Structural and Electrical Characterization of LaSrAl1−xMgxO4−δ Layered Perovskites Obtained by Mechanical Synthesis
Abstract
:1. Introduction
2. Experimental Methodology
3. Results and Discussion
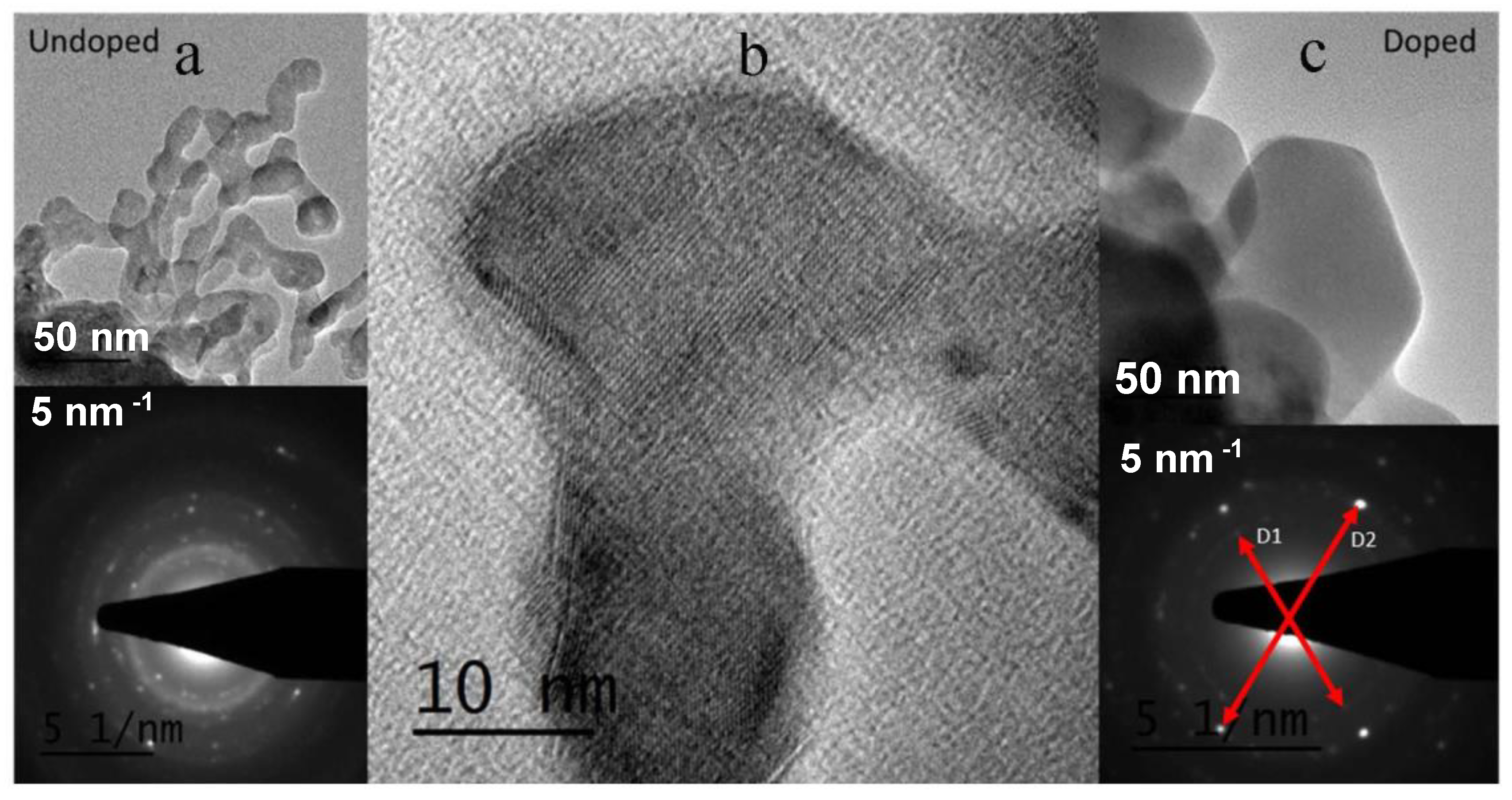
3.1. Structural Characterization
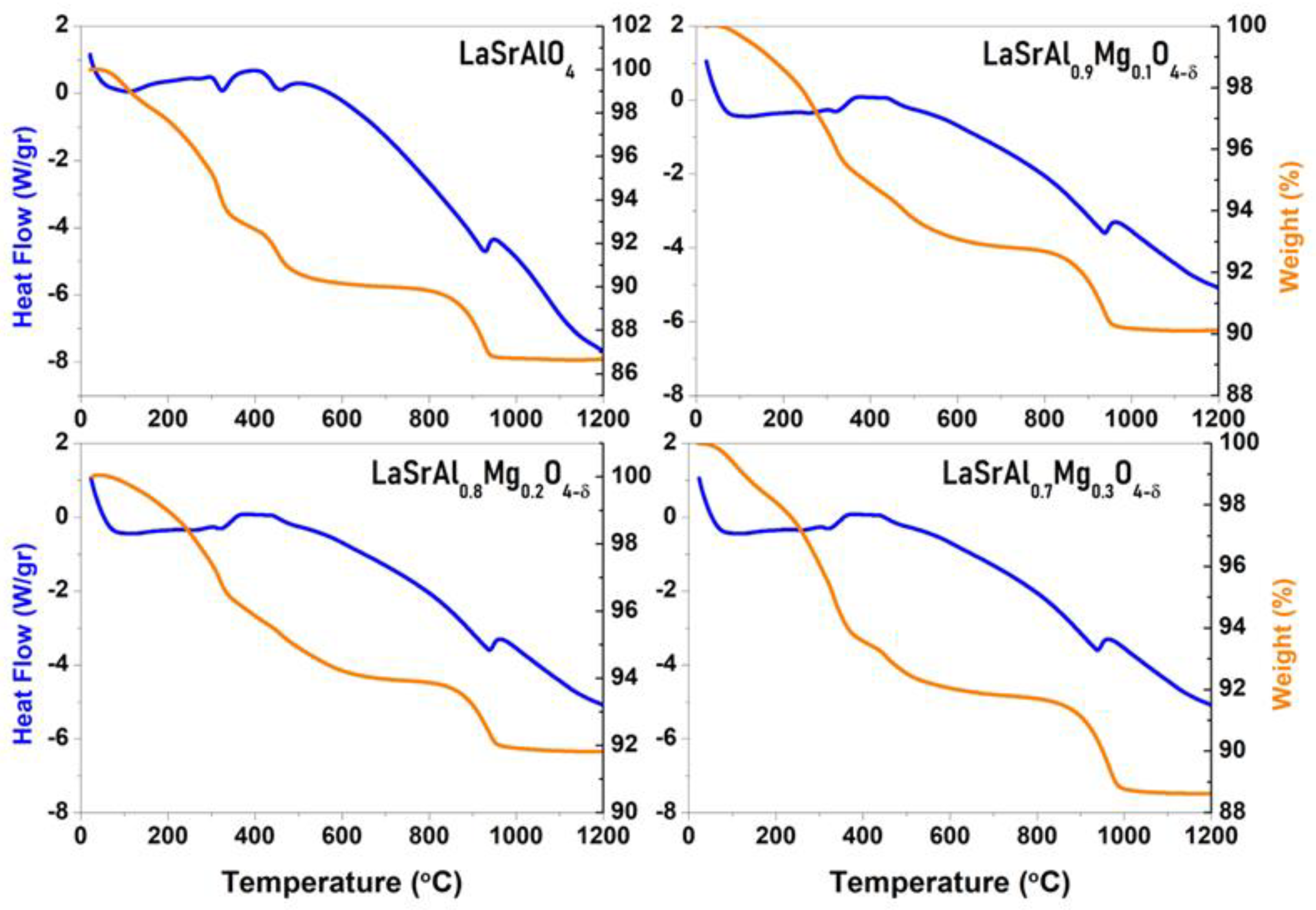
3.2. Thermal Stability
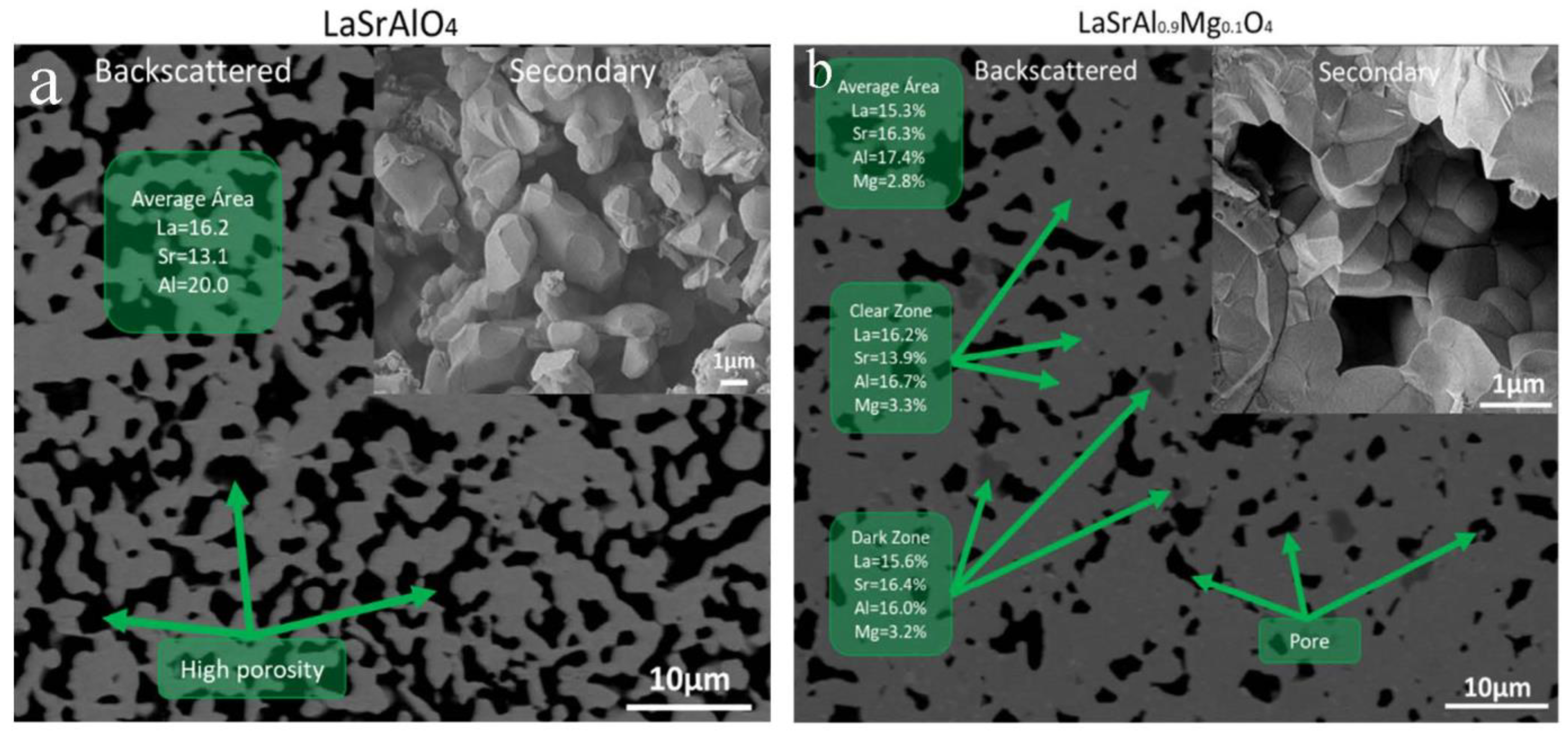
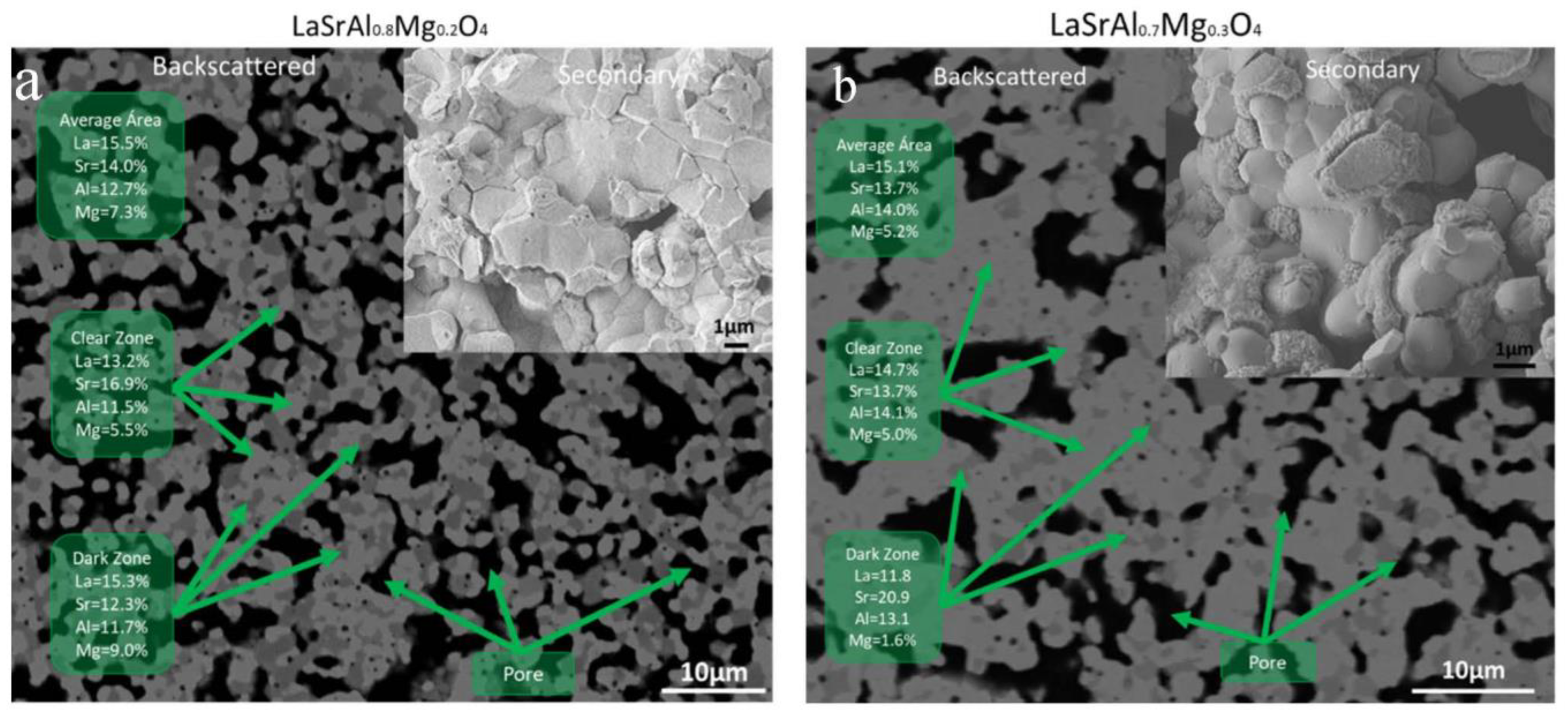
3.3. Microstructure
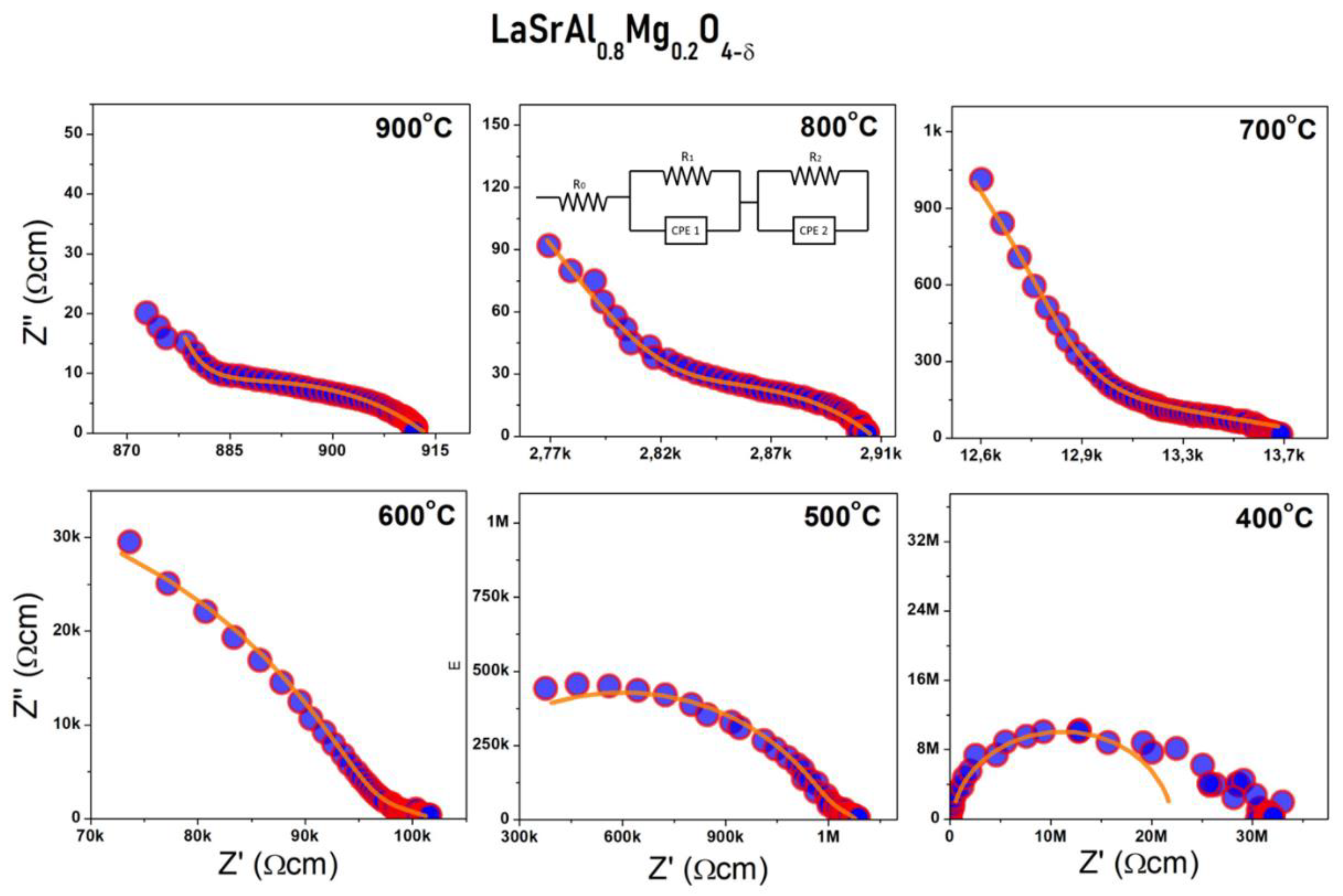
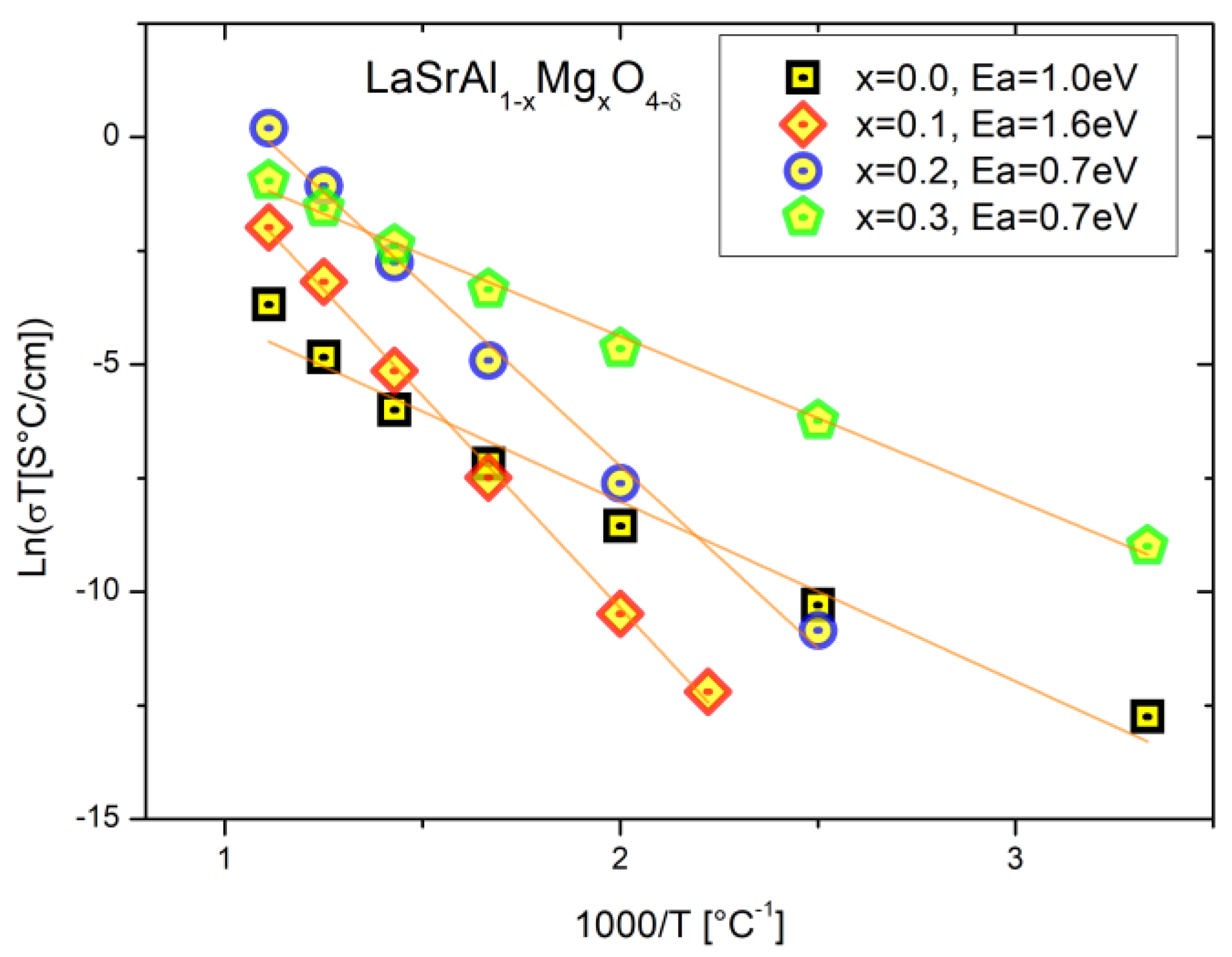
3.4. Electric Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AbouSeada, N.; Hatem, T.M. Climate action: Prospects of green hydrogen in Africa. Energy Rep. 2022, 8, 3873–3890. [Google Scholar] [CrossRef]
- Wyss, A.M.; Berger, S.; Baumgartner, T.; Knoch, D. Reactions to warnings in the climate commons. J. Environ. Psychol. 2021, 78, 101689. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Takahashi, T.; Steele, B.C.H. Oxygen Ion Conductors. High Conduct. Solid Ion. Conduct. 2013, 6, 402–446. [Google Scholar] [CrossRef]
- Arslan, O.; Acikkalp, E.; Genc, G. A multi-generation system for hydrogen production through the high-temperature solid oxide electrolyzer integrated to 150 MW coal-fired steam boiler. Fuel 2022, 315, 123201. [Google Scholar] [CrossRef]
- Palomba, V.; Ferraro, M.; Frazzica, A.; Vasta, S.; Sergi, F.; Antonucci, V. Experimental and numerical analysis of a SOFC-CHP system with adsorption and hybrid chillers for telecommunication applications. Appl. Energy 2018, 216, 620–633. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell—A comprehensive review. Fuel Process. Technol. 2022, 235, 107380. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Solid State Zirconia-based solid electrolytes: Microstructure, stability and ionic conductivity. Solid State Ion 1992, 52, 23–32. [Google Scholar] [CrossRef]
- Honorato, R.; Honorato, D.; Tramontin, M.; Raymundo, M. Compatibility Analyses of BICUVOX. 10 as a Cathode in Yttria-stabilized Zirconia Electrolytes for Usage in Solid Oxide Fuel Cells. Mater. Res. 2014, 17, 1173–1179. [Google Scholar]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 8 C. Solid State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1−xInO4+δ. J. Mater. Chem. A Mater. 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Mariño, C.; Basbus, J.; Alonso, J.A.; Troncoso, L. Structural characterization and electrochemical properties of (La,Sr)(Al,Mg)O4−δ perovskites. New J. Chem. 2020, 44, 11608–11614. [Google Scholar] [CrossRef]
- Mariño, C.; Basbus, J.; Larralde, A.L.; Alonso, J.A.; Fernández-Díaz, M.T.; Troncoso, L. Structural, electrical characterization and oxygen-diffusion paths in LaSrGa1−xMgxO4−δ (x = 0.0–0.2) layered perovskites: An impedance spectroscopy and neutron diffraction study. New J. Chem. 2021, 45, 10248–10256. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Review of perovskite-structure related cathode materials for solid oxide fuel cells. Ceram. Int. 2020, 46, 5521–5535. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Neutron Powder Diffraction; Oxford University Press: Oxford, UK, 1993; Volume 192, pp. 55–69. [Google Scholar]
- Ragoisha, G.A. Challenge for electrochemical impedance spectroscopy in the dynamic world. J. Solid State Electrochem. 2020, 24, 2171–2172. [Google Scholar] [CrossRef]
- Zvereva, I.A.; Popova, V.F.; Vagapov, D.A.; Toikka, A.M.; Gusarov, V.V. Kinetics of formation of ruddlesden-popper phases: I. mechanism of La2SrAl2O7 Formation. Russ J. Gen Chem. 2001, 71, 1181–1185. [Google Scholar] [CrossRef]
- Popova, V.F.; Tugova, E.A.; Zvereva, I.A.; Gusarov, V.V. Phase equilibria in the LaAlO3-LaSrAlO4 system. Glass Phys. Chem. 2004, 30, 564–567. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A general method to synthesize and sinter bulk ceramics in seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef] [PubMed]

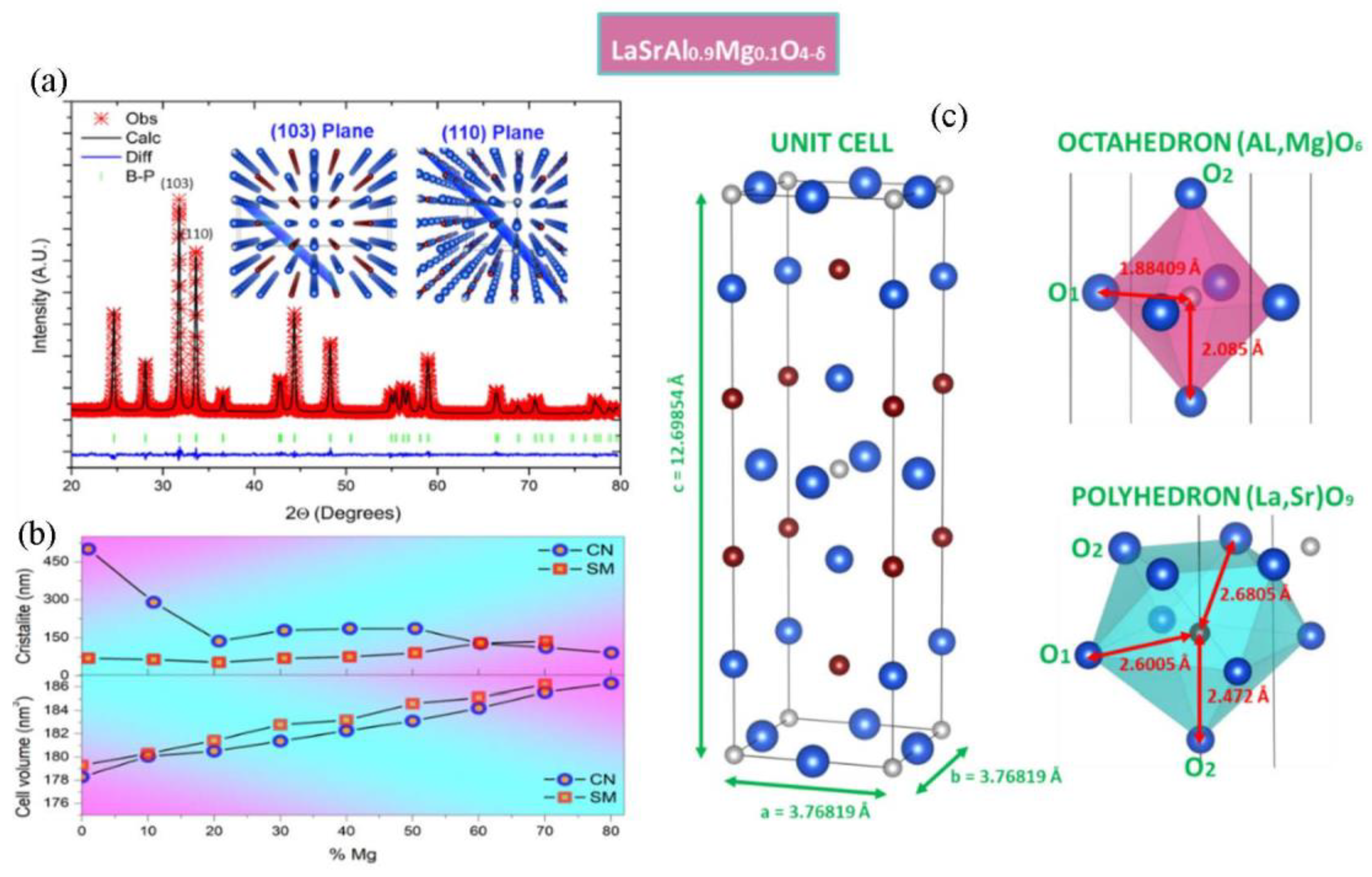
| LaSrAl1−xMgxO4 | x = 0.0 | x = 0.1 | x = 0.2 | x = 0.3 |
|---|---|---|---|---|
| a[Å] | 3.7606 (7) | 3.76819 (11) | 3.7770 (2) | 3.7897 (3) |
| c[Å] | 12.678 (3) | 12.6985 (4) | 12.7174 (8) | 12.727 (1) |
| V[Å3] | 179.29 (6) | 180.31 (1) | 181.43 (2) | 182.78 (2) |
| La/Sr (0 0 z) | ||||
| z | 0.3569 (3) | 0.35885 (9) | 0.35901 (14) | 0.3599 (2) |
| focc | 1.0 | 1.0 | 1.0 | 1.0 |
| Al/Mg (½ ½ ½) | ||||
| focc | 1.0/0.0 | 0.9/0.1 | 0.8/0.2 | 0.7/0.3 |
| O1 (½ 0 ½) | ||||
| B [Å2] | - | 2.3 (5) | 1.4 (7) | 1.7 (9) |
| focc | 2.0 | 2.00 (0) | 2.00(0) | 2.00 (0) |
| O2 (0 0 z) | ||||
| z | 0.163 (2) | 0.1642 (5) | 0.1642 (8) | 0.1662 (1) |
| B [Å2] | - | 1.7 (4) | 0.9 (5) | 0.3 (7) |
| focc | 2.0 | 2.00 (0) | 2.00 (0) | 2.00 (0) |
| Reliability | parameters | |||
| χ2 | 2.77 | 1.57 | 2.43 | 6.37 |
| Rp (%) | 2.57 | 4.73 | 6.20 | 7.43 |
| Rwp (%) | 3.49 | 5.95 | 8.40 | 12.2 |
| RBragg (%) | 5.77 | 2.78 | 4.84 | 4.53 |
| Distance [Å] | LaSrAlO4 | LaSrAl0.9Mg0.1O4 | LaSrAl0.8Mg0.2O4 | LaSrAl0.7Mg0.3O4 |
|---|---|---|---|---|
| La/Sr-O1(x4) | 2.613 (3) | 2.6005 (8) | 2.6041 (12) | 2.6019 (17) |
| La/Sr-O2 | 2.46 (2) | 2.472 (6) | 2.477 (10) | 2.464 (13) |
| La/Sr-O2(x4) | 2.671 (2) | 2.6805 (7) | 2.6870 (11) | 2.7004 (16) |
| 2.623 (2) | 2.622 (1) | 2.627 (2) | 2.630 (3) | |
| Al/Mg-O1(x4) | 1.8803 (4) | 1.88409 (5) | 1.88853 (9) | 1.89485 (10) |
| Al/Mg-O2(x2) | 2.07 (2) | 2.085 (6) | 2.088 (10) | 2.117 (13) |
| 1.944 (7) | 1.951 (2) | 1.955 (4) | 1.969 (22) | |
| O1-O1(x4) | 2.6591 (4) | 2.66451 (5) | 2.67078 (9) | 2.67972 (10) |
| O2-O1(x4) | 2.793 (17) | 2.810 (5) | 2.816 (8) | 2.841 (9) |
| O2-O2(x4) | 3.46 (2) | 3.442 (6) | 3.449 (9) | 3.423 (11) |
| Al/Mg-O1-Al/Mg | 180.0 | 180.0 | 180.0 | 180.0 |
| Al/Mg-O2-Al/Mg | 180.0 | 180.0 | 180.0 | 180.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariño, C.; Serafini, D.; Basbus, J.; Alonso, J.A.; Troncoso, L. Structural and Electrical Characterization of LaSrAl1−xMgxO4−δ Layered Perovskites Obtained by Mechanical Synthesis. Materials 2023, 16, 7564. https://doi.org/10.3390/ma16247564
Mariño C, Serafini D, Basbus J, Alonso JA, Troncoso L. Structural and Electrical Characterization of LaSrAl1−xMgxO4−δ Layered Perovskites Obtained by Mechanical Synthesis. Materials. 2023; 16(24):7564. https://doi.org/10.3390/ma16247564
Chicago/Turabian StyleMariño, Carlos, Daniel Serafini, Juan Basbus, José Antonio Alonso, and Loreto Troncoso. 2023. "Structural and Electrical Characterization of LaSrAl1−xMgxO4−δ Layered Perovskites Obtained by Mechanical Synthesis" Materials 16, no. 24: 7564. https://doi.org/10.3390/ma16247564
APA StyleMariño, C., Serafini, D., Basbus, J., Alonso, J. A., & Troncoso, L. (2023). Structural and Electrical Characterization of LaSrAl1−xMgxO4−δ Layered Perovskites Obtained by Mechanical Synthesis. Materials, 16(24), 7564. https://doi.org/10.3390/ma16247564







