In Vitro Investigation of Gelatin/Polycaprolactone Nanofibers in Modulating Human Gingival Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Cell Isolation
2.3. Preparation of GPP and VSCM
2.4. Cell Morphology and Adhesion
2.5. Cell Proliferation/Viability and Metabolic Activity Assays
2.6. RT-qPCR and ELISA
2.7. Statistical Analysis
3. Results
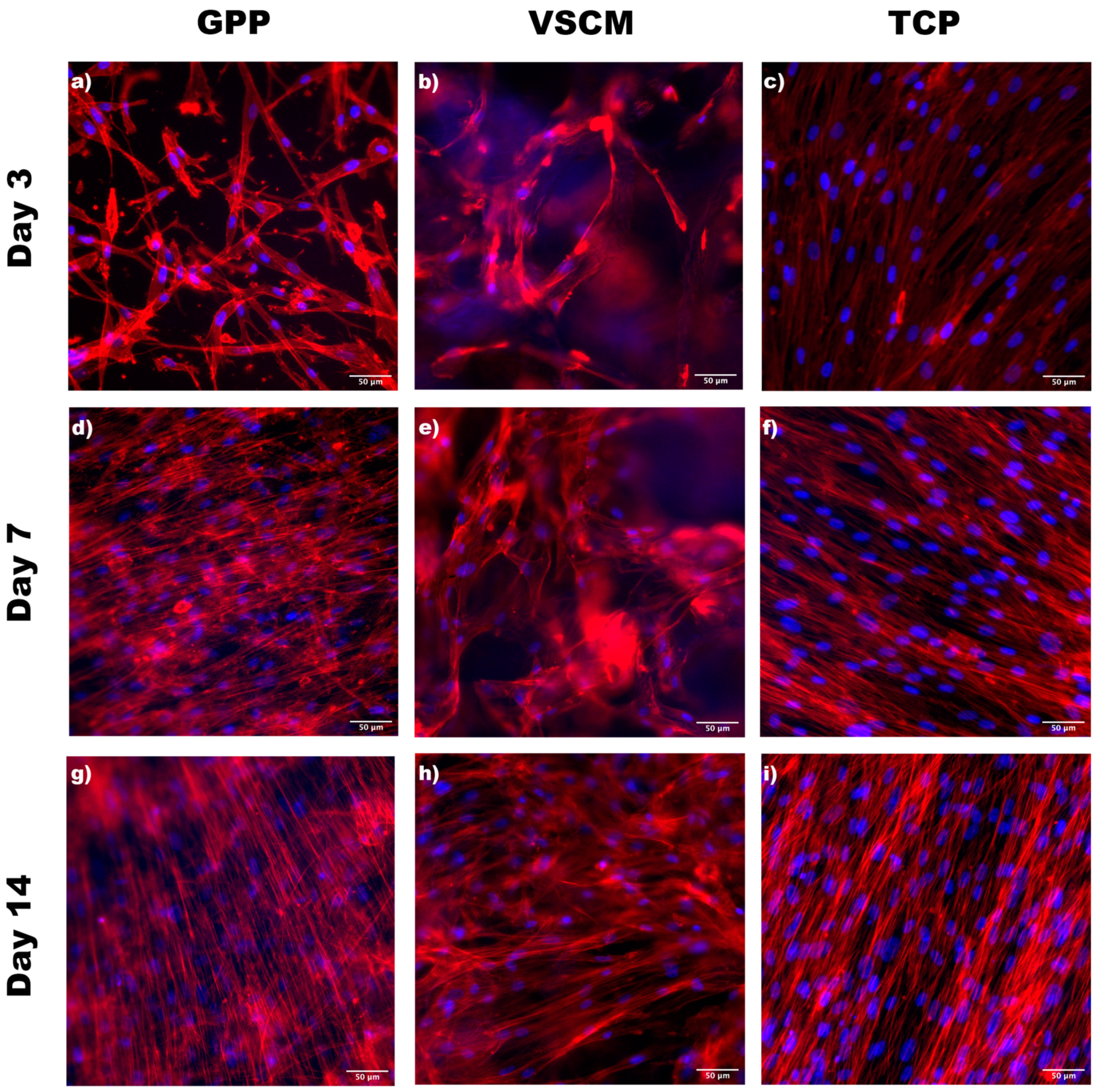
3.1. Cell Morphology and Adhesion
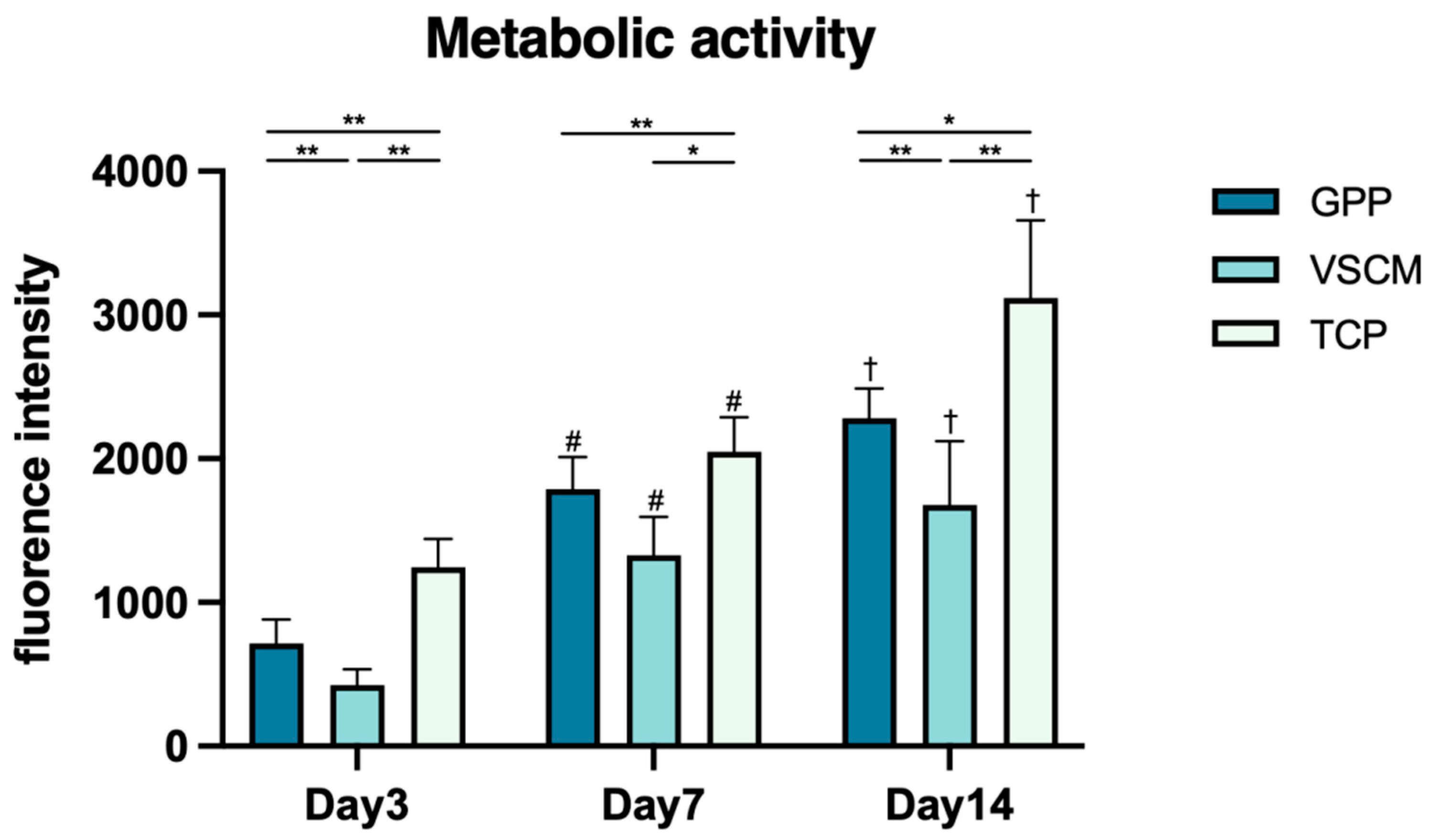
3.2. Cell Proliferation/Viability and Metabolic Activity
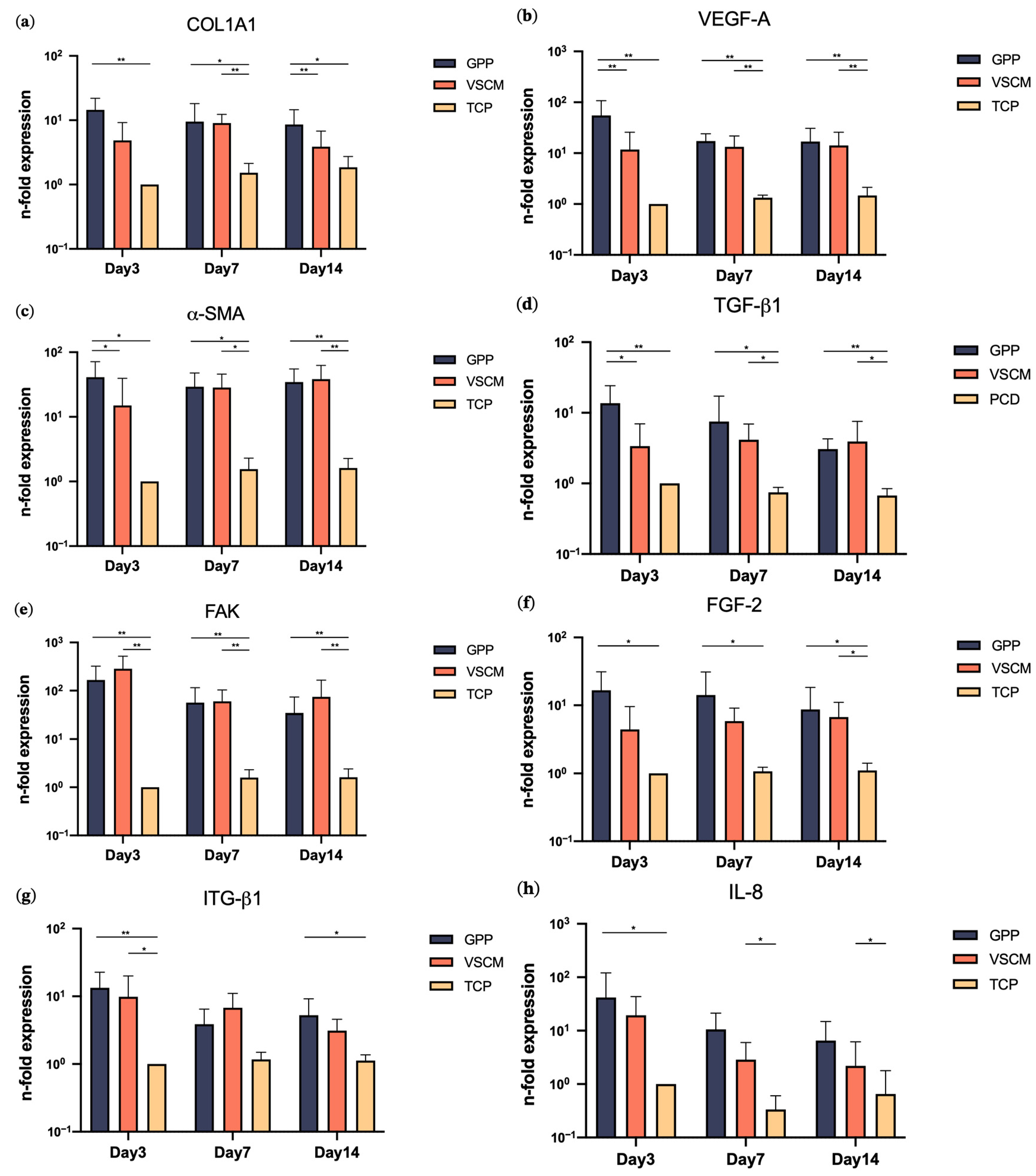
3.3. Effects of GPP and VSCM on Gene Expression
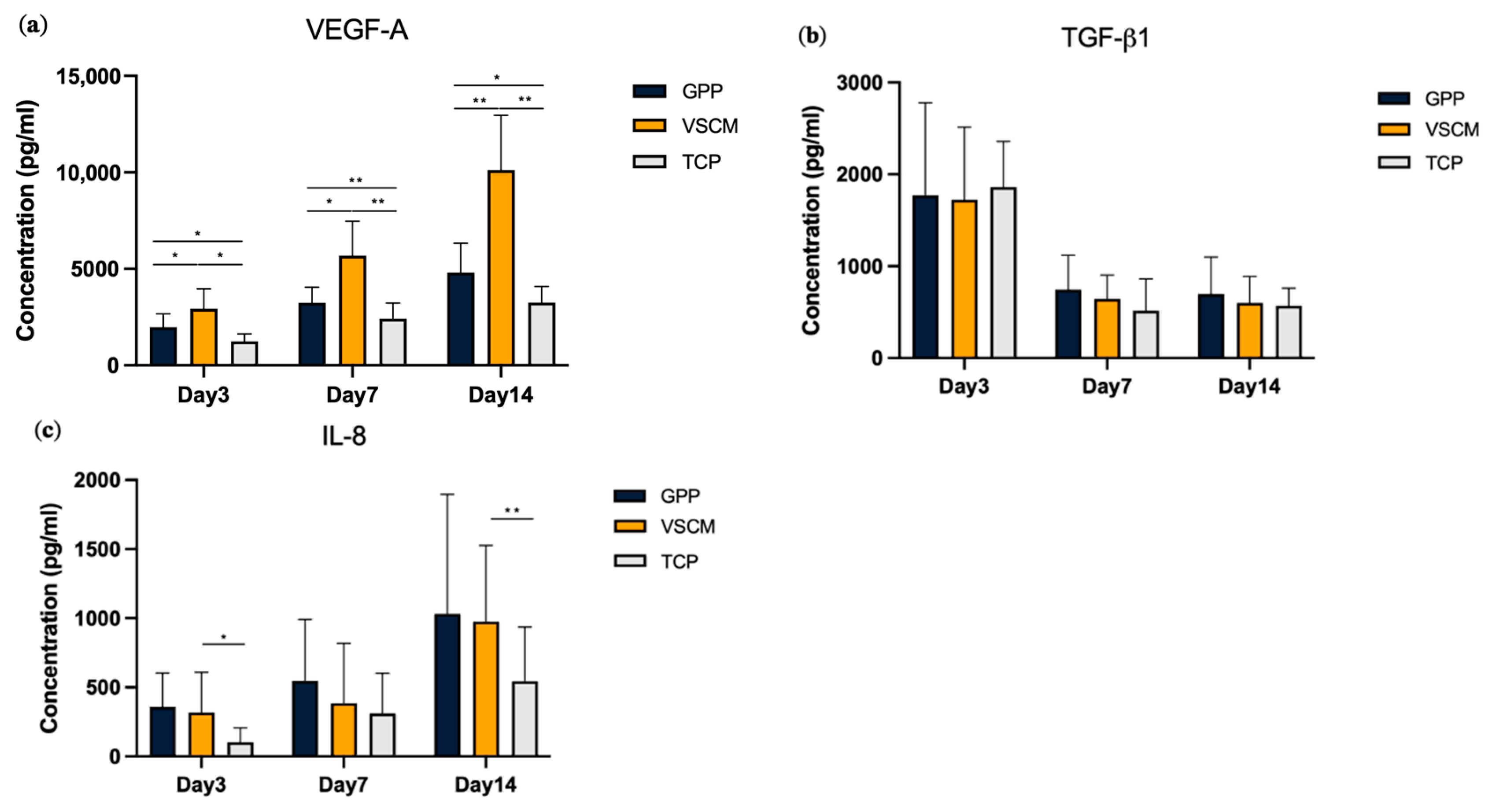
3.4. Effects of GPP and VSCM on the Protein Production by hG-MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef] [PubMed]
- Kassab, M.M.; Cohen, R.E. The etiology and prevalence of gingival recession. J. Am. Dent. Assoc. 2003, 134, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Stetler, K.J.; Bissada, N.F. Significance of the width of keratinized gingiva on the periodontal status of teeth with submarginal restorations. J. Periodontol. 1987, 58, 696–700. [Google Scholar] [CrossRef]
- Tugnait, A.; Clerehugh, V. Gingival recession-its significance and management. J. Dent. 2001, 29, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S190–S198. [Google Scholar] [CrossRef]
- Cairo, F. Periodontal plastic surgery of gingival recessions at single and multiple teeth. Periodontol. 2000 2017, 75, 296–316. [Google Scholar] [CrossRef]
- Zucchelli, G.; Mounssif, I. Periodontal plastic surgery. Periodontol. 2000 2015, 68, 333–368. [Google Scholar] [CrossRef]
- Friedman, N.; Levine, H.L. Mucogingival Surgery: Current Status. J. Periodontol. 1964, 35, 5–21. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Cortellini, P.; Pellegrini, G.; Nieri, M.; Bonaccini, D.; Allegri, M.; Bouchard, P.; Cairo, F.; Conforti, G.; Fourmousis, I.; et al. Xenogenic collagen matrix or autologous connective tissue graft as adjunct to coronally advanced flaps for coverage of multiple adjacent gingival recession: Randomized trial assessing non-inferiority in root coverage and superiority in oral health-related quality of life. J. Clin. Periodontol. 2018, 45, 78–88. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Stahli, A.; Guldener, K.; Sculean, A. A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 4051. [Google Scholar] [CrossRef]
- Mathes, S.H.; Wohlwend, L.; Uebersax, L.; von Mentlen, R.; Thoma, D.S.; Jung, R.E.; Gorlach, C.; Graf-Hausner, U. A bioreactor test system to mimic the biological and mechanical environment of oral soft tissues and to evaluate substitutes for connective tissue grafts. Biotechnol. Bioeng. 2010, 107, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, M.; Jung, R.E.; Hammerle, C.H.; Husler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, M.; Skurska, A.; Podlewski, L.; Milewski, R.; Pietruski, J. Clinical evaluation of Miller class I and II recessions treatment with the use of modified coronally advanced tunnel technique with either collagen matrix or subepithelial connective tissue graft: A randomized clinical study. J. Clin. Periodontol. 2019, 46, 86–95. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Mehwish, N.; Chen, Y.; Zaeem, M.; Wang, Y.; Lee, B.H.; Deng, H. Novel biohybrid spongy scaffolds for fabrication of suturable intraoral graft substitutes. Int. J. Biol. Macromol. 2022, 214, 617–631. [Google Scholar] [CrossRef]
- Lashkari, M.; Rahmani, M.; Yousefpoor, Y.; Ahmadi-Zeidabadi, M.; Faridi-Majidi, R.; Ameri, Z.; Salary, M.; Azizi, S.; Shahabi, A.; Rahi, A.; et al. Cell-based wound dressing: Bilayered PCL/gelatin nanofibers-alginate/collagen hydrogel scaffold loaded with mesenchymal stem cells. Int. J. Biol. Macromol. 2023, 239, 124099. [Google Scholar] [CrossRef]
- Angarano, M.; Schulz, S.; Fabritius, M.; Vogt, R.; Steinberg, T.; Tomakidi, P.; Friedrich, C.; Mülhaupt, R. Layered Gradient Nonwovens of In Situ Crosslinked Electrospun Collagenous Nanofibers Used as Modular Scaffold Systems for Soft Tissue Regeneration. Adv. Funct. Mater. 2013, 23, 3277–3285. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Nakatsuka, N.; Young, T.D.; Taghdiri, N.; Ansari, S.; Aghaloo, T.; Li, S.; Khademhosseini, A.; Weiss, P.S.; et al. Hierarchically Patterned Polydopamine-Containing Membranes for Periodontal Tissue Engineering. ACS Nano 2019, 13, 3830–3838. [Google Scholar] [CrossRef]
- Williamson, M.R.; Adams, E.F.; Coombes, A.G. Gravity spun polycaprolactone fibres for soft tissue engineering: Interaction with fibroblasts and myoblasts in cell culture. Biomaterials 2006, 27, 1019–1026. [Google Scholar] [CrossRef]
- Coombes, A.G.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Safi, I.N.; Al-Shammari, A.M.; Ul-Jabbar, M.A.; Hussein, B.M.A. Preparing polycaprolactone scaffolds using electrospinning technique for construction of artificial periodontal ligament tissue. J. Taibah Univ. Med. Sci. 2020, 15, 363–373. [Google Scholar] [CrossRef]
- Kusirisin, T.; Suwanprateeb, J.; Buranawat, B. Polycaprolactone versus collagen membrane and 1-year clinical outcomes: A randomized controlled trial. Clin. Implant. Dent. Relat Res. 2023, 25, 330–342. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Jalaja, K.; Kumar, P.R.A.; Dey, T.; Kundu, S.C.; James, N.R. Modified dextran cross-linked electrospun gelatin nanofibres for biomedical applications. Carbohydr. Polym. 2014, 114, 467–475. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef]
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized Alginate-Gelatin Hydrogel: A Favorable Matrix for Growth and Osteogenic Differentiation of Adipose-Derived Stem Cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Madjd, Z.; Pezeshki-Modaress, M.; Khosrowpour, Z.; Farshi, P.; Eini, L.; Kiani, J.; Seifi, M.; Kundu, S.C.; Ghods, R.; et al. Bioengineering of fibroblast-conditioned polycaprolactone/gelatin electrospun scaffold for skin tissue engineering. Artif. Organs 2022, 46, 1040–1054. [Google Scholar] [CrossRef]
- Prado-Prone, G.; Silva-Bermudez, P.; Bazzar, M.; Focarete, M.L.; Rodil, S.E.; Vidal-Gutierrez, X.; Garcia-Macedo, J.A.; Garcia-Perez, V.I.; Velasquillo, C.; Almaguer-Flores, A. Antibacterial composite membranes of polycaprolactone/gelatin loaded with zinc oxide nanoparticles for guided tissue regeneration. Biomed. Mater. 2020, 15, 035006. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Angarano, M.; Fabritius, M.; Mulhaupt, R.; Dard, M.; Obrecht, M.; Tomakidi, P.; Steinberg, T. Nonwoven-based gelatin/polycaprolactone membrane proves suitability in a preclinical assessment for treatment of soft tissue defects. Tissue Eng. Part A 2014, 20, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Janjic, K.; Agis, H.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Effects of collagen membranes and bone substitute differ in periodontal ligament cell microtissues and monolayers. J. Periodontol. 2022, 93, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Blufstein, A.; Abhari, S.Y.; Koch, C.; Gahn, J.; Schaffer, C.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Response of Human Mesenchymal Stromal Cells from Periodontal Tissue to LPS Depends on the Purity but Not on the LPS Source. Mediat. Inflamm. 2020, 2020, 8704896. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef]
- Andrukhov, O. Toll-Like Receptors and Dental Mesenchymal Stromal Cells. Front. Oral. Health 2021, 2, 648901. [Google Scholar] [CrossRef]
- Guo, B.; Tang, C.; Wang, M.; Zhao, Z.; Shokoohi-Tabrizi, H.A.; Shi, B.; Andrukhov, O.; Rausch-Fan, X. In vitro biocompatibility of biohybrid polymers membrane evaluated in human gingival fibroblasts. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2590–2598. [Google Scholar] [CrossRef]
- Blufstein, A.; Behm, C.; Kubin, B.; Gahn, J.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Anti-apoptotic effects of human gingival mesenchymal stromal cells on polymorphonuclear leucocytes. Oral Dis. 2022, 28, 777–785. [Google Scholar] [CrossRef]
- Wehner, C.; Laky, M.; Shokoohi-Tabrizi, H.A.; Behm, C.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Effects of Er:YAG laser irradiation of different titanium surfaces on osteoblast response. J. Mater. Sci. Mater. Med. 2021, 32, 22. [Google Scholar] [CrossRef]
- Blufstein, A.; Behm, C.; Nguyen, P.Q.; Rausch-Fan, X.; Andrukhov, O. Human periodontal ligament cells exhibit no endotoxin tolerance upon stimulation with Porphyromonas gingivalis lipopolysaccharide. J. Periodontal. Res. 2018, 53, 589–597. [Google Scholar] [CrossRef]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Gelatin-polycaprolactone-nanohydroxyapatite electrospun nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111588. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, W.; Sun, B.; Li, H.; Wu, T.; Ke, Q.; Huang, C.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. A comparison of nanoscale and multiscale PCL/gelatin scaffolds prepared by disc-electrospinning. Colloids Surf B Biointerfaces 2016, 146, 632–641. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Jiang, L.; Huang, A.; Wang, X.F.; Li, Q.; Turng, L.S. Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 901–908. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yuan, J.; Shen, J. Differences in cytocompatibility between collagen, gelatin and keratin. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Ontoria-Oviedo, I.; Castellano, D.; Sepulveda, P.; Ribes-Greus, A. Polycaprolactone/gelatin-based scaffolds with tailored performance: In vitro and in vivo validation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110296. [Google Scholar] [CrossRef] [PubMed]
- Abraham, L.C.; Zuena, E.; Perez-Ramirez, B.; Kaplan, D.L. Guide to collagen characterization for biomaterial studies. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 264–285. [Google Scholar] [CrossRef]
- Alvarez Perez, M.A.; Guarino, V.; Cirillo, V.; Ambrosio, L. In vitro mineralization and bone osteogenesis in poly(epsilon-caprolactone)/gelatin nanofibers. J. Biomed. Mater. Res. A 2012, 100, 3008–3019. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, S.M.; Kang, H.Y.; Min, H.J.; Lee, R.; Ikram, M.; Subhan, F.; Jin, S.W.; Jeong, Y.H.; Kwak, J.Y.; et al. Bioactive fish collagen/polycaprolactone composite nanofibrous scaffolds fabricated by electrospinning for 3D cell culture. J. Biotechnol. 2015, 205, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Zhao, X.K.; Cheng, Y.; Liang Cheng, M.; Yu, L.; Mu, M.; Li, H.; Liu, Y.; Zhang, B.; Yao, Y.; Guo, H.; et al. Focal Adhesion Kinase Regulates Fibroblast Migration via Integrin beta-1 and Plays a Central Role in Fibrosis. Sci. Rep. 2016, 6, 19276. [Google Scholar] [CrossRef]
- Bashiri, Z.; Zahiri, M.; Allahyari, H.; Esmaeilzade, B. Proliferation of human spermatogonial stem cells on optimized PCL/Gelatin nanofibrous scaffolds. Andrologia 2022, 54, e14380. [Google Scholar] [CrossRef]
- Ronnov-Jessen, L.; Petersen, O.W. A function for filamentous alpha-smooth muscle actin: Retardation of motility in fibroblasts. J. Cell Biol. 1996, 134, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. Interaction of human smooth muscle cells with nanofibrous scaffolds: Effect of fiber orientation on cell adhesion, proliferation, and functional gene expression. J. Biomed. Mater. Res. A 2015, 103, 2236–2250. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, R.; Orsini, G.; Iezzi, G.; Scarano, A.; Rubini, C.; Pecora, G.; Piattelli, A. Bone regeneration with calcium sulfate: Evidence for increased angiogenesis in rabbits. J. Oral Implantol. 2002, 28, 273–278. [Google Scholar] [CrossRef]
- Patel, Z.S.; Ueda, H.; Yamamoto, M.; Tabata, Y.; Mikos, A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm. Res. 2008, 25, 2370–2378. [Google Scholar] [CrossRef]
- Bekic, M.; Radanovic, M.; Dokic, J.; Tomic, S.; Erakovic, M.; Radojevic, D.; Duka, M.; Markovic, D.; Markovic, M.; Ismaili, B.; et al. Mesenchymal Stromal Cells from Healthy and Inflamed Human Gingiva Respond Differently to Porphyromonas gingivalis. Int. J. Mol. Sci. 2022, 23, 3510. [Google Scholar] [CrossRef]
- Fu, X.; Wang, H. Spatial arrangement of polycaprolactone/collagen nanofiber scaffolds regulates the wound healing related behaviors of human adipose stromal cells. Tissue Eng. Part A 2012, 18, 631–642. [Google Scholar] [CrossRef]
- Ramanathan, G.; Muthukumar, T.; Tirichurapalli Sivagnanam, U. In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur. J. Pharmacol. 2017, 814, 45–55. [Google Scholar] [CrossRef]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef]
- Tabata, Y.; Miyao, M.; Ozeki, M.; Ikada, Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J. Biomater. Sci. Polym. Ed. 2000, 11, 915–930. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Kim, J.; Ma, T. Autocrine fibroblast growth factor 2-mediated interactions between human mesenchymal stem cells and the extracellular matrix under varying oxygen tension. J. Cell Biochem. 2013, 114, 716–727. [Google Scholar] [CrossRef]
- Malik, A.F.; Hoque, R.; Ouyang, X.; Ghani, A.; Hong, E.; Khan, K.; Moore, L.B.; Ng, G.; Munro, F.; Flavell, R.A.; et al. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc. Natl. Acad. Sci. USA 2011, 108, 20095–20100. [Google Scholar] [CrossRef]
- Islam, M.M.; AbuSamra, D.B.; Chivu, A.; Argueso, P.; Dohlman, C.H.; Patra, H.K.; Chodosh, J.; Gonzalez-Andrades, M. Optimization of Collagen Chemical Crosslinking to Restore Biocompatibility of Tissue-Engineered Scaffolds. Pharmaceutics 2021, 13, 832. [Google Scholar] [CrossRef]
- Vozzi, G.; Corallo, C.; Carta, S.; Fortina, M.; Gattazzo, F.; Galletti, M.; Giordano, N. Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: In vitro evidences. J. Biomed. Mater. Res. A 2014, 102, 1415–1421. [Google Scholar] [CrossRef]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Crone, W.C.; Peng, X.F.; Turng, L.S. Electrospinning of unidirectionally and orthogonally aligned thermoplastic polyurethane nanofibers: Fiber orientation and cell migration. J. Biomed. Mater. Res. A 2015, 103, 593–603. [Google Scholar] [CrossRef]
- McNamara, L.E.; Burchmore, R.; Riehle, M.O.; Herzyk, P.; Biggs, M.J.; Wilkinson, C.D.; Curtis, A.S.; Dalby, M.J. The role of microtopography in cellular mechanotransduction. Biomaterials 2012, 33, 2835–2847. [Google Scholar] [CrossRef]
- Agis, H.; Collins, A.; Taut, A.D.; Jin, Q.; Kruger, L.; Gorlach, C.; Giannobile, W.V. Cell population kinetics of collagen scaffolds in ex vivo oral wound repair. PLoS ONE 2014, 9, e112680. [Google Scholar] [CrossRef]
- Yao, R.; He, J.; Meng, G.; Jiang, B.; Wu, F. Electrospun PCL/Gelatin composite fibrous scaffolds: Mechanical properties and cellular responses. J. Biomater. Sci. Polym. Ed. 2016, 27, 824–838. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Zhao, Z.; Rausch, M.A.; Behm, C.; Shokoohi-Tabrizi, H.A.; Andrukhov, O.; Rausch-Fan, X. In Vitro Investigation of Gelatin/Polycaprolactone Nanofibers in Modulating Human Gingival Mesenchymal Stromal Cells. Materials 2023, 16, 7508. https://doi.org/10.3390/ma16247508
Tian Z, Zhao Z, Rausch MA, Behm C, Shokoohi-Tabrizi HA, Andrukhov O, Rausch-Fan X. In Vitro Investigation of Gelatin/Polycaprolactone Nanofibers in Modulating Human Gingival Mesenchymal Stromal Cells. Materials. 2023; 16(24):7508. https://doi.org/10.3390/ma16247508
Chicago/Turabian StyleTian, Zhiwei, Zhongqi Zhao, Marco Aoqi Rausch, Christian Behm, Hassan Ali Shokoohi-Tabrizi, Oleh Andrukhov, and Xiaohui Rausch-Fan. 2023. "In Vitro Investigation of Gelatin/Polycaprolactone Nanofibers in Modulating Human Gingival Mesenchymal Stromal Cells" Materials 16, no. 24: 7508. https://doi.org/10.3390/ma16247508
APA StyleTian, Z., Zhao, Z., Rausch, M. A., Behm, C., Shokoohi-Tabrizi, H. A., Andrukhov, O., & Rausch-Fan, X. (2023). In Vitro Investigation of Gelatin/Polycaprolactone Nanofibers in Modulating Human Gingival Mesenchymal Stromal Cells. Materials, 16(24), 7508. https://doi.org/10.3390/ma16247508





