Abstract
Marine biofouling is a worldwide problem in marine systems. Nowadays, innovative non-toxic antifouling and fouling-release materials are highly desirable. In this study, a strategy for preparing antifouling and fouling-release materials via one-step dip coating is reported. Copolymers were synthesized via the polymerization of a monomer with catechol sticky functional groups and four monomers with antifouling- or fouling-release functional groups, respectively. The copolymers could assemble onto different material surfaces, such as metals and plastics, using biomimetic catechol groups via multivalent complex bonding. The catechol groups were helpful for adhesion onto the surfaces, while the other functional groups endowed the coatings with antifouling or fouling-release properties. The effects of modifying the substrates using these copolymer coatings were verified via X-ray photoelectron spectroscopy; images of Chlorella cell and Ulva zoospore settlement were taken using a microscope and scanning electron microscope. The copolymer-coated surfaces, especially the surface modified by DOPA–PSPMA, displayed the best antifouling activity, and surface modification via DOPA–PTMETH was shown to be the most effective for producing the fouling-release property in the settlement assay.
1. Introduction
Biofouling presents micro- and macroorganisms or their excretion products on surfaces, which has become a widespread problem for marine vessels and infrastructure [1,2,3]. Many traditional effective antifouling methods, such as toxic-release coatings, have been restricted by legislation because of their significant adverse environmental effects [4]. Therefore, innovative non-toxic antifouling materials are highly desirable [5,6,7,8,9,10].
Surface chemical modification is one of the key approaches for endowing marine vessels with antifouling properties. Many materials with universal antifouling functions have been screened out [10,11,12,13,14]. Antifouling surfaces prepared via grafting polymers from initiators or grafting polymers with functional groups have been reported [8,10,15,16,17]. Chilkoti et al. prepared poly-(OEGMA) using SI–ATRP to resist protein adhesion [18]. Jiang et al. discussed antimicrobial cationic surfaces that can effectively kill bacterial [19]. Researchers have found that hydrophilic polymers such as poly(ethylene glycol) and zwitterionic polymers have significant antifouling properties [20,21,22,23]. Copolymers were also regularly reported for antifouling applications [14,24]. Liu et al. reported the synthesis of zwitterionic–phosphonic copolymers for the efficient antifouling surface settlement of diverse biomedical metals [25]. Li et al. reported that a polyether–thiourea–siloxane copolymer was synthesized by introducing thiourea and some ether groups into the MQ silicone resin polymer, which showed the most effective antifouling ability, via free radical polymerization [26]. Guo et al. designed a new surface-active copolymer based on hydrophilic polyvinylpyrrolidone and PDMS and then incorporated it into a crosslinked PDMS matrix to create a surface-renewable antifouling coating [27]. Xu et al. used an innovative photopolymerization technique to develop sulfur-containing polymer-grafted antifouling surfaces [28].
Although these approaches have been shown to be effective for modifying polymers onto material surfaces, specific interactions between interfacial modifiers (such as thiolate on noble metals and organosilane and polyamines on oxides) are also required, which are laborious and costly and cannot be easily used in practical applications [29]. Effective and simple one-step methods are strongly required. Catechol is the side chain of DOPA, which has widely been found in mussel adhesive proteins. It has been shown to be very important in surface-independent anchor molecules [30,31,32]. Many studies have shown that catechol groups can form covalent bonds and hydrogen bonds, and they also had strong physical interactions with material surfaces in [33]. The high concentration of DOPA at the adhesive interface showed the possible mechanism of enhancing adhesion. This specific study has stimulated significant interest in exploiting catechol for enhancing the interfacial adhesion of material surfaces. Polymers with catechol groups showed strong interfacial adhesion strength on many material surfaces, especially on wet surfaces, in [34]. Jiang et al. reported an antifouling polyampholyte polymer with catechol groups that was formed polymer coating and prevented protein adsorption [35]. Xiong et al. synthesized copolymers with different topologies, antifouling blocks, and terminal poly(dopamine-acrylamide) anchoring blocks and also used them for antiprotein absorption, antibacterial settlement, and antimarine fouling [36]. Park et al. presented highly antibiofouling multiloop polyethers functionalized with mussel–biomimic catechol groups with varying loop dimensions [37]. Sathyan et al. reported the synthesis of zwitterionic copolymers and their ability to form antifouling coatings on porous hydroxyapatite (used as a mimic of dental coatings) [38]. Yeon et al. synthesized a zwitterionic dopamine derivative containing catechol and amine groups and also demonstrated its excellent antifouling material surface by showing how it could control the oxidation of ZW–DOPA [39].
In this study, four kinds of copolymers containing antifouling pendant side groups (including hydrophilic groups, cationic surfactant functional groups, anionic surfactant functional groups, and hydrophobic groups) and catechol adhesive groups via polymerization were obtained. Settlement tests with Chlorella cells and Ulva zoospores were carried out to investigate the antifouling and fouling-release properties of the copolymer-modified material surfaces.
2. Materials and Methods
2.1. Materials and Characterization
3-Hydroxytyramine hydrochloride, α,α′-Azobisisobutyronitrile (AIBN), 3-sulfopropyl methacrylate potassium salt (SPMA(K)), 3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluorooctyl methacrylate (TMETH), 2-(methacryloyloxy) ethyl-trimethylammonium chloride (METAC), and oligoethylene glycol monomethyl ether methacrylate (OEGMA, average Mn 500, 50 wt. % in H2O) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Methacryloyl chloride was obtained from Alfa Aesar. Chemical reagents of Na2B4O7·10H2O and Na2CO3·H2O were obtained from Chinese Tianjin Chemical Reagents Corp. (Tianjin, China).
Chlorella cells and Ulva zoospores were obtained from Marine Biology Institute of Shandong Province (Jinan, China), and their suspensions were prepared using the standard method.
Information on the chemical compositions of the surfaces of the copolymer-modified substrates were characterized via XPS (PHI-5702, Phisical Electronics Corporation, Chanhassen, MN, USA) using Al KR radiation. The molecular weights of the copolymers were determined using GPC (Dawn Heleos System, Wyatt Technology, Santa Barbara, CA, USA) in water and DMF. The molecular weights were determined relative to the narrow molecular weight polystyrene standard. Optical images of cell and zoospore settlement on the surfaces the substrates were obtained using a microscope (BX51, Olympus Corporation, Tokyo, Japan). Micrographs of cells and zoospores were taken via SEM (JSM-5600LV, JEOL Ltd., Tokyo, Japan). All the samples were treated with 2.5% glutaraldehyde before being characterized via SEM. Coating thickness was measured using spectroscopic ellipsometry (UVISEL, Horiba Jobin Yvon, Montpellier, France) using a He-Ne laser at a fixed angle of 70°. Ten measurements were recorded per sample.
2.2. The Synthetic Method of Monomer
N-(3,4-dihydroxyphenyl)ethyl methacrylamide (DOPAMA) was prepared as shown in Scheme 1 [40]. Next, 3.83 g Na2B4O7·10H2O and 100 mL water were added into a 250 mL round-bottomed flask. Subsequently, 1.9 g dopamine·HCl was added after the solution had been degassed in an Ar atmosphere for more than 15 min, and the mixture was stirred for 10 min; this was followed by adjusting pH to 10 using Na2CO3·H2O.
The solution was cooled in an ice-water bath, and 1.05 g of methacryloyl chloride was added dropwise. The reaction solution was stirred for 24 h under an Ar atmosphere at 25 °C and then acidified to pH 2 using 6 M hydrochloric acid aqueous solution before being extracted using 100 mL ethyl acetate three times. The organic extracts were dried using anhydrous Na2SO4, and the brown solid was obtained via distillation under reduced pressure. The pure solid DOPAMA was then obtained using silica gel column chromatography.
2.3. Polymerization Methods
As shown in Scheme 1, PDOPA–PMETAC was synthetized via the polymerization reaction of METAC solution and DOPAMA. Briefly, 8 mL METAC solution, 44 mg DOPAMA, and 7.2 mg AIBN were all dissolved in 5 mL pure water and 5 mL methanol. Then, the mixed solution was charged using N2 for 10 min and kept in a N2 atmosphere for 1 h via stirring at 30 °C. The precipitation was obtained in methanol. The PDOPA–PMETAC copolymer was washed with methanol three times and then dried in a vacuum oven for 24 h.
PDOPA–POEGMA was synthetized via the precipitation polymerization reaction of the OEGMA solution and DOPAMA. Briefly, 5 mL OEGMA, 44 mg DOPAMA, and 7.2 mg AIBN were dissolved in 5 mL methanol. Then, the mixed solution was charged using N2 for 10 min and kept in a N2 atmosphere for 1.5 h via stirring at 35 °C. The PDOPA–POEGMA copolymer was washed with methanol three times and then dried in a vacuum oven for 24 h.
PDOPA–PSPMA was synthetized via the polymerization reaction of SPMA(K) powder and DOPAMA. Briefly, 0.96 g SPMA(K), 22 mg DOPAMA, and 7.2 mg AIBN were dissolved in 6 mL pure water and 4mL methanol. Then, the mixed solution was charged using N2 for 10 min and kept in a N2 atmosphere for 1 h via stirring at 30 °C. The precipitation was obtained in methanol. The PDOPA–PSPMA copolymer was washed with methanol three times and then dried in a vacuum oven for 24 h.
PDOPA–PTMETH was synthetized via the polymerization of TMETH liquid and DOPAMA. Briefly, 0.86 g TMETH, 88 mg DOPAMA, and 7.2 mg AIBN were dissolved in 20 mL methanol. Then, the mixed solution was charged using N2 for 10 min and kept in a N2 atmosphere for 5 h via stirring at 45 °C. The obtained PDOPA–PMETAC copolymer was washed with methanol three times and then dried in a vacuum oven for 24 h.
The number average molecular weight and molecular weight distribution values of the synthetized copolymers are shown in Table 1.

Table 1.
Number average molecular weight and molecular weight distribution values of synthetized copolymers.
Table 1.
Number average molecular weight and molecular weight distribution values of synthetized copolymers.
| Copolymer | Mn (g mol−1) | D |
|---|---|---|
| PDOPA–PMETAC | 7900 | 1.17 |
| PDOPA–POEGMA | 9800 | 1.21 |
| PDOPA–PSPMA | 7200 | 1.12 |
| PDOPA–PTMETH | 5600 | 1.28 |
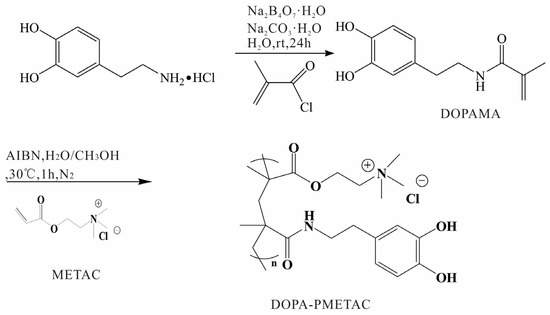
Scheme 1.
Synthesis of the PDOPA–PMETAC copolymer.
2.4. Surface Modification by Copolymer Coatings on Substrates
Si, Cu, Fe, Ti, and PTFE wafers were all cleaned in pure methanol via sonication for 10 min. Then, all the wafers were immersed in 20 mg/mL copolymer solution for 12 h at 25 °C. After that, all the coating modified wafers were rinsed in water and methanol, respectively, and then dried in a vacuum oven.
2.5. Settlement and Adhesion Tests of Cells and Zoospores
All the modified silicon wafer samples were immersed in distilled water for 12 h and then transferred to artificial seawater for 1 h for the settlement and adhesion tests. All these samples were left on glass containers individually, and then the containers were filled with 20 mL 1.1 × 106 cells mL−1 Chlorella suspension and 7.8 × 105 spores mL−1 Ulva zoospores. All the samples were kept in Chlorella cell and Ulva zoospore suspensions for 3 h and then rinsed in new artificial seawater to remove unattached cells or spores. The cells or zoospores were counted based on observing 30 random fields of the 5 replicates for each substrate sample. All the removal rate data were obtained by exposing these substrate samples to 35 kPa water impact pressure and calculated from the settlement cells and zoospores density between these substrate samples before and after exposure to water.
3. Results and Discussion
3.1. Chemical Compositions of the Surfaces
Surface modification on Si wafer substrates by four copolymers was characterized via XPS. Figure 1 illustrates that the XPS spectra of PDOPA–POEGMA-, PDOPA–PMETAC-, PDOPA–PSPMA-, and PDOPA–PTMETH-modified substrates showed the presence of C, O, N elements. The XPS spectrum of the PDOPA–PSPMA coating exhibited the S2p signal at 162.5 eV, originating from the C-S bond; the XPS spectrum of the PDOPA-PTMETH-modified substrates exhibited the F1s signal at 689.1 eV from the C-F covalent bond. Ellipsometry was used to measures the dry thickness of the PDOPA–POEGMA, PDOPA–PMETAC, PDOPA–PSPMA, and PDOPA–PTMETH coatings on the Si wafers after being immersed in 20 mg/mL copolymer solution for 12 h at 25 °C, and the values were 7.48 ± 2.27 nm, 5.38 ± 1.86 nm, 4.63 ± 1.55 nm, and 3.79 ± 1.93 nm, respectively. It is clear that all these substrate surfaces had been successfully modified by these copolymer coatings. The authors of [32,33] indicated that the catechol side chains could anchor the polymers onto surfaces, and with this and the results from Figure 1 in mind, the functional groups of POEGMA, PSPMA, PMETAC, and PTMETH were proved to be on the surfaces of the PDOPA–POEGMA, PDOPA–PMETAC, PDOPA–PSPMA, and PDOPA–PTMETH coatings, respectively.
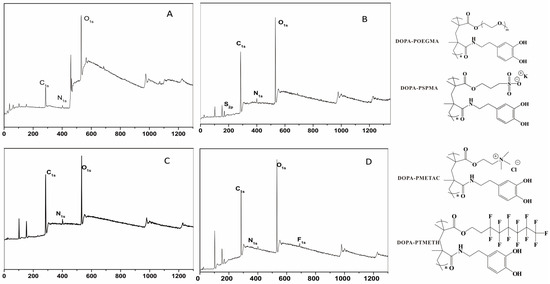
Figure 1.
The XPS spectra of the (A) PDOPA–POEGMA, (B) PDOPA–PSPMA, (C) PDOPA–PMETAC, and (D) PDOPA–PTMETH coatings.
Figure 2 shows the XPS spectra of the copolymer coatings on the Cu wafer substrate, Fe wafer substrate, Ti wafer substrate, and PTFE wafer substrate. Surface modification was proved by the S2p, N1s, and F1s signals. Adhesion onto metal and plastic substrates was also provided by the catechol groups. It is shown that the copolymers could be easily used on many material substrate surfaces, containing general marine artificial materials.
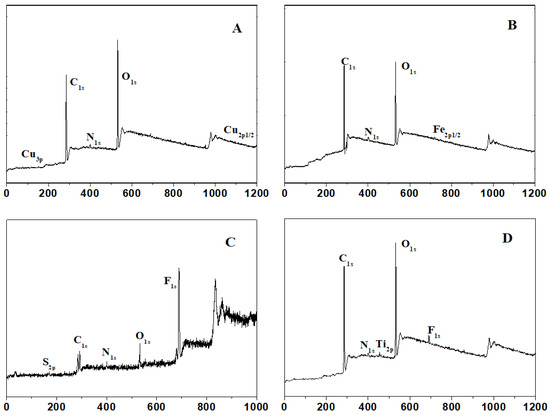
Figure 2.
The XPS spectra of (A) a PDOPA–POEGMA-modified Cu substrate, (B) a PDOPA–PMETAC-modified Fe substrate, (C) a PDOPA–PSPMA-modified PTFE substrate, and (D) a PDOPA–PTMETH-modified Ti substrate.
3.2. Antifouling and Fouling-Release Performances
Table 2 shows the relationship between the reduced settlement ratios and growth removal percentages of the cells/spores with the molar ratios of the DOPAMA and OEGMA/SPMA(K)/METAC/TMETH monomers. It is obvious that the copolymers synthetized by 1:20 of DOPAMA and OEGMA, 1:30 of DOPAMA and SPMA(K), 1:20 of DOPAMA and METAC, and 1:20 of DOPAMA and TMETH monomers showed the best antifouling and fouling-release properties. In these copolymers, the catechol groups from the DOPAMA monomer provided strong interfacial adhesion strength to the surfaces, the hydrophilic groups, cationic surfactant functional groups, and anionic surfactant functional groups, and the hydrophobic groups of the DOPAMA and OEGMA/SPMA(K)/METAC/TMETH monomers provided antifouling and fouling-release properties. Dense copolymer coatings should have appropriate quantities of catechol groups in order to be strongly modified on the substrate and provide excellent antifouling and fouling-release performances.

Table 2.
Reduced settlement ratios and growth removal percentages of cells/spores of different samples.
Chlorella cell and Ulva zoospore settlement tests were used to verify the antifouling properties of all of the substrates. Chlorella have no flagella; they settle by and adhere to the substrate surface via proteins. Ulva zoospores have flagella to swim onto the proper surface and then settle [6]. The results of the settlement test on the Si wafer substrate surface and the copolymer-modified substrate surfaces are shown in Figure 3. It is shown that compared with the control Si wafer substrate, the DOPA–POEGMA-, DOPA–PMETAC-, DOPA–PSPMA-, and DOPA–PTMETH-modified substrates can significantly inhibit the settlement of Chlorella cells and Ulva zoospores, which could reduce 83 ± 3%, 91 ± 3%, 78 ± 3%, 45 ± 3% Chlorella cell settlement, respectively, and 76 ± 4%, 89 ± 3%, 77 ± 3%, 48 ± 4% Ulva zoospore settlement, respectively. The density values of the Chlorella cells (147 cells mm−2) and Ulva zoospores (101 spores mm−2) of the DOPA–PSPMA-modified substrate was the lowest, which means that the DOPA–PSPMA-modified substrate was the most effective in protecting against cell and spore settlement, while the DOPA–PTMETH-modified substrate was worse than all of the other substrates. Overall, the modifying copolymer coatings can efficiently inhibit cell and zoospore settlement and adhesion.
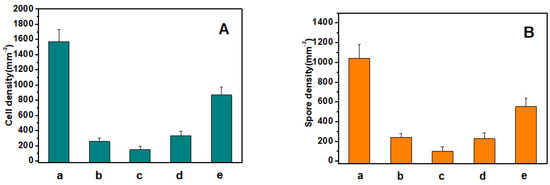
Figure 3.
(A) Chlorella cells. (B) Settlement density values of Ulva zoospores on (a) the Si wafer substrate, (b) the PDOPA–POEGMA–modified wafer substrate, (c) the PDOPA–PSPMA–modified wafer substrate, (d) the PDOPA–PMETAC–modified wafer substrate, and (e) the PDOPA–PTMETH–modified wafer substrate.
Figure 4 shows the cell and spore removal percentages of different substrates via water scouring. It is shown that compared with the control Si wafer substrate, the PDOPA–POEGMA-, PDOPA–PMETAC-, PDOPA–PSPMA-, and PDOPA–PTMETH-modified substrates could enhance the removal of Chlorella cells and Ulva zoospores much more efficiently, improving the removal percentage of Chlorella cells by 14 ± 1%, 18 ± 3%, 15 ± 3%, 49 ± 2%, respectively, and improving the removal percentage of Ulva zoospores by 15 ± 2%, 17 ± 1%, 16 ± 2%, 50 ± 2%, respectively. The copolymer coatings allowed the adhesive cells and zoospores to be easily released. Because of the low surface energy of the F element, the PDOPA–PTMETH-modified substrate was the most effective in releasing cells and zoospores via water shear force; there were almost no cells or spores on this substrate’s surface after exposure to water scouring. Therefore, these copolymer coatings, especially the PDOPA–PTMETH coating, could strongly improve the fouling-release performance of material substrate surfaces.
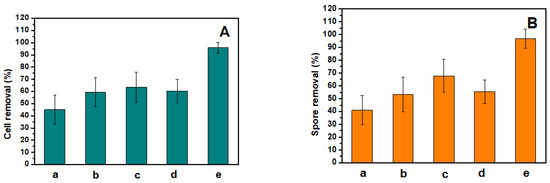
Figure 4.
The removal percentages of (a) the Si wafer substrate, (b) the PDOPA-POEGMA-modified wafer substrate, (c) the PDOPA–PSPMA-modified wafer substrate, (d) the PDOPA-PMETAC-modified wafer substrate, and (e) the PDOPA-PTMETH-modified wafer substrate after the settlement of (A) Chlorella cells and (B) Ulva zoospores.
These findings are corroborated by the images in Figure 5 and Figure 6. The Chlorella cell and Ulva zoospore settlement densities on the DOPA–POEGMA-modified, DOPA–PMETAC-modified, DOPA–PSPMA-modified, and DOPA–PTMETH-modified substrate surfaces were much lower than those on the control Si wafer substrate surfaces (as shown in Figure 4A and Figure 5A). There were few single cells and zoospores on the PDOPA–PSPMA-modified substrate, and there were much more cell and zoospore colonies on the Si wafer substrate surface.
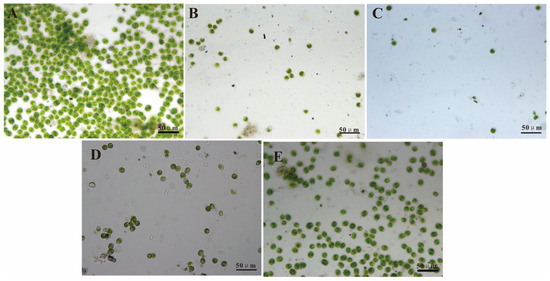
Figure 5.
Images of Chlorella cells settled for 3 h on (A) the Si wafer substrate, (B) the PDOPA–POEGMA-modified substrate, (C) the PDOPA–PSPMA-modified substrate, (D) the PDOPA–PMETAC-modified substrate, and (E) the PDOPA–PTMETH-modified substrate.
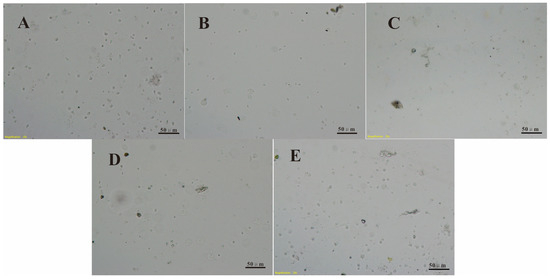
Figure 6.
Images of Ulva zoospores settled for 3 h on (A) the Si wafer substrate, (B) the PDOPA–POEGMA-modified substrate, (C) the PDOPA–PSPMA-modified substrate, (D) the PDOPA–PMETAC-modified substrate, and (E) the PDOPA–PTMETH-modified substrate.
The PDOPA–PSPMA-modified Si wafer substrate was scratched by tweezers to create a gap of about 20 μm without coating. After 3 h of Chlorella cell settlement, as shown in the images above, the only two colonies of Chlorella cells were both in the gap without the copolymer coating, while few individual cells could be observed on the coating area. The Chlorella cells have no flagella; they can only settle on surface through the action of gravity, adhere on the substrates via protein, and then continuously reproduce through cell division [6]. The schematic on the right of Figure 7 shows the reasoning behind the image on the left of the figure; if Chlorella cells settled in the gap area without PDOPA–PSPMA modification, they could easily adhere to the surface and reproduce through cell division along the gap to form cell colonies, while if Chlorella cells settled in the area modified with PDOPA–PSPMA, adhesion would be inhibited by the copolymer coating, meaning that they also could not reproduce.
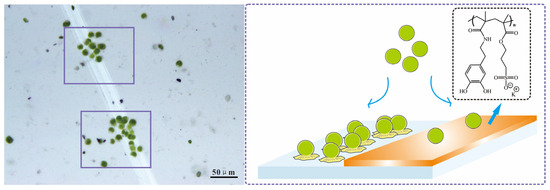
Figure 7.
Image and schematic representation of Chlorella cells settled on a surface prone to biofouling and a substrate surface modified with the PDOPA–PSPMA coating.
Representative SEM images of Chlorella cells settled on a surface prone to biofouling and a surface modified by the PDOPA–PSPMA coating are shown in Figure 8. In Figure 8A, the Chlorella cells have adhered onto the surface via the secretion of an adhesive glycoprotein [41] and created cell colonies; the cells were flat, and there were circles of blot around them. Figure 8B shows Chlorella cells on the PDOPA–PSPMA-modified substrate that have been separated from the substrate surface; no trace of a glycoprotein was present on the substrate surface. All these phenomena prove that these copolymer coatings, especially PDOPA–PSPMA, were very effective in inhibiting cell settlement. As shown in Figure 8C, after water scouring, there were few Chlorella cells on and clear glycoprotein traces on the PDOPA–PTMETH-modified substrate, which also proved that it had a significant fouling-release property.
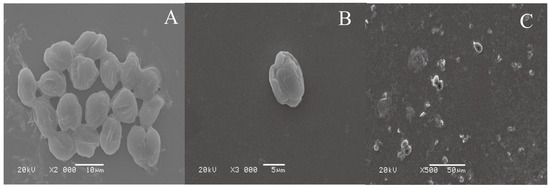
Figure 8.
SEM images of Chlorella cells settled on (A) the Si wafer substrate, (B) the PDOPA–PSPMA-modified Si wafer substrate, and (C) the PDOPA–PTMETH-modified Si wafer substrate after water scouring (impact pressure of 35 kPa).
4. Conclusions
For this paper, four kinds of copolymers containing catechol anchoring groups were prepared for preparing coatings with antibiofouling and fouling-release properties via a one-step procedure method. The catechol anchoring groups provided strong immobilization for the copolymer coatings onto the substrate surfaces, while the hydrophilic groups, cationic surfactant functional groups, anionic surfactant functional groups, and hydrophobic groups enhanced the antifouling and fouling-release properties. These copolymer coatings, especially the PDOPA–PSPMA coating, could be effective in inhibiting the settlement and adhesion of biofouling cells/spores; the PDOPA–PTMETH coating significantly enhanced the fouling-release property of the material’s substrate surface. These copolymer coatings can be used to modify the surfaces of various material substrates, as they contain general marine artificial materials such as metals and plastics. In conclusion, this new approach for easily producing materials with antifouling and fouling-release properties will be a very useful for practical applications.
Author Contributions
Conceptualization, F.W. and W.Y.; methodology, F.W. and W.Y.; data curation, F.W., W.Y., R.T., L.Z. and C.F.; funding acquisition, F.W. and C.F.; formal analysis, F.W., W.Y., R.T., L.Z. and C.F.; investigation, F.W., W.Y., R.T. and L.Z.; validation, R.T. and L.Z.; writing—original draft, F.W., W.Y. and C.F.; writing—review and editing, F.W., W.Y. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of China (Grant No. 51909128).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magin, C.M.; Finlay, J.A.; Clay, G.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Antifouling Performance of Cross-linked Hydrogels: Refinement of an Attachment Model. Biomacromolecules 2011, 12, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I.S.; Schiffman, J.D. Current and Emerging Approaches to Engineer Antibacterial and Antifouling Electrospun Nanofibers. Materials 2018, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, P.A.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. J. Mar. Sci. Eng. 2020, 8, 27. [Google Scholar] [CrossRef]
- Alzieu, C. Impact of tributyltin on marine invertebrates. Ecotoxicology 2000, 9, 71–76. [Google Scholar] [CrossRef]
- de Nys, R.; Steinberg, P.D. Linking marine biology and biotechnology. Curr. Opin. Biotechnol. 2002, 13, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Marechal, J.P.; Hellio, C.; Sebire, M.; Clare, A.S. Settlement behaviour of marine invertebrate larvae measured by EthoVision 3.0. Biofouling 2004, 20, 211–217. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A.; Pickett-Heaps, J.D.; Wetherbee, R. Primary Adhesion of Enteromorpha (Chlorophyta, Ulvales) Propagules: Quantitative Settlement Studies and Video Microscopy. J. Phycol. 1997, 33, 938–947. [Google Scholar] [CrossRef]
- Zhao, W.W.; Wu, Z.Q.; Liu, Y.M.; Dai, P.; Hai, G.J.; Liu, F.; Shang, Y.; Cao, Z.Y.; Yang, W.F. Research Progress of Natural Products and Their Derivatives in Marine Antifouling. Materials 2023, 16, 6190. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, W.C.; Zhang, L.Q.; Vinokurov, V.; Stavitskaya, A.; Lvov, Y. Development of Marine Antifouling Epoxy Coating Enhanced with Clay Nanotubes. Materials 2019, 12, 4195. [Google Scholar] [CrossRef]
- Sun, X.H.; Li, Q.; Guo, Z.R.; Wang, K.; Gui, T.J.; Gao, C.L. Study on the Core-Shell Reversion of PSBMA-b-PLMA Nanoparticles for the Fabrication of Antifouling Coatings. ACS Appl. Mater. Interfaces 2019, 11, 21323–21333. [Google Scholar] [CrossRef]
- Feng, S.J.; Wang, Q.; Gao, Y.; Huang, Y.G.; Qing, F.L. Synthesis and Characterization of a Novel Amphiphilic Copolymer Capable as Anti-Biofouling Coating Material. J. Appl. Polym. Sci. 2009, 114, 2071–2078. [Google Scholar] [CrossRef]
- Weinman, C.J.; Finlay, J.A.; Park, D.; Paik, M.Y.; Krishnan, S.; Sundaram, H.S.; Dimitriou, M.; Sohn, K.E.; Callow, M.E.; Callow, J.A.; et al. ABC Triblock Surface Active Block Copolymer with Grafted Ethoxylated Fluoroalkyl Amphiphilic Side Chains for Marine Antifouling/Fouling-Release Applications. Langmuir 2009, 25, 12266–12274. [Google Scholar] [CrossRef] [PubMed]
- Dinu, C.Z.; Zhu, G.; Bale, S.S.; Anand, G.; Reeder, P.J.; Sanford, K.; Whited, G.; Kane, R.S.; Dordick, J.S. Enzyme-Based Nanoscale Composites for Use as Active Decontamination Surfaces. Adv. Funct. Mater. 2010, 20, 392–398. [Google Scholar] [CrossRef]
- Romeu, M.J.; Mergulhao, F. Development of Antifouling Strategies for Marine Applications. Microorganisms 2023, 11, 34. [Google Scholar] [CrossRef]
- Huang, J.Y.; Koepsel, R.R.; Murata, H.; Wu, W.; Lee, S.B.; Kowalewski, T.; Russell, A.J.; Matyjaszewski, K. Nonleaching antibacterial glass surfaces via “Grafting Onto”: The effect of the number of quaternary ammonium groups on biocidal activity. Langmuir 2008, 24, 6785–6795. [Google Scholar] [CrossRef]
- Lee, S.B.; Koepsel, R.R.; Morley, S.W.; Matyjaszewski, K.; Sun, Y.J.; Russell, A.J. Permanent, nonleaching antibacterial surfaces. 1. Synthesis by atom transfer radical polymerization. Biomacromolecules 2004, 5, 877–882. [Google Scholar] [CrossRef]
- Hou, A.L.; Wang, S.Y.; Lin, W.P.; Kuo, W.H.; Wang, T.J.; Wang, M.J. Surface Antifouling Modification on Polyethylene Filtration Membranes by Plasma Polymerization. Materials 2020, 13, 13. [Google Scholar] [CrossRef]
- Ma, H.W.; Wells, M.; Beebe, T.P.; Chilkoti, A. Surface-initiated atom transfer radical polymerization of oligo(ethylene glycol) methyl methacrylate from a mixed self-assembled monolayer on gold. Adv. Funct. Mater. 2006, 16, 640–648. [Google Scholar] [CrossRef]
- Zhang, Z.; Finlay, J.A.; Wang, L.F.; Gao, Y.; Callow, J.A.; Callow, M.E.; Jiang, S.Y. Polysulfobetaine-Grafted Surfaces as Environmentally Benign Ultralow Fouling Marine Coatings. Langmuir 2009, 25, 13516–13521. [Google Scholar] [CrossRef]
- Zhang, Z.; Chao, T.; Chen, S.F.; Jiang, S.Y. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Cao, Z.Q. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ding, X.M.; Sun, J. Zwitterionic Polypeptoids: A Promising Class of Antifouling Bioinspired Materials. Materials 2022, 15, 4498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Shen, T.; Ling, J. Synthesis and properties of polypeptoid-containing block copolymers: A review. J. Polym. Sci. 2021, 59, 2946–2958. [Google Scholar] [CrossRef]
- Liu, H.W.; Liu, L.; Jiang, X.F.; Fan, J.; Peng, W.; Liu, P.M.; Yang, T.; Chen, H.L.; Jiang, W.; Yin, G.Y.; et al. Rational design of a zwitterionic-phosphonic copolymer for the surface antifouling modification of multiple biomedical metals. J. Mat. Chem. B 2019, 7, 4055–4065. [Google Scholar] [CrossRef]
- Li, M.Y.; Nan, L.Y.; Zhang, B.X.; Kong, J.J.; Wang, Y.F.; Ba, M. Polyether-Thiourea-Siloxane Copolymer Based on H-Bonding Interaction for Marine Antifouling. Molecules 2023, 28, 16. [Google Scholar] [CrossRef]
- Guo, H.S.; Liu, X.M.; Zhao, W.Q.; Xie, C.H.; Zhu, Y.N.; Wen, C.Y.; Li, Q.S.; Sui, X.J.; Yang, J.; Zhang, L. A polyvinylpyrrolidone-based surface-active copolymer for an effective marine antifouling coating. Prog. Org. Coat. 2021, 150, 105975. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.Y.; Chang, Y.X.; Zhang, Y.H.; Peng, H.; Whittaker, A.K.; Fu, C.K. Antifouling and Antibacterial Surfaces Grafted with Sulfur-Containing Copolymers. ACS Appl. Mater. Interfaces 2022, 14, 41400–41411. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W.M. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Xi, P.X.; Cheng, K.; Sun, X.L.; Zeng, Z.Z.; Sun, S.H. Magnetic Fe3O4 nanoparticles coupled with a fluorescent Eu complex for dual imaging applications. Chem. Commun. 2012, 48, 2952–2954. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wu, J.F.; Avery, C.W.; Mizutani, M.; Jiang, X.M.; Kamigaito, M.; Chen, Z.; Xi, C.W.; Kuroda, K. Immobilization of Amphiphilic Polycations by Catechol Functionality for Antimicrobial Coatings. Langmuir 2011, 27, 4010–4019. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, K.D.; Pyo, K.B.; Park, S.Y.; Lee, H. Catechol-Grafted Poly(ethylene glycol) for PEGylation on Versatile Substrates. Langmuir 2010, 26, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Xue, H.; Gao, C.L.; Zhang, F.B.; Jiang, S.Y. Nonfouling Polyampholytes from an Ion-Pair Comonomer with Biomimetic Adhesive Groups. Macromolecules 2010, 43, 14–16. [Google Scholar] [CrossRef]
- Zar, J.H.; Hall, P. Biostatistical Analysis, 4th ed.; Pearson College Div: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Xiong, C.X.; Xiong, W.J.; Mu, Y.B.; Pei, D.F.; Wan, X.B. Mussel-inspired polymeric coatings with the antifouling efficacy controlled by topologies. J. Mater. Chem. B 2022, 10, 9295–9304. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Park, J.; Choi, W.; Hong, J.; Lee, D.W.; Kim, B.S. Mussel-Inspired Multiloop Polyethers for Antifouling Surfaces. Biomacromolecules 2021, 22, 5173–5184. [Google Scholar] [CrossRef]
- Sathyan, A.; Kurtz, I.; Rathore, P.; Emrick, T.; Schiffman, J.D. Using Catechol and Zwitterion-Functionalized Copolymers to Prevent Dental Bacterial Adhesion. ACS Appl. Bio Mater. 2023, 6, 2905–2915. [Google Scholar] [CrossRef]
- Yeon, D.K.; Ko, S.; Jeong, S.; Hong, S.P.; Kang, S.M.; Cho, W.K. Oxidation-Mediated, Zwitterionic Polydopamine Coatings for Marine Antifouling Applications. Langmuir 2019, 35, 1227–1234. [Google Scholar] [CrossRef]
- Wan, F.; Pei, X.W.; Yu, B.; Ye, Q.; Zhou, F.; Xue, Q.J. Grafting Polymer Brushes on Biomimetic Structural Surfaces for Anti-Algae Fouling and Foul Release. ACS Appl. Mater. Interfaces 2012, 4, 4557–4565. [Google Scholar] [CrossRef]
- Callow, J.A.; Osborne, M.P.; Callow, M.E.; Baker, F.; Donald, A.M. Use of environmental scanning electron microscopy to image the spore adhesive of the marine alga Enteromorpha in its natural hydrated state. Colloid Surf. B Biointerfaces 2003, 27, 315–321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).