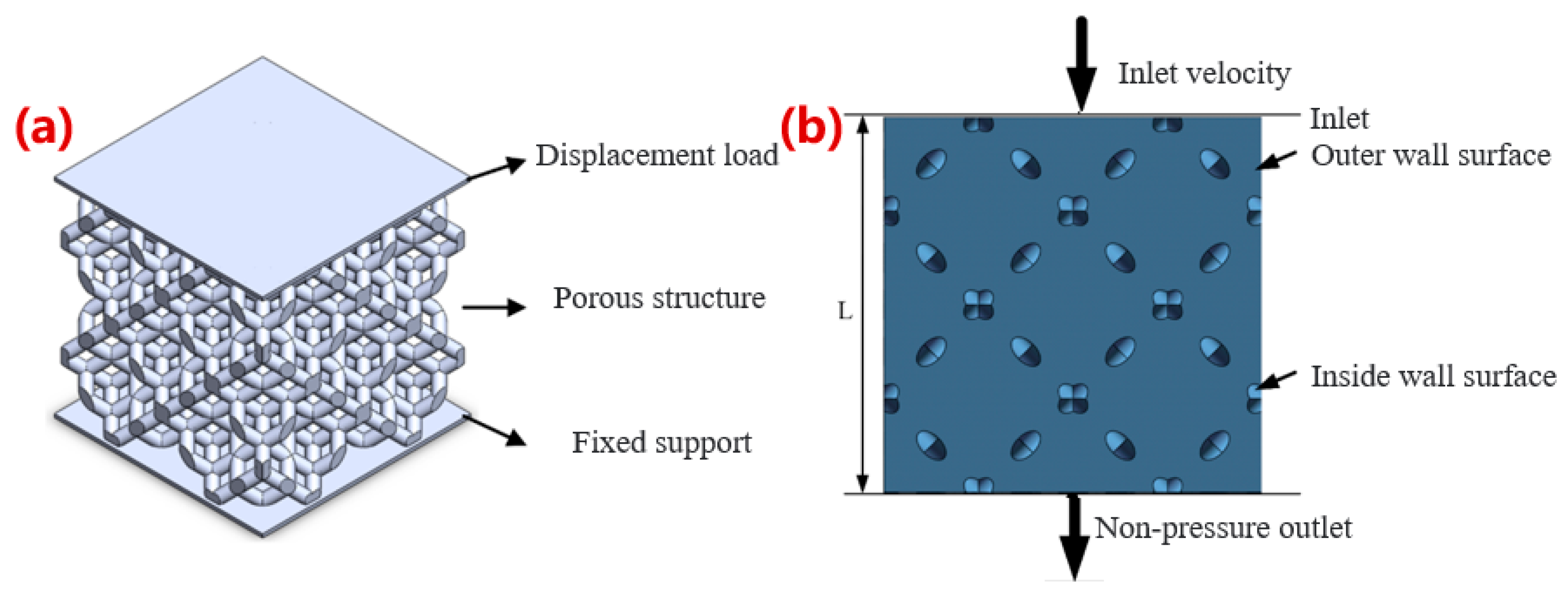
2.2. Simulation of Mechanical Properties and Permeability for Optimization
2.2.1. Mechanical Properties Simulation
2.2.2. Permeability Simulation
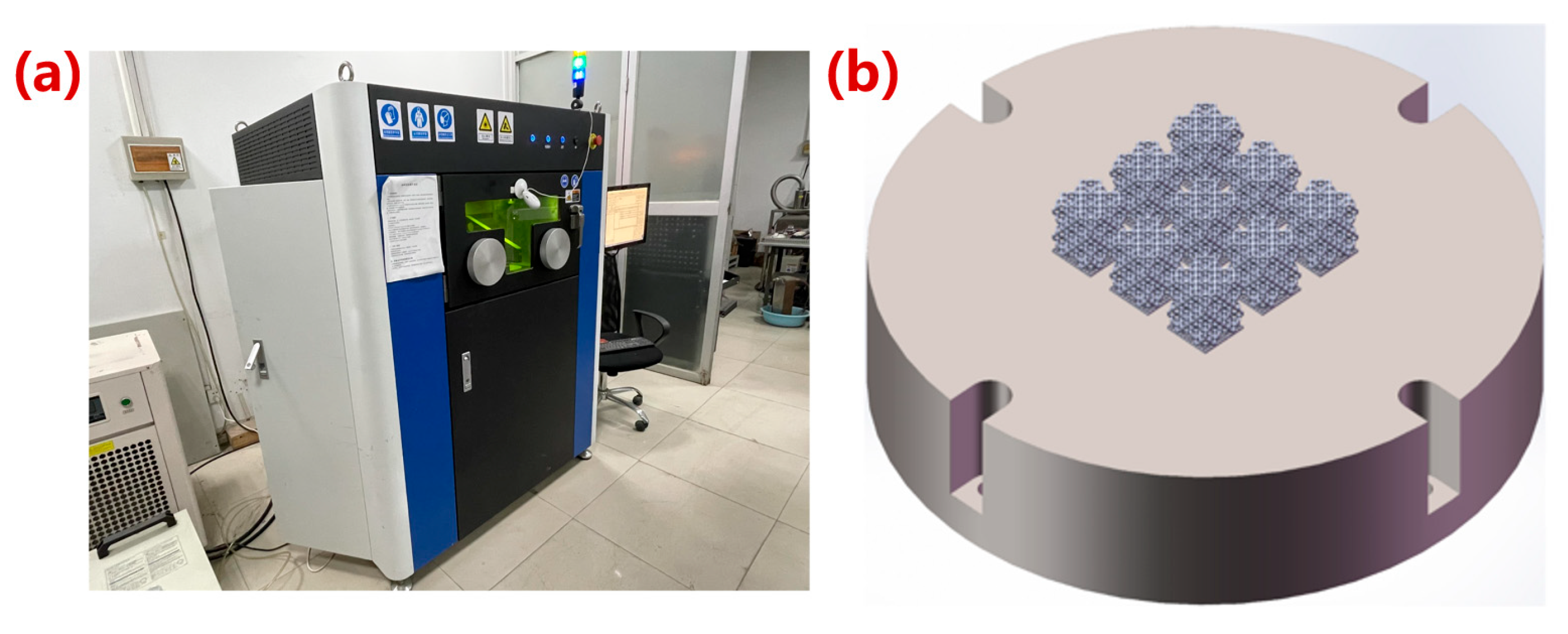
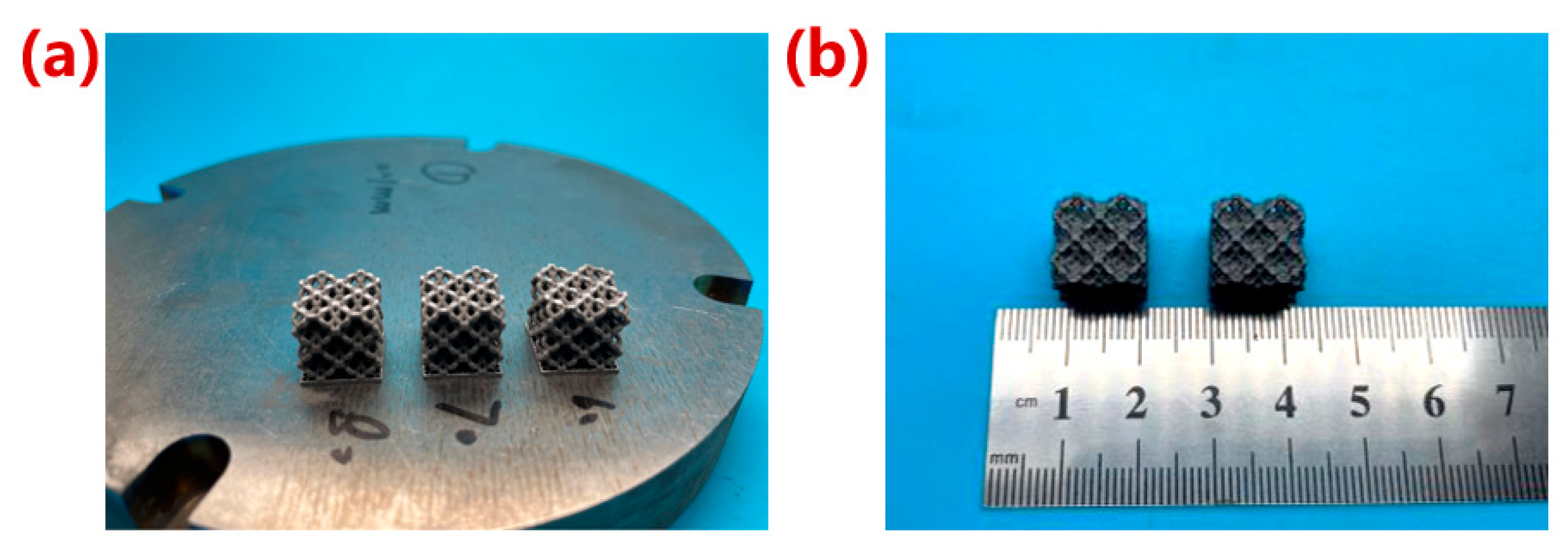
2.3. Manufacturing of Porous Ta
2.4. Evaluation of Properties of Porous Ta Optimization
2.4.1. Microstructure Observation
2.4.2. Mechanics Performance Testing
2.4.3. Permeability Testing
2.5. Statistical Analysis Method
3. Results and Discussion
3.1. Simulation Analysis Results
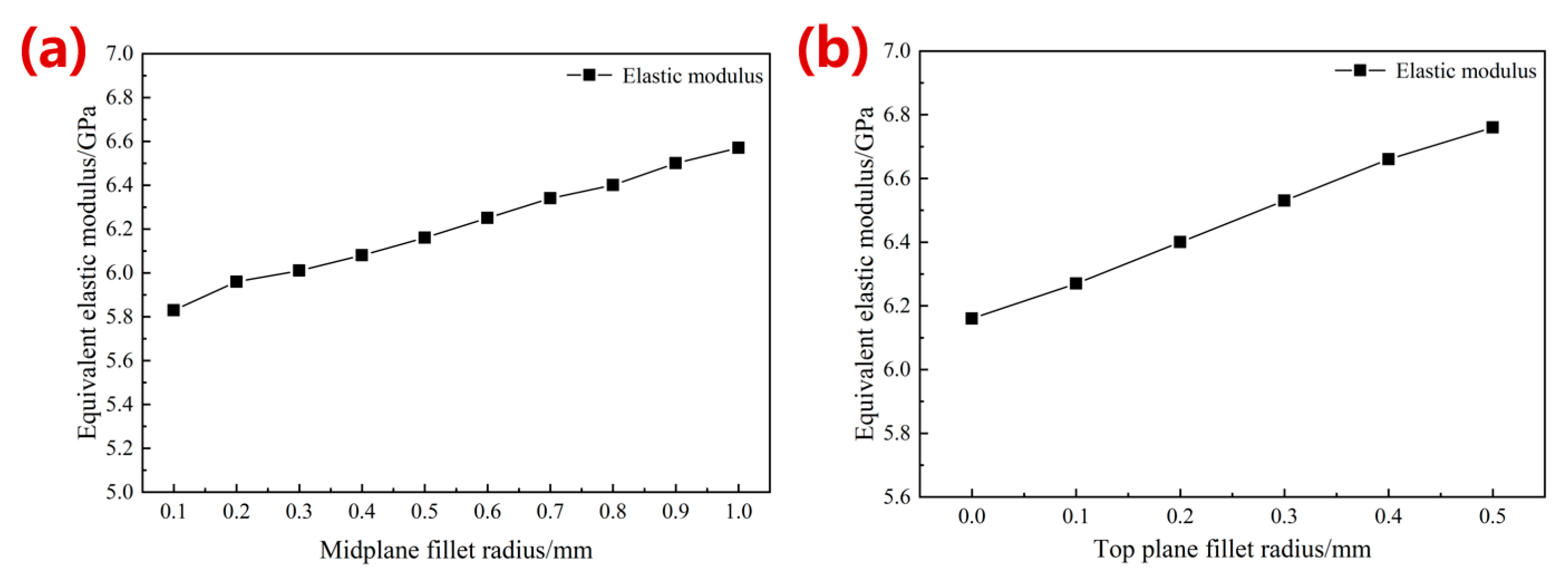
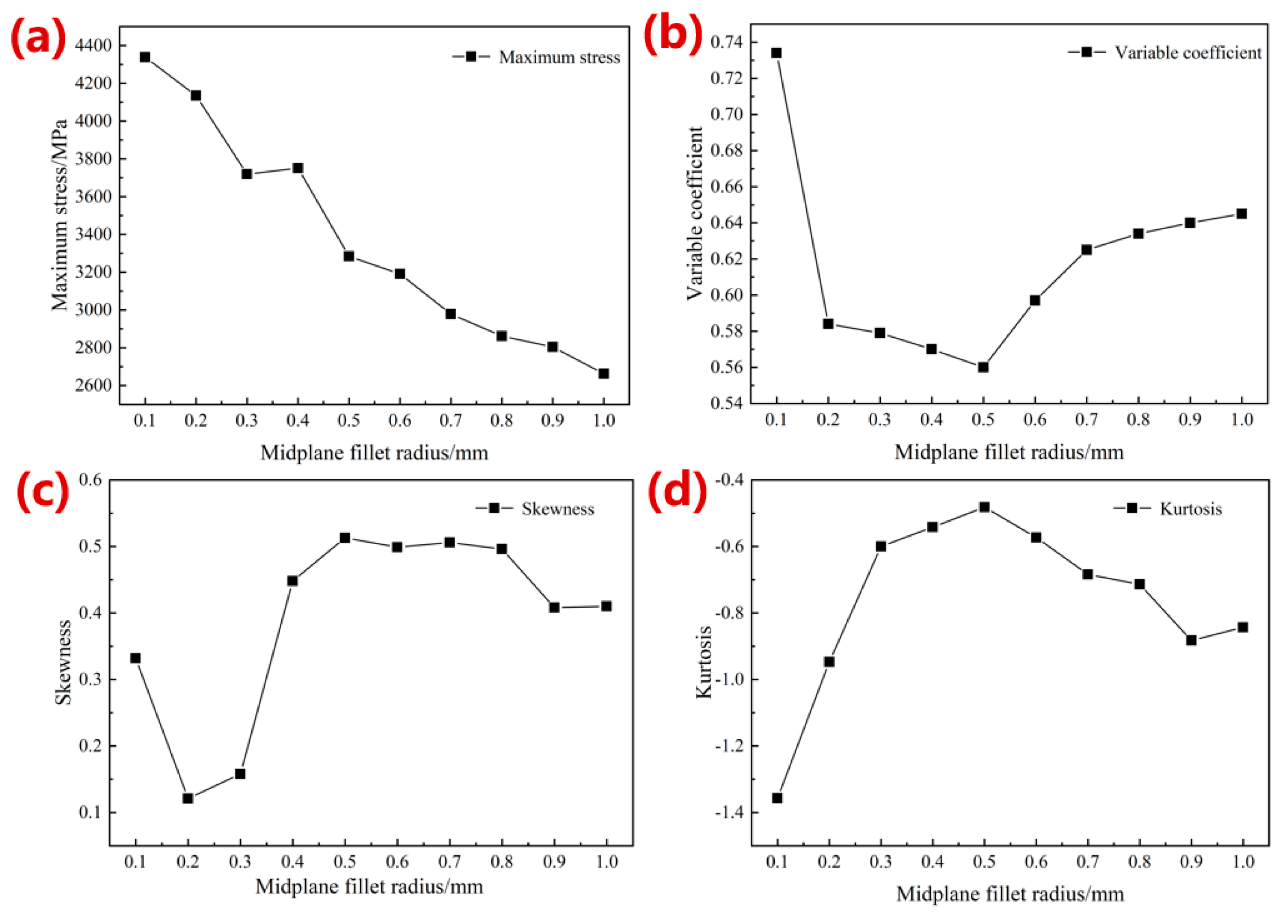
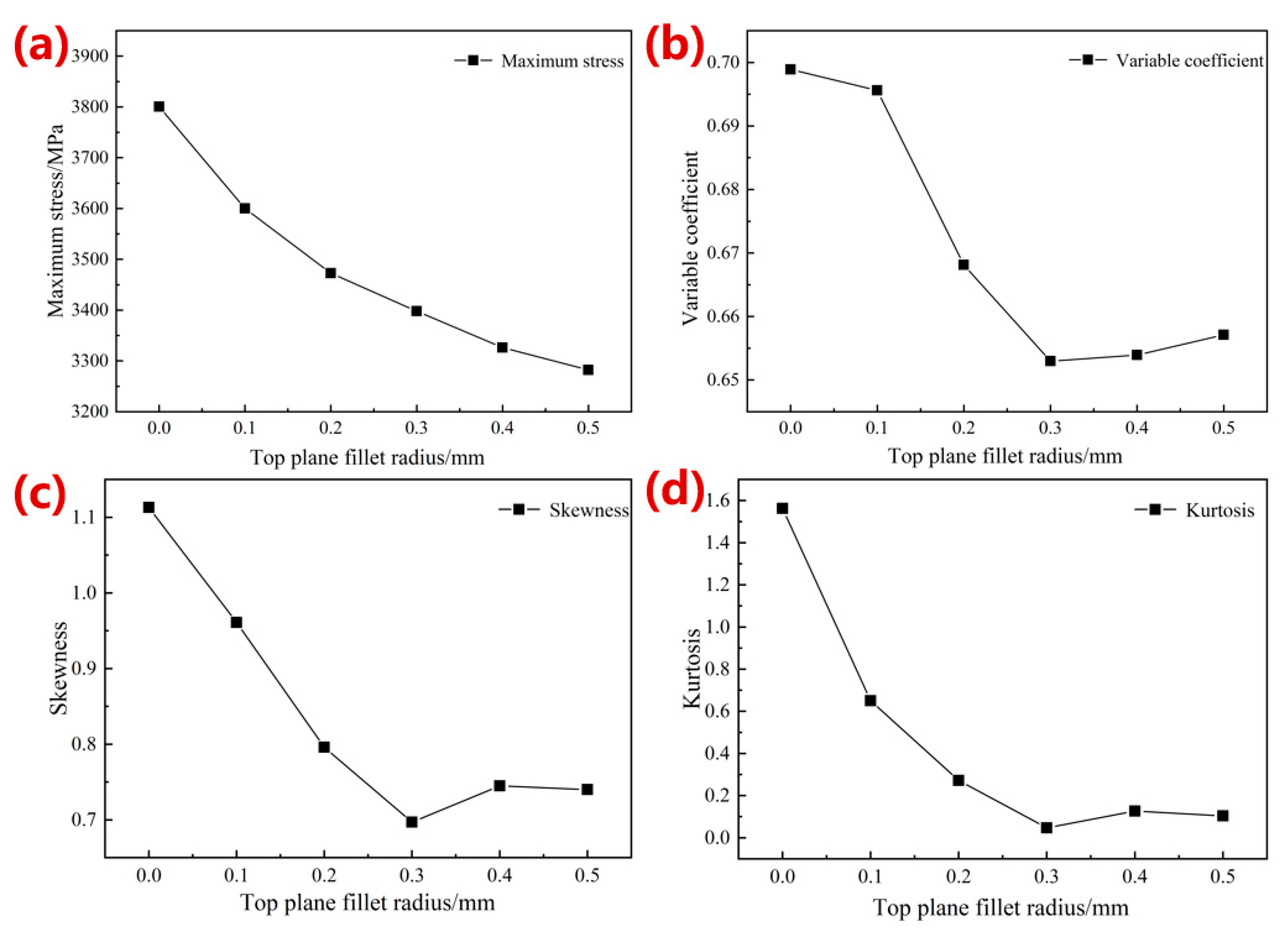
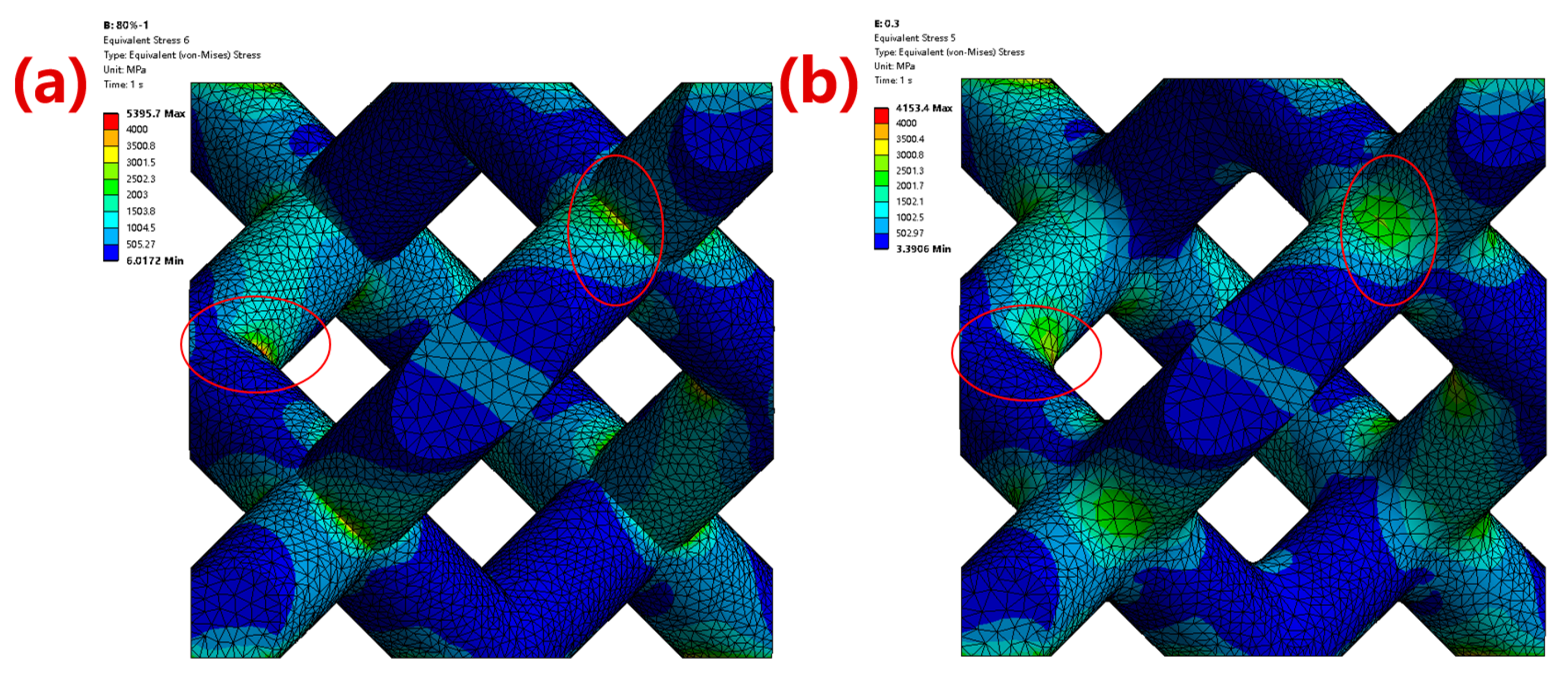
3.1.1. Simulation Results of Mechanical Properties
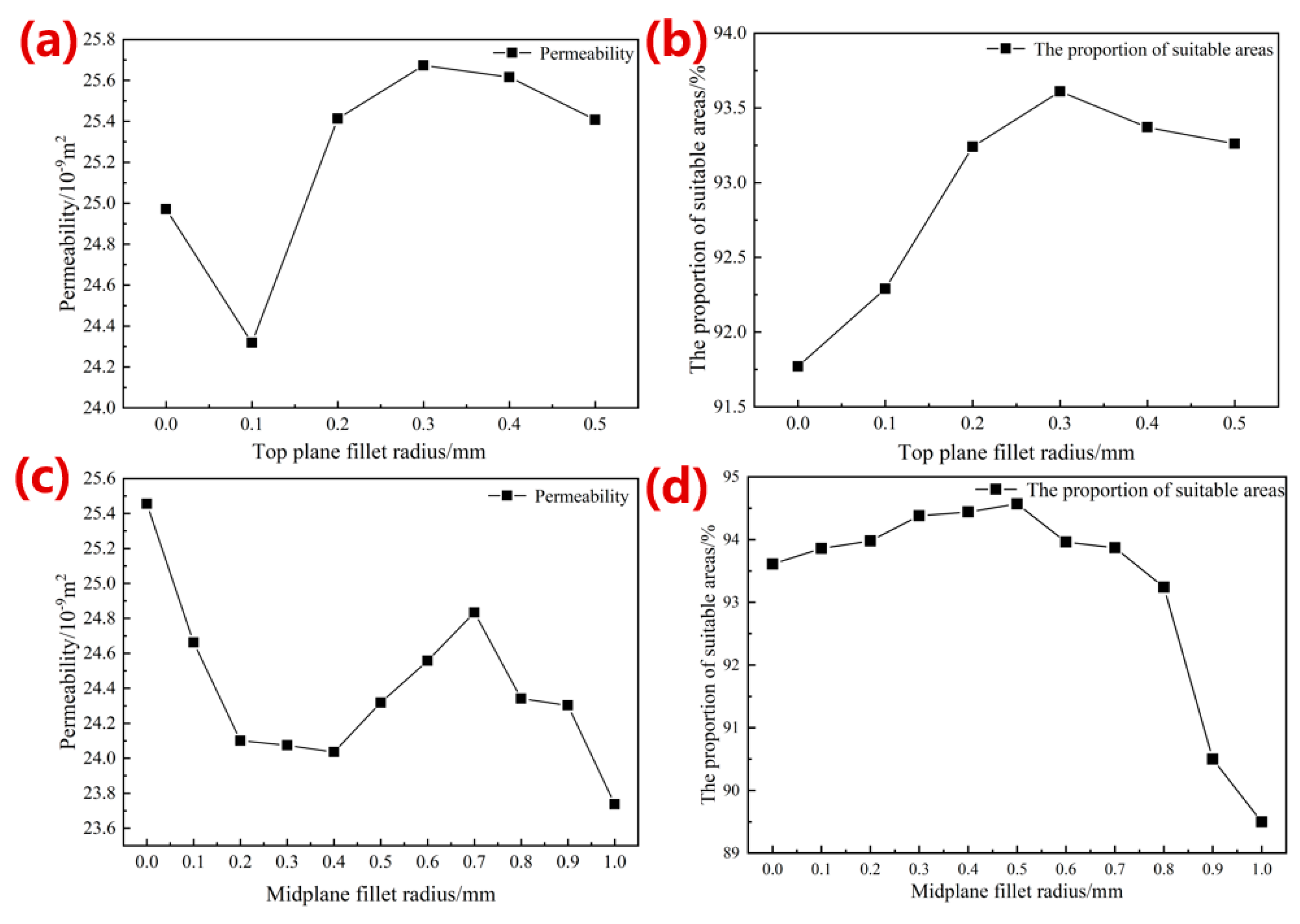
3.1.2. Simulation Results of Permeability Performance
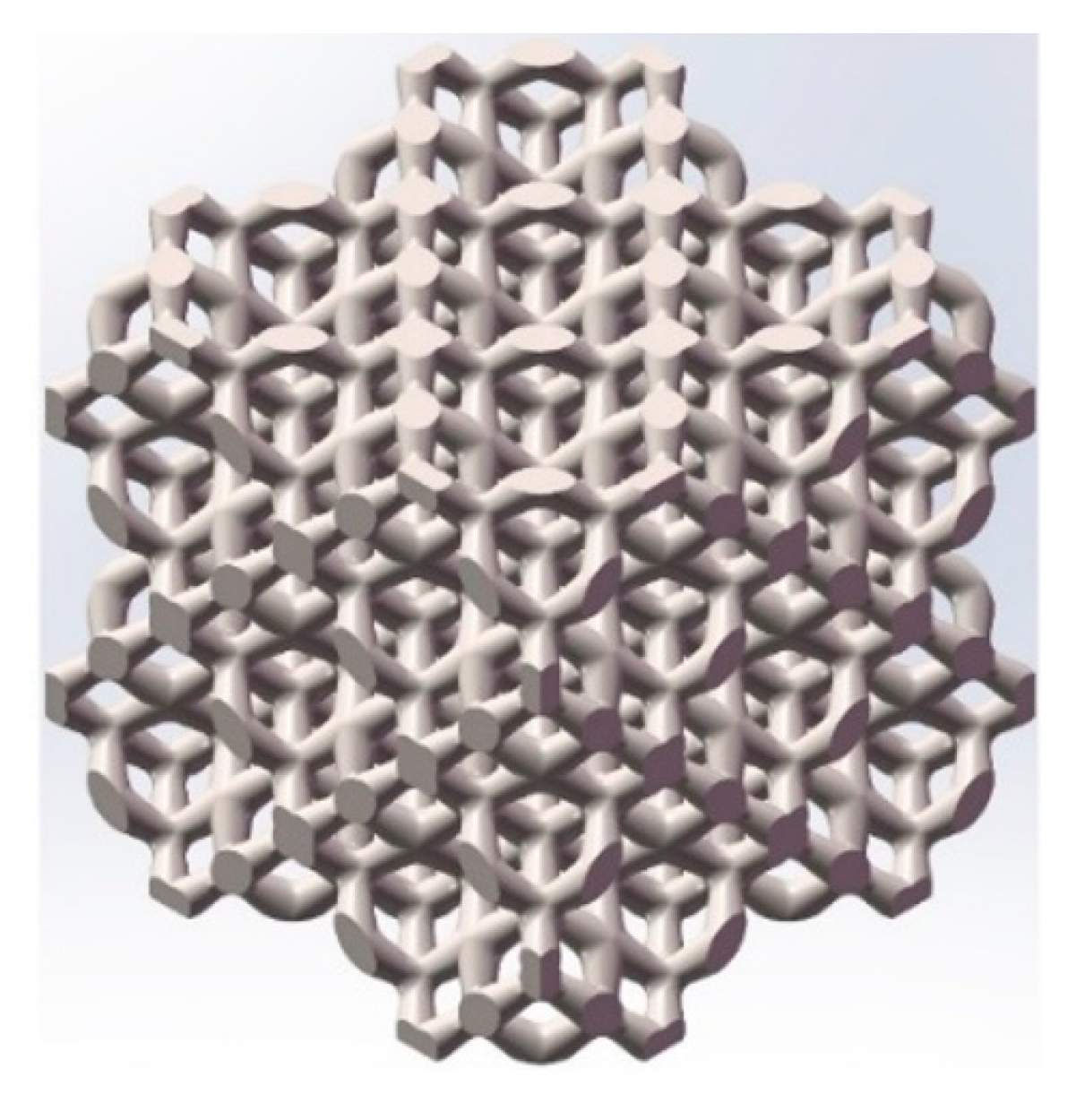
3.2. Porous Model after Microstructure Optimization for Manufacturing
3.3. Morphology of Porous Ta with Optimized Microstructure
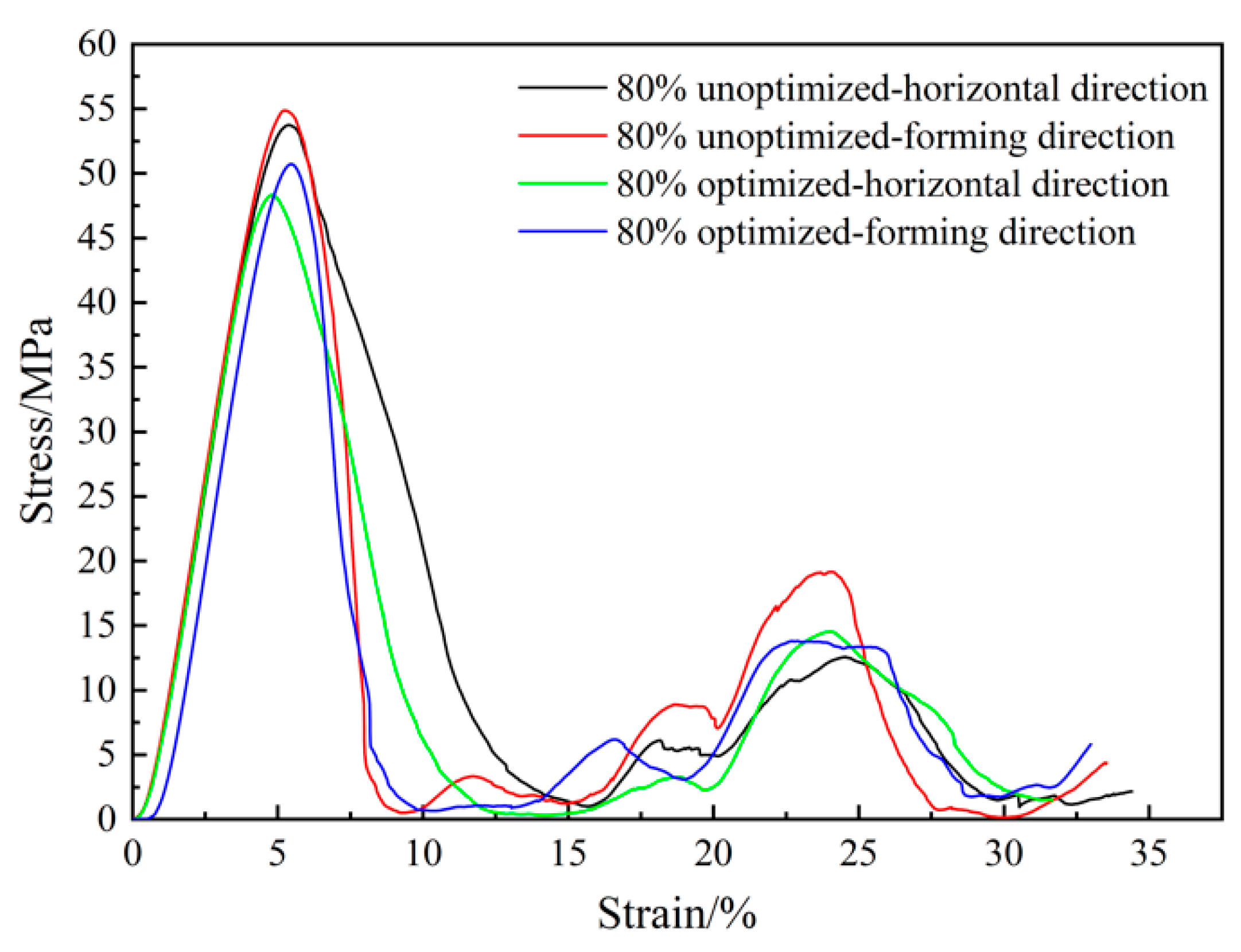
3.4. Mechanical Test Results of Porous Ta with Optimized Microstructure
3.5. Permeability Test Results of Porous Ta with Optimized Microstructure
4. Conclusions
- (1)
- The optimized parameters of the porous diamond structure are as follows: the fillet radius of the top plane is 0.50 mm, and the radius of the midplane fillet is 0.30 mm. The equivalent elastic modulus of the 80%-porosity cell structure is increased by 13.96%, the maximum stress is reduced by 39.17%, and the proportion of the suitable area for cell growth is increased by 1.15%.
- (2)
- The elastic modulus in the forming and horizontal directions of the porous Ta before and after fillet optimization are increased from 1.298 ± 0.006 GPa and 1.352 ± 0.007 GPa to 1.328 ± 0.002 GPa and 1.384 ± 0.006 GPa, representing increases of 2.31% and 2.37%, respectively. At the same time, the permeability is increased by 8.25% from 6.63 ± 0.16 × 10−9 m2 to 7.18 ± 0.18 × 10−9 m2.
- (3)
- The yield strength of the optimized porous Ta samples prepared by LPBF has a relatively obvious increase of 10.39%. This might be due to the fact that the porous Ta after the fillet optimization of can effectively reduce the stress concentration effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wauthle, R.; van der Stok, J.; Yavari, S.A.; Van Humbeeck, J.; Kruth, J.-P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jin, X.; Yang, J.; Zhang, D.; Zhang, S.; Zhang, F.; Chen, H. Porous structure and compressive failure mechanism of additively manufactured cubic-lattice tantalum scaffolds. Mater. Today Adv. 2021, 12, 100183. [Google Scholar] [CrossRef]
- Dong, C.; Bi, X.; Yu, J.; Liu, R.; Zhang, Q. Microstructural evolution and sintering kinetics during spark plasma sintering of pure tantalum powder. J. Alloys Compd. 2019, 781, 84–92. [Google Scholar] [CrossRef]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J. Bone Jt. Surg. Br. Vol. 1999, 81, 907–914. [Google Scholar] [CrossRef]
- Gruen, T.A.; Poggie, R.A.; Lewallen, D.G.; Hanssen, A.D.; Lewis, R.J.; O’Keefe, T.J.; Stulberg, S.D.; Sutherland, C.J. Radiographic evaluation of a monoblock acetabular component: A multicenter study with 2-to 5-year results. J. Arthroplast. 2005, 20, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Austin, M.S. Use of porous tantalum cones in revision total knee arthroplasty. Oper. Tech. Orthop. 2012, 22, 209–221. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Nasser, S.; Poggie, R.A. Revision and salvage patellar arthroplasty using a porous tantalum implant. J. Arthroplast. 2004, 19, 562–572. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J. Preparation of porous Ta and its application as bone implant materials. Acta Met. Sin. 2017, 53, 1303–1310. (In Chinese) [Google Scholar]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; et al. Porous tantalum scaffolds: Fabrication, structure, properties, and orthopedic applications. Mater. Des. 2021, 210, 110095. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.; Qiu, J. The osteogenic effects of porous Tantalum and Titanium alloy scaffolds with different unit cell structure. Colloids Surf. B Biointerfaces 2022, 210, 112229. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Xiong, Y.; Zhang, H.; Dong, L.; Li, J.; Li, X. Mechanical properties and biocompatibility of radial gradient porous titanium/tantalum prepared by SLM. Rare Met. Mater. Eng. 2021, 50, 249–254. (In Chinese) [Google Scholar]
- Wang, X.; Zhu, Z.; Xiao, H.; Luo, C.; Luo, X.; Lv, F.; Liao, J.; Huang, W. Three-Dimensional, MultiScale, and Interconnected Trabecular Bone Mimic Porous Tantalum Scaffold for Bone Tissue Engineering. ACS Omega 2020, 5, 22520–22528. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jin, X.; Gao, H.; Zhang, D.; Chen, H.; Zhang, S.; Li, X. Additive manufacturing of trabecular tantalum scaffolds by laser powder bed fusion: Mechanical property evaluation and porous structure characterization. Mater. Charact. 2020, 170, 110694. [Google Scholar] [CrossRef]
- Pligovka, A. Reflectant photonic crystals produced via porous-alumina-assisted-anodizing of Al/Nb and Al/Ta systems. Surf. Rev. Lett. 2021, 28, 2150055. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef]
- Lv, J.; Jia, Z.; Li, J.; Wang, Y.; Yang, J.; Xiu, P.; Zhang, K.; Cai, H.; Liu, Z. Electron Beam Melting Fabrication of Porous Ti6Al4V Scaffolds: Cytocompatibility and Osteogenesis. Adv. Eng. Mater. 2015, 17, 1391–1398. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, Y.; Wang, M.; Liu, Z.; Liu, C. Parametric design of hip implant with gradient porous structure. Front. Bioeng. Biotechnol. 2022, 10, 850184. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Jiao, C.; Liang, H.; Xie, D.; Shen, L.; Liu, Z. Analysis of mechanical properties and permeability of trabecular-like porous scaffold by additive manufacturing. Front. Bioeng. Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef] [PubMed]
- Urschel, K.; Tauchi, M.; Achenbach, S.; Dietel, B. Investigation of wall shear stress in cardiovascular research and in clinical practice—from bench to bedside. Int. J. Mol. Sci. 2021, 22, 5635. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Habibovic, P.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, Y.; Qin, X.; Lv, N.; Tong, Z.; Sun, C.; Li, D. Optimization of Laser Powder Bed Fusion Process for Forming Porous Ta Scaffold. Metals 2023, 13, 1764. [Google Scholar] [CrossRef]
- ISO 13314:2011; Mechanical testing of metals—Ductility testing—Compression test for porous and cellular metals. ISO: Geneva, Switzerland, 2011.
- Cianetti, F.; Palmieri, M.; Braccesi, C.; Morettini, G. Correction formula approach to evaluate fatigue damage induced by non-Gaussian stress state. Procedia Struct. Integr. 2018, 8, 390–398. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, X.L.; Zhang, Y.Y. Influence of roughness parameters skewness and kurtosis on fatigue life under mixed elastohydrodynamic lubrication point contacts. J. Tribol. 2014, 136, 031503. [Google Scholar] [CrossRef]
- Niesłony, A.; Böhm, M.; Owsiński, R. Crest factor and kurtosis parameter under vibrational random loading. Int. J. Fatigue 2021, 147, 106179. [Google Scholar] [CrossRef]


| Parameters | Radius |
|---|---|
| Middle-plane fillets | 0.1 mm~1.0 mm (interval 0.1 mm) |
| Top-plane fillets | 0.1 mm~0.5 mm (interval 0.1 mm) |
| Structure Type | Elasticity Modulus (GPa) | Yield Strength (MPa) | ||
|---|---|---|---|---|
| Horizontal Direction | Forming Direction | Horizontal Direction | Forming Direction | |
| Original structure | 1.352 ± 0.007 | 1.298 ± 0.006 | 53.981 ± 0.124 | 49.771 ± 0.091 |
| Optimized structure | 1.384 ± 0.053 | 1.328 ± 0.009 | 59.590 ± 0.053 | 54.226 ± 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qin, X.; Lv, N.; Gao, L.; Sun, C.; Tong, Z.; Li, D. Microstructure Optimization for Design of Porous Tantalum Scaffolds Based on Mechanical Properties and Permeability. Materials 2023, 16, 7568. https://doi.org/10.3390/ma16247568
Wang Y, Qin X, Lv N, Gao L, Sun C, Tong Z, Li D. Microstructure Optimization for Design of Porous Tantalum Scaffolds Based on Mechanical Properties and Permeability. Materials. 2023; 16(24):7568. https://doi.org/10.3390/ma16247568
Chicago/Turabian StyleWang, Yikai, Xiao Qin, Naixin Lv, Lin Gao, Changning Sun, Zhiqiang Tong, and Dichen Li. 2023. "Microstructure Optimization for Design of Porous Tantalum Scaffolds Based on Mechanical Properties and Permeability" Materials 16, no. 24: 7568. https://doi.org/10.3390/ma16247568
APA StyleWang, Y., Qin, X., Lv, N., Gao, L., Sun, C., Tong, Z., & Li, D. (2023). Microstructure Optimization for Design of Porous Tantalum Scaffolds Based on Mechanical Properties and Permeability. Materials, 16(24), 7568. https://doi.org/10.3390/ma16247568






