Poly(glycerol itaconate) Crosslinking via the aza-Michael Reaction—A Preliminary Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. NMR
2.2. FTIR
2.3. DSC Analysis
2.4. TG Analysis
2.5. Acid Number (AN)
- V—the volume of 1 M NaOH solution used to titrate the sample [cm3];
- V0—the volume of 1 M NaOH used for blank titration [cm3];
- MNaOH—the titer of the solution for the titration (1 M);
- 56.1—the molar mass of KOH [g/mol];
- m—the weight of the sample [g].
2.6. Ester Number (EN)
- V—the volume of 1 M HCl solution used to titrate the sample [cm3];
- V0—the volume of 1 M HCl used for blank titration [cm3];
- MHCl—the titer of the solution for the titration (1 M);
- 56.1—the molar mass of KOH [g/mol];
- m—the weight of the sample [g].
2.7. Esterification Degree by Titration (EDtit)
- EN—ester number;
- AN—acid number.
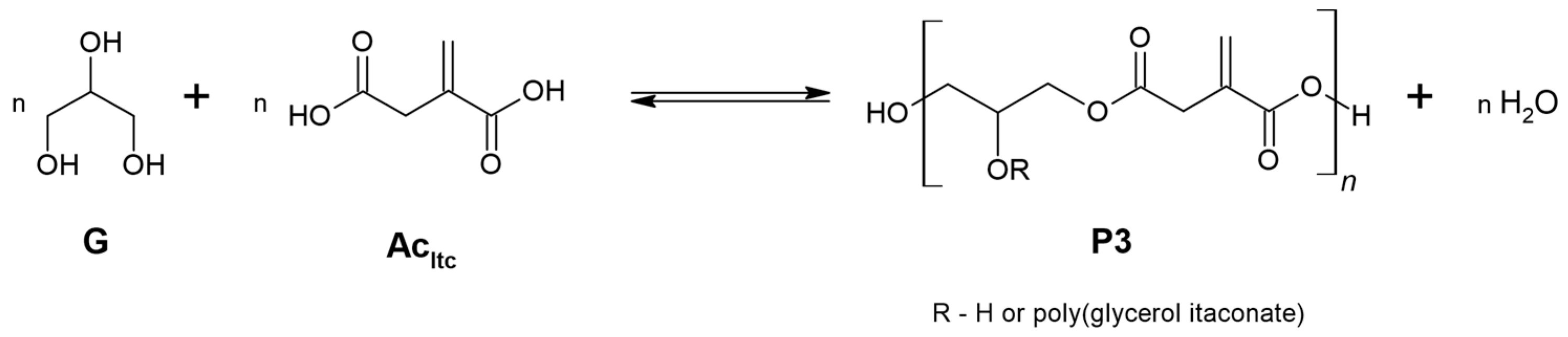
2.8. PGItc Syntheses Procedure
2.9. Aza-Michael Addition Procedure
- 1:2 (proton of the amine group:C=C bond) means that eight times less amine than PGItc was used molarly for the reaction, making ½ diamine hydrogen atom per C=C bond of PGItc;
- 1:1 (proton of the amine group:C=C bond) means that four times less amine than PGItc was used molarly for the reaction, making one diamine hydrogen atom per C=C bond of PGItc;
- 2:1 (proton of the amine group:C=C bond) means that twice as much amine as PGItc was used molarly for the reaction, making two diamine hydrogen atoms per C=C bond of PGItc;
- 4:1 (proton of the amine group:C=C bond) means that molar as much amine as PGItc was used in the reaction, making four diamine hydrogen atoms per C=C bond of PGItc; and
- 8:1 (proton of the amine group:C=C bond) means that twice as much amine as PGItc was used molarly for the reaction, so there are 8 diamine hydrogen atoms per C=C bond of PGItc.
2.10. PGItc End Groups Protection with Tert-Butanol (t-BuOH)
3. Results
3.1. PGItc Syntheses
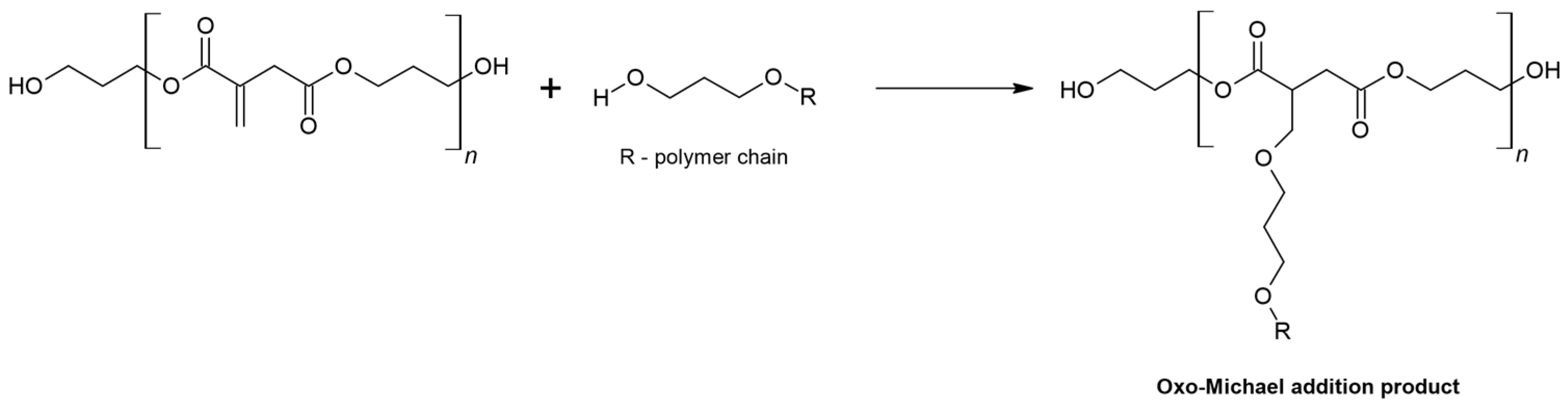
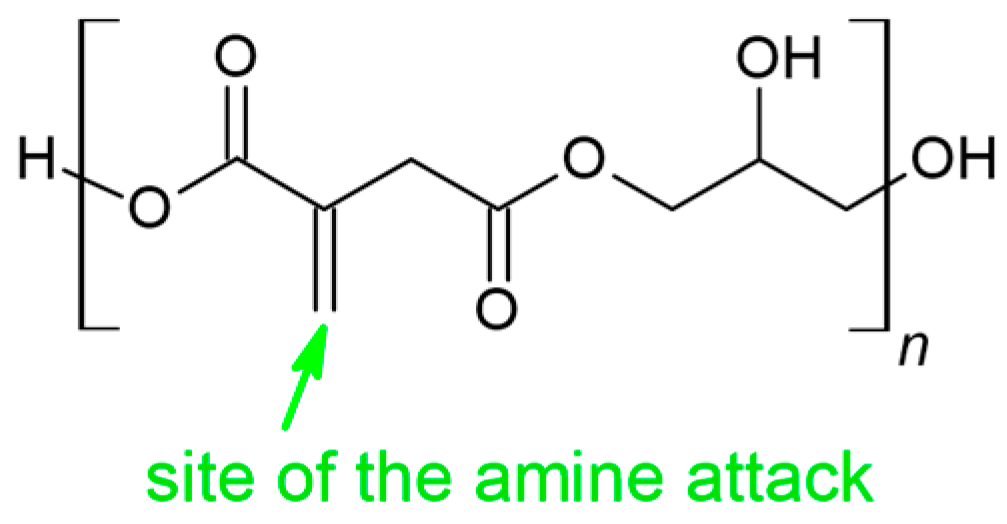
3.2. Aza-Michael Reactions
- The crosslinking product should have a dense consistency (high viscosity)—the higher the viscosity, the better the addition product is crosslinked.
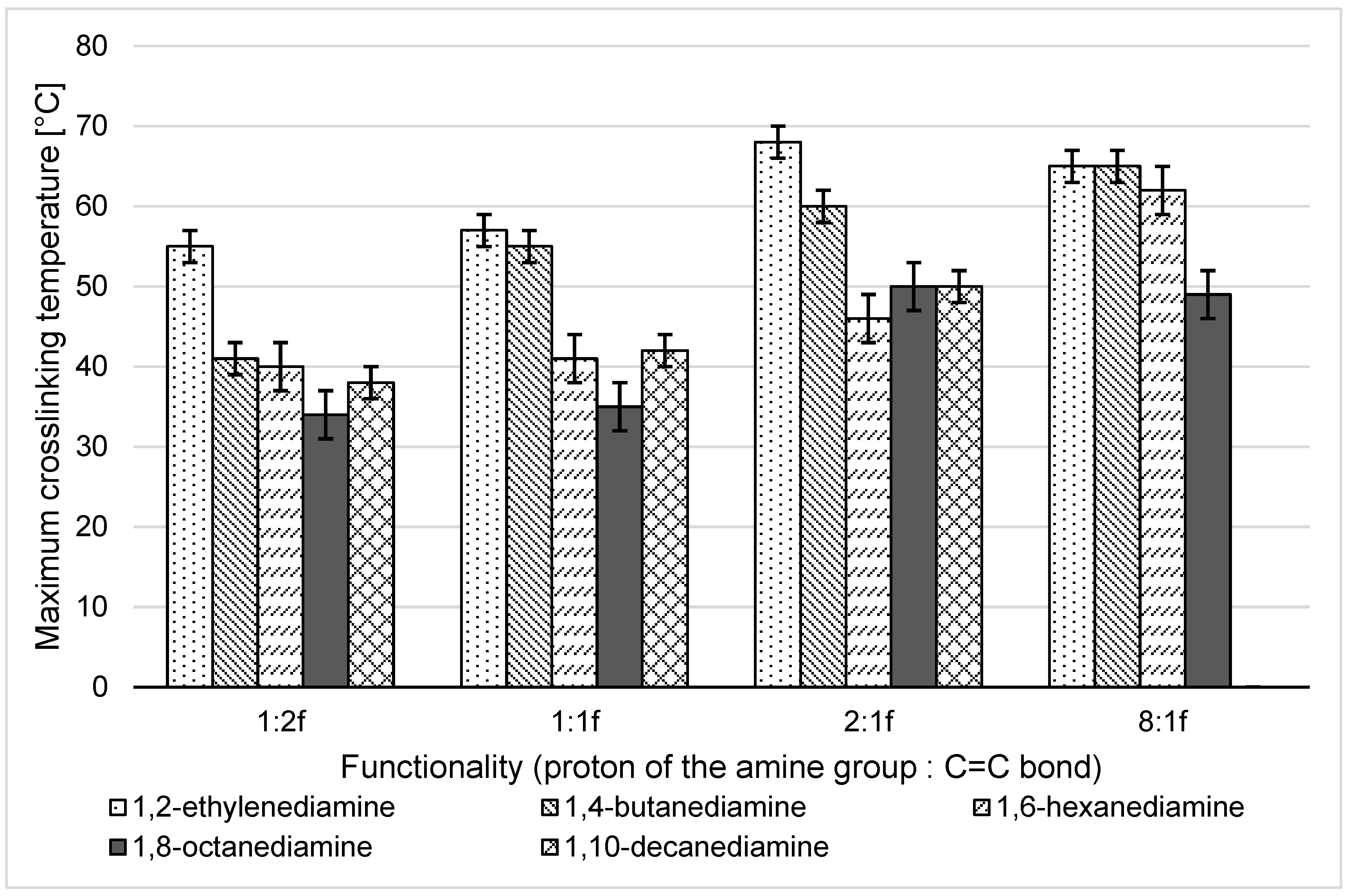
- The crosslinking temperature should not exceed the temperature of 50 °C to use the addition in tissue engineering—the higher the temperature of the addition reaction, the more likely cell death is to occur.
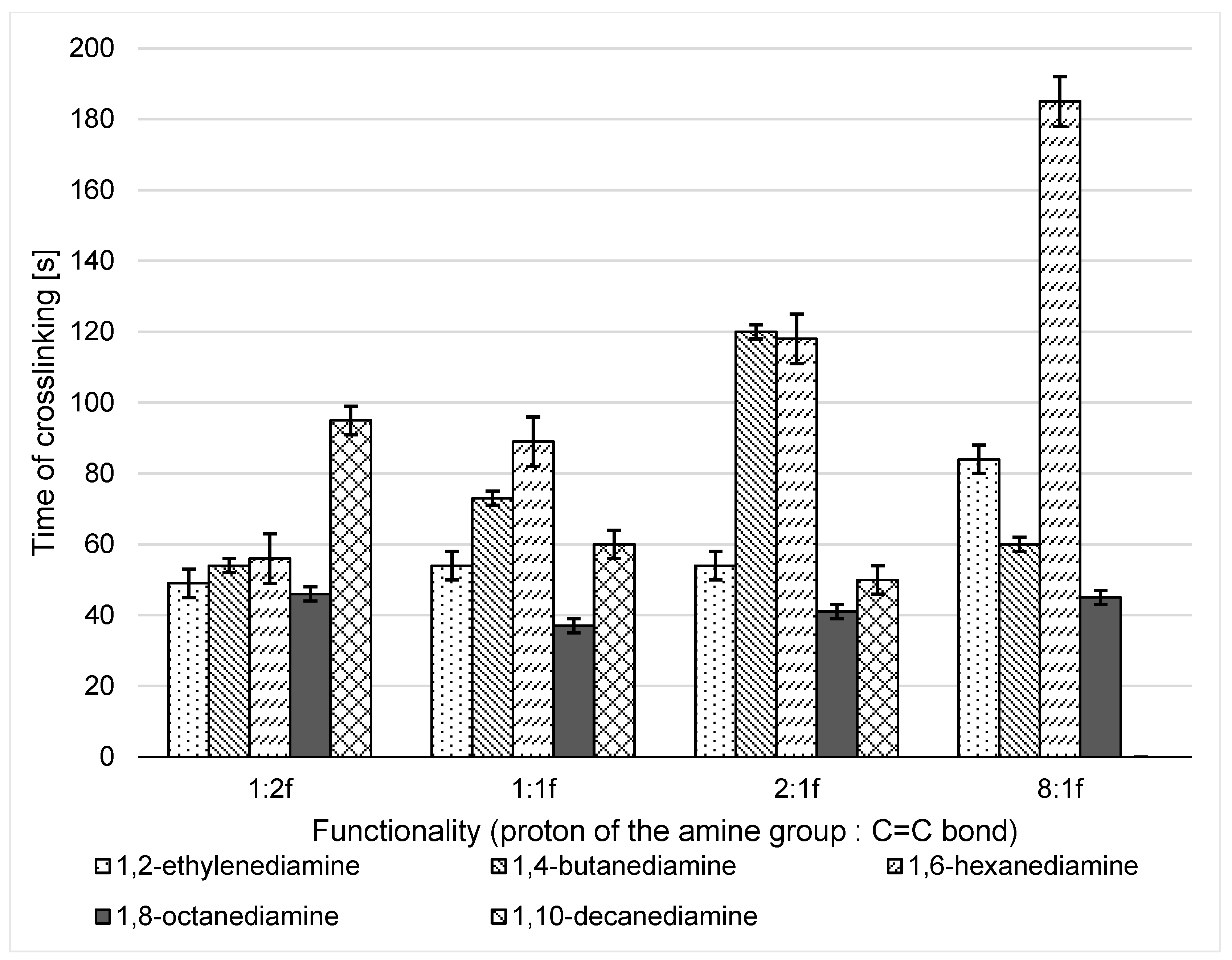
- Crosslinking time should be as short as possible—to reduce the time of discomfort for the potential patient.
3.2.1. Analysis of Temperature and Time of aza-Michael Addition and the Viscosity of the Adducts
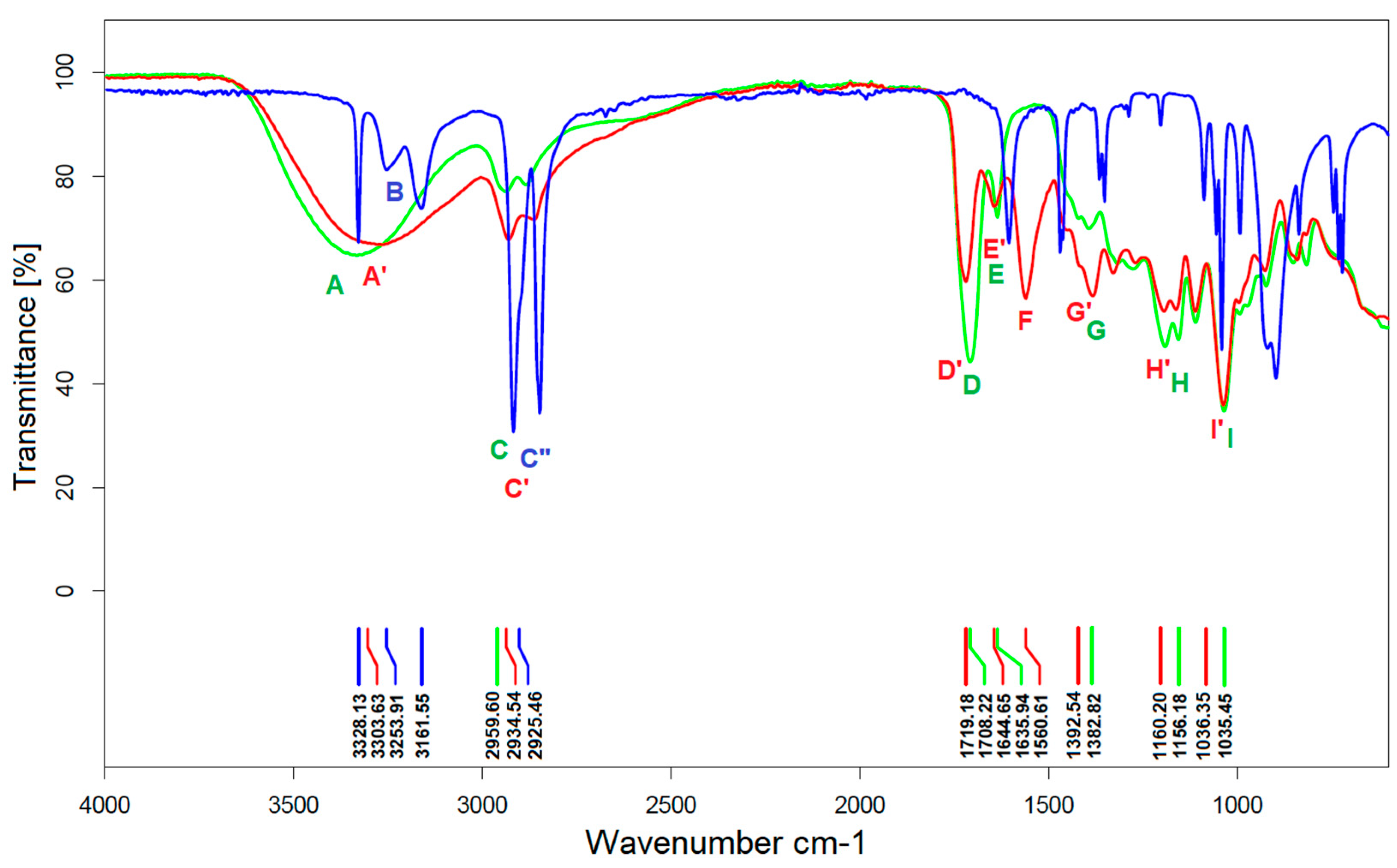
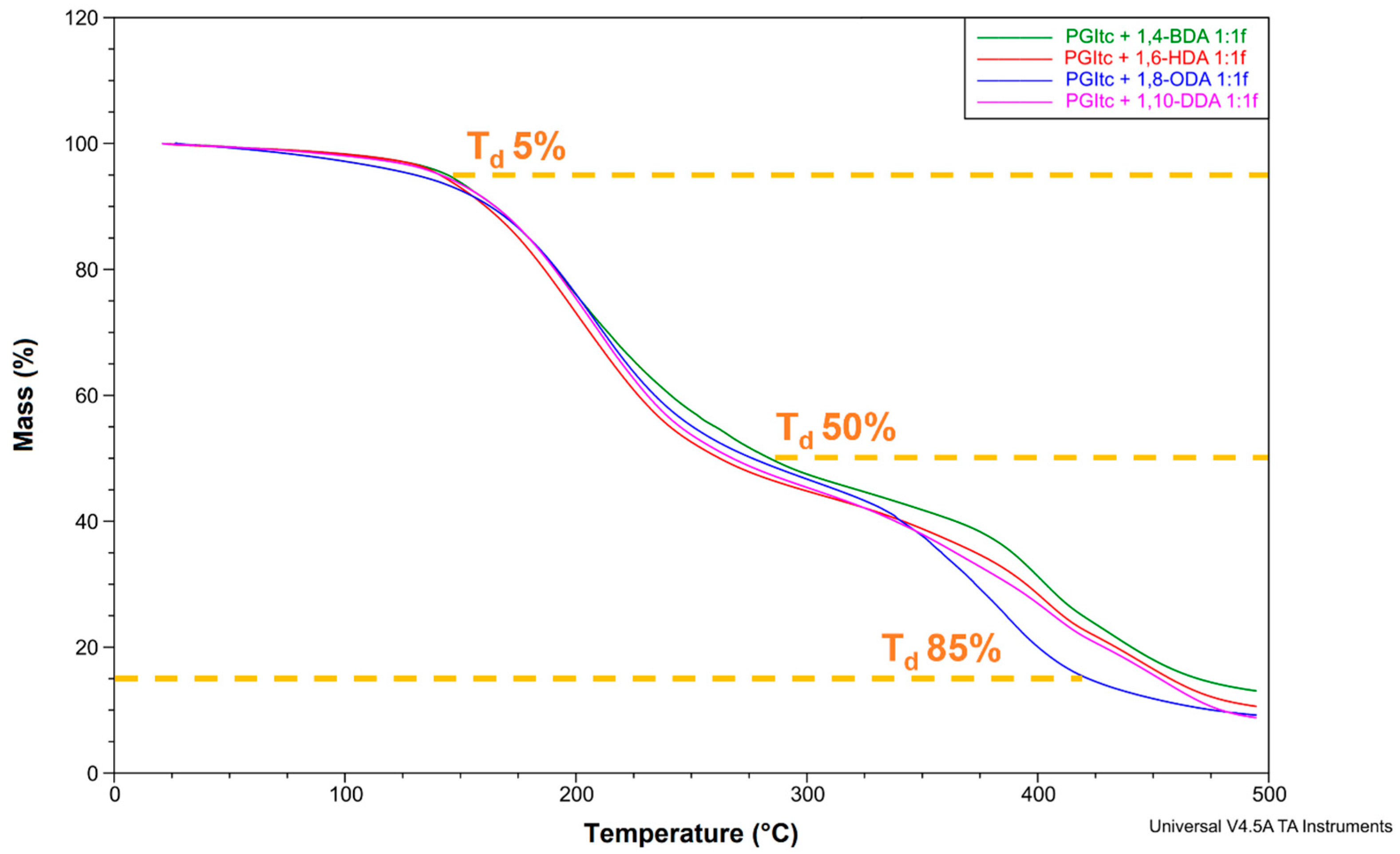
3.2.2. FTIR Analysis
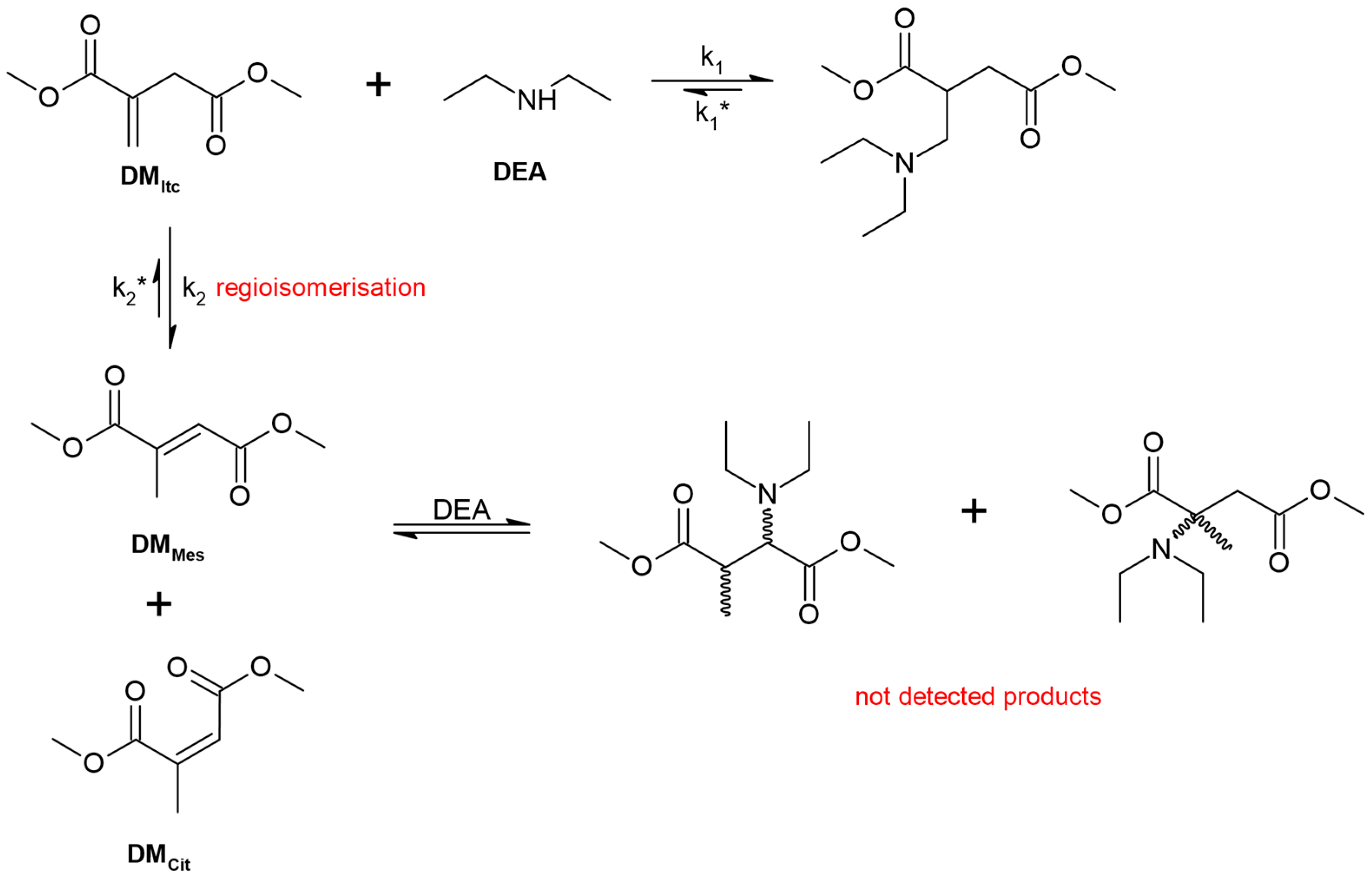
- On the spectrum of the adduct, the stretching vibration of the N-H bonds of the free amine group is invisible, indicating that the amine has completely reacted with the C=C bonds of PGItc.
- The shift to larger values of the wavenumbers of D’ vibrations relative to D indicates an aza-Michael addition.
- A partial rearrangement of the C=C double bonds occurs. The weakening of the E’ vibration of the addition product compared to the E band of PGItc can be seen. However, due to the minor differences in the intensity of these vibrations, it isn’t easy to compare them reliably.
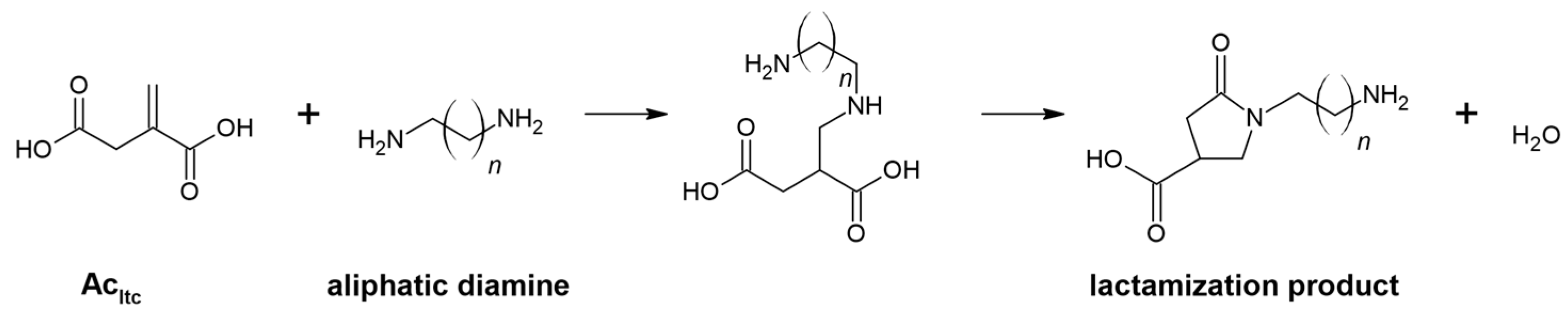
- The presence of an F vibration in the adduct indicates that an undesired reaction occurred in the reaction system between the end group (-COOH) of the polymer and the amine group of the diamine. The crosslinking products were soluble in water. In addition, the crosslinking reactions occurred without an external heat source, so there was only a small possibility for a lactamization reaction.
3.2.3. NMR Analysis
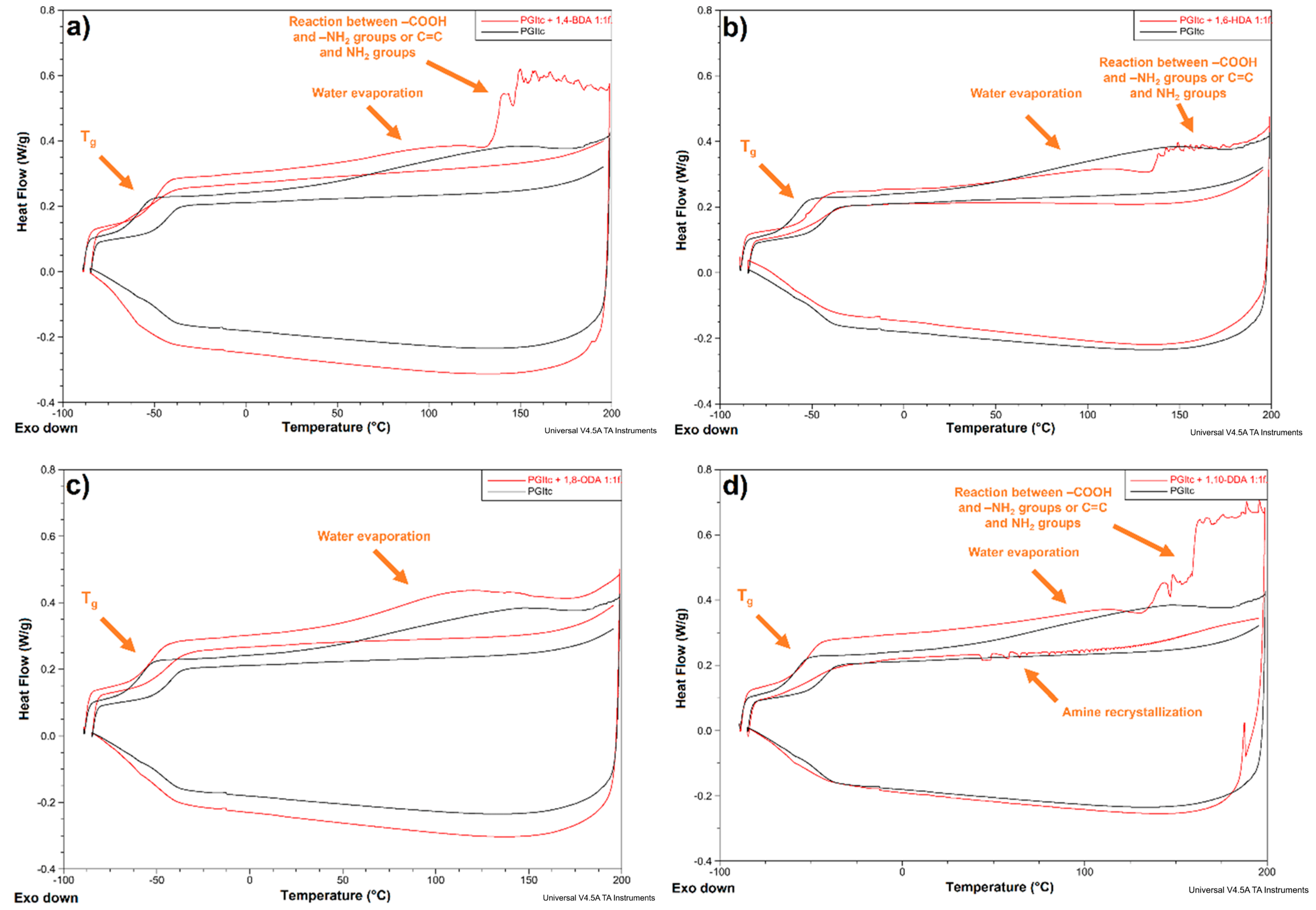
3.2.4. Differential Scanning Calorimetry Analysis
3.2.5. Thermogravimetric Analysis
3.3. PGItc End-Group Protection with Tert-Butanol
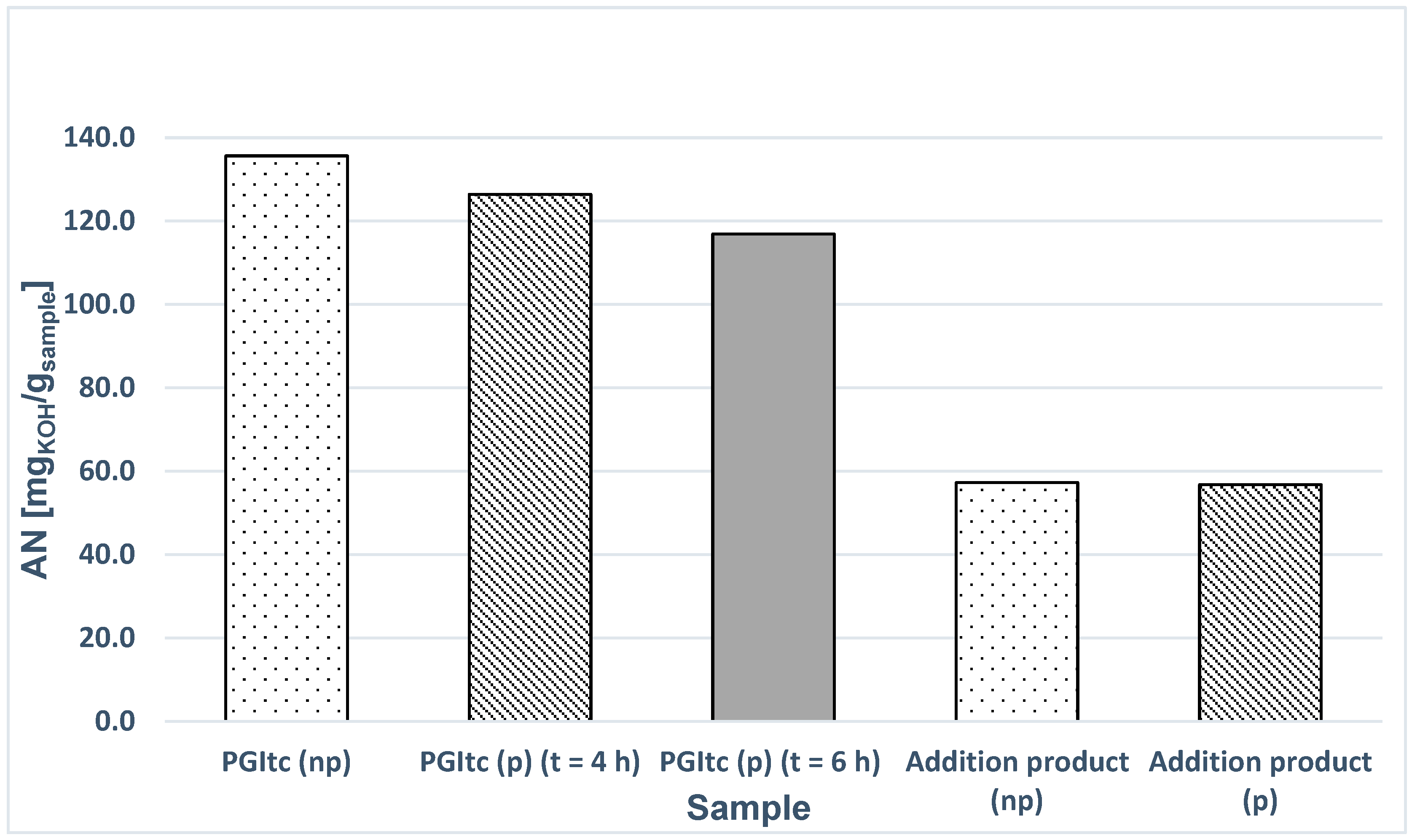
- Attempts to secure the PGItc end groups were unsuccessful. The differences between the AN values of the unprotected and protected samples are insignificant. It indicates the lack of reaction between the PGItc end group and t-BuOH.
- The increase in the synthesis time of PGItc has little effect on the obtained AN value.
- Performing the crosslinking reaction of protected PGItc with 1,8-ODA does not result in significant changes in the AN value compared to the crosslinked unprotected sample.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Willke, T.; Vorlop, K.D. Biotechnological Production of Itaconic Acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.C.; Magalhães, A.I.; Soccol, C.R. Biobased Itaconic Acid Market and Research Trends-Is It Really a Promising chemical? Chim. Oggi/Chem. Today 2018, 36, 56–58. [Google Scholar]
- Kolankowski, K.; Gadomska-Gajadhur, A.; Wrzecionek, M.; Ruśkowski, P. Mathematically Described Model of Poly(Glycerol Maleate) Cross-Linking Process Using Triethylenetetramine Addition. Polym. Adv. Technol. 2022, 33, 1298–1306. [Google Scholar] [CrossRef]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J. Synthesis of Unsaturated Polyester Resins from Various Bio-Derived Platform Molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef] [PubMed]
- Daglar, O.; Ozcan, B.; Gunay, U.S.; Hizal, G.; Tunca, U.; Durmaz, H. Extremely Rapid Postfunctionalization of Maleate and Fumarate Main Chain Polyesters in the Presence of TBD. Polymer 2019, 182, 121844. [Google Scholar] [CrossRef]
- Farmer, T.J.; Macquarrie, D.J.; Comerford, J.W.; Pellis, A.; Clark, J.H. Insights into Post-Polymerisation Modification of Bio-Based Unsaturated Itaconate and Fumarate Polyesters via Aza-Michael Addition: Understanding the Effects of C=C Isomerisation. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Genest, A.; Portinha, D.; Fleury, E.; Ganachaud, F. The Aza-Michael Reaction as an Alternative Strategy to Generate Advanced Silicon-Based (Macro)Molecules and Materials. Prog. Polym. Sci. 2017, 72, 61–110. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Y.; Lei, Y.; Zhang, L.; Zhou, W.Y.; Rabie, A.B.M.; Zhao, J. Biobased Poly(Propylene Sebacate) as Shape Memory Polymer with Tunable Switching Temperature for Potential Biomedical Applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Zhu, J.; Liu, X. Synthesis of Bio-Based Unsaturated Polyester Resins and Their Application in Waterborne Uv-Curable Coatings. Prog. Org. Coat. 2015, 87, 197–203. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Han, L.; Zhang, L.; Zhu, J.; Liu, X. Polyesters Derived from Itaconic Acid for the Properties and Bio-Based Content Enhancement of Soybean Oil-Based Thermosets. Green Chem. 2015, 17, 2383–2392. [Google Scholar] [CrossRef]
- Pellis, A.; Hanson, P.A.; Comerford, J.W.; Clark, J.H.; Farmer, T.J. Enzymatic Synthesis of Unsaturated Polyesters: Functionalization and Reversibility of the Aza-Michael Addition of Pendants. Polym. Chem. 2019, 10, 843–851. [Google Scholar] [CrossRef]
- Shou, Y.; Campbell, S.B.; Lam, A.; Lausch, A.J.; Santerre, J.P.; Radisic, M.; Davenport Huyer, L. Toward Renewable and Functional Biomedical Polymers with Tunable Degradation Rates Based on Itaconic Acid and 1,8-Octanediol. ACS Appl. Polym. Mater. 2021, 3, 1943–1955. [Google Scholar] [CrossRef]
- Sakai, M. Studies of the Isomerization of Unsaturated Carboxylic Acids. II. The Thermal Rearrangement of Citraconic Acid to Itaconic Acid in Aqueous Solutions. Bull. Chem. Soc. Jpn. 1976, 49, 219–223. [Google Scholar] [CrossRef]
- Teramoto, N.; Ozeki, M.; Fujiwara, I.; Shibata, M. Crosslinking and Biodegradation of Poly (Butylene Succinate) Prepolymers Containing Itaconic or Maleic Acid Units in the Main Chain. Appl. Polym. 2004, 95, 1473–1480. [Google Scholar] [CrossRef]
- Popovic, A.R.; Nikolic, V.B.; Spasojevic, P.M. Simple One-Pot Synthesis of Fully Bio-Based Unsaturated Polyester Resins Based on Itaconic Acid. Biomacromolecules 2017, 18, 3881–3891. [Google Scholar] [CrossRef]
- Takasu, A.; Ito, M.; Inai, Y.; Hirabayashi, T.; Nishimura, Y. Synthesis of Biodegradable Polyesters by Ring-Opening Copolymerization of Cyclic Anhydrides Containing a Double Bond with 1, 2-Epoxybutane and One-Pot Preparation of the Itaconic Acid-Based Polymeric Network. Polymer 1999, 31, 961–969. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly(Glycerol-Co-Diacids) Polyesters: From Glycerol Biorefinery to Sustainable Engineering Applications, A Review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693. [Google Scholar] [CrossRef]
- Agach, M.; Delbaere, S.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V. Characterization, Stability and Ecotoxic Properties of Readily Biodegradable Branched Oligoesters Based on Bio-Sourced Succinic Acid and Glycerol. Polym. Degrad. Stab. 2012, 97, 1956–1963. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Smith, P.B. Rational Synthesis of Hyperbranched Poly(Ester)S. Ind. Eng. Chem. Res. 2017, 56, 1661–1670. [Google Scholar] [CrossRef]
- Schoon, I.; Kluge, M.; Eschig, S.; Robert, T. Catalyst Influence on Undesired Side Reactions in the Polycondensation of Fully Bio-Based Polyester Itaconates. Polymers 2017, 9, 693. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J. Hyperbranched Polymers from A2 + B3 Strategy: Recent Advances in Description and Control of Fine Topology. Polym. Chem. 2016, 7, 3643–3663. [Google Scholar] [CrossRef]
- Lang, K.; Sánchez-Leija, R.J.; Gross, R.A.; Linhardt, R.J. Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers 2020, 12, 2969. [Google Scholar] [CrossRef] [PubMed]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels-Alder Reaction in Polymer Chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef] [PubMed]
- Korytkowska-Walach, A.; Smiga-Matuszowicz, M.; Lukaszczyk, J. Polymeric In Situ Forming Systems for Biomedical Applications. Part II. Injectable Hydrogel Systems. Polimery 2015, 60, 435–447. [Google Scholar] [CrossRef]
- Luleburgaz, S.; Hizal, G.; Durmaz, H.; Tunca, U. Modification of Electron Deficient Polyester via Huisgen/Passerini Sequence. Polymer 2017, 127, 45–51. [Google Scholar] [CrossRef]
- Kato, J.; Seo, A.; Kiso, K.; Kudo, K.; Shiraishi, S. Synthesis of 2,8-Dioxaspiro [4.5]Decane-1,3,7,9-Tetrone and the Reactions with Amines. Bull. Chem. Soc. Jpn. 1999, 72, 1075–1081. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.F.; Chan, S.H.; Chan, A.S.C.; Kwong, F.Y. Catalyst-Free Aza-Michael Addition of Azole to β,γ-Unsaturated- α-Keto Ester: An Efficient Access to C-N Bond Formation. Tetrahedron Lett. 2012, 53, 2887–2889. [Google Scholar] [CrossRef]
- Ouhichi, R.; Sacha, P.; Bougarech, A.; Abid, S.; Abid, M.; Robert, T. First Example of Unsaturated Poly(Ester Amide)s Derived From Itaconic Acid and Their Application as Bio-Based UV-Curing Polymers. Appl. Sci. 2020, 10, 2163. [Google Scholar] [CrossRef]
- Moore, O.B.; Hanson, P.A.; Comerford, J.W.; Pellis, A.; Farmer, T.J. Improving the Post-Polymerization Modification of Bio-Based Itaconate Unsaturated Polyesters: Catalyzing Aza-Michael Additions with Reusable Iodine on Acidic Alumina. Front. Chem. 2019, 7, 501. [Google Scholar] [CrossRef]
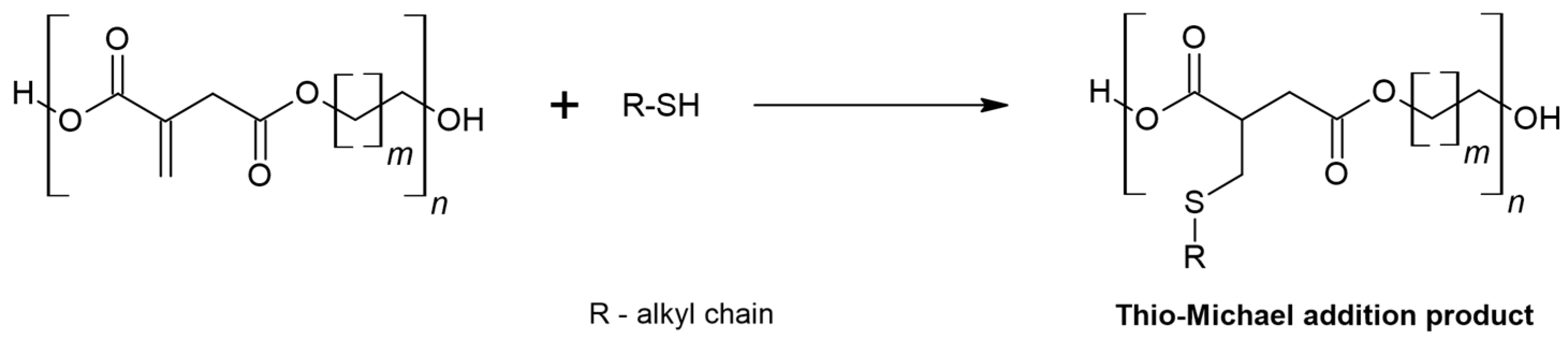
- Gunay, U.S.; Cetin, M.; Daglar, O.; Hizal, G.; Tunca, U.; Durmaz, H. Ultrafast and Efficient Aza- and Thiol-Michael Reactions on a Polyester Scaffold with Internal Electron Deficient Triple Bonds. Polym. Chem. 2018, 9, 3037–3054. [Google Scholar] [CrossRef]
- Farmer, T.J.; Comerford, J.W.; Pellis, A.; Robert, T. Post-Polymerization Modification of Bio-Based Polymers: Maximizing the High Functionality of Polymers Derived from Biomass. Polym. Int. 2018, 67, 775–789. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Z.; Yang, P.; Xu, Y.; Zhang, W.; Liu, B.; Lu, M.; Chang, H.; Ding, T.; Xu, H. Thio-Michael Addition of α,β-Unsaturated Amides Catalyzed by Nmm-Based Ionic Liquids. RSC Adv. 2017, 7, 43104–43113. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Kolankowski, K.; Miętus, M.; Ruśkowski, P.; Gadomska-Gajadhur, A. Optimisation of Glycerol and Itaconic Anhydride Polycondensation. Molecules 2022, 27, 4627. [Google Scholar] [CrossRef] [PubMed]
- Ilica, R.A.; Kloetzer, L.; Galaction, A.I.; Caşcaval, D. Fumaric Acid: Production and Separation. Biotechnol. Lett. 2019, 41, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Trotta, J.T.; Watts, A.; Wong, A.R.; Lapointe, A.M.; Hillmyer, M.A.; Fors, B.P. Renewable Thermosets and Thermoplastics from Itaconic Acid. ACS Sustain. Chem. Eng. 2019, 7, 2691–2701. [Google Scholar] [CrossRef]
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of Enzymatically Synthesized Rigid Poly(itaconate)s via Post-Polymerization Aza-Michael Addition of Primary Amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar] [CrossRef]
- Day, D.M.; Farmer, T.J.; Sherwood, J.; Clark, J.H. An Experimental Investigation into the Kinetics and Mechanism of the Aza-Michael Additions of Dimethyl Itaconate. Tetrahedron 2022, 121, 132921. [Google Scholar] [CrossRef]
- Noordzij, G.J.; Wilsens, C.H.R.M. Cascade Aza-Michael Addition-Cyclizations; Toward Renewable and Multifunctional Carboxylic Acids for Melt-Polycondensation. Front. Chem. 2019, 7, 729. [Google Scholar] [CrossRef]
- Mutsuji, S.; Haruo, K.; Yasumasa, S.; Uchino, N. Studies of the Isomerization of Unsaturated Carboxylic Acids. IV. Base-Catalyzed Rearrangements of β,γ-Unsaturated Esters to α,β-Isomers. Bull. Chem. Soc. Jpn. 1978, 51, 2970–2972. [Google Scholar] [CrossRef]
- Farmer, T.J.; Clark, J.H.; Macquarrie, D.J.; Ogunjobi, J.K.; Castle, R.L. Post-Polymerisation Modification of Bio-Derived Unsaturated Polyester Resins via Michael Additions of 1,3-Dicarbonyls. Polym. Chem. 2016, 7, 1650–1658. [Google Scholar] [CrossRef]
- Lv, A.; Li, Z.L.; Du, F.S.; Li, Z.C. Synthesis, Functionalization, and Controlled Degradation of High Molecular Weight Polyester from Itaconic Acid via ADMET Polymerization. Macromolecules 2014, 47, 7707–7716. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Technical Report; U.S. Department of Energy: Oak Ridge, TN, USA, 2004.
- Jahandideh, A.; Esmaeili, N.; Muthukumarappan, K. Synthesis and Characterization of Novel Star-Shaped Itaconic Acid Based Thermosetting Resins. J. Polym. Environ. 2017, 26, 2072–2085. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Recent Advances in Biotechnological Itaconic Acid Production, and Application for a Sustainable Approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, C.; Yang, F.; Zeng, Y.X.; Sun, P.; Liu, P.; Li, X. Itaconate is a Lysosomal Inducer That Promotes Antibacterial Innate Immunity. Mol. Cell 2022, 82, 2844–2857.e10. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.U.; Zhang, X.; Zhang, H.; Lin, X.; Chen, X.; Zhang, Y.; Lin, X.; Huang, L.; Zhuge, Q. Dimethyl Itaconate Inhibits LPS-Induced Microglia Inflammation and Inflammasome-Mediated Pyroptosis via Inducing Autophagy and Regulating the Nrf-2/HO-1 Signaling Pathway. Mol. Med. Rep. 2021, 24, 672. [Google Scholar] [CrossRef]
- Hooftman, A.; O’Neill, L.A.J. The Immunomodulatory Potential of the Metabolite Itaconate. Trends Immunol. 2019, 40, 687–698. [Google Scholar] [CrossRef]
- Jacob, P.L.; Ruiz Cantu, L.A.; Pearce, A.K.; He, Y.; Lentz, J.C.; Moore, J.C.; Machado, F.; Rivers, G.; Apebende, E.; Fernandez, M.R.; et al. Poly (Glycerol Adipate) (PGA) Backbone Modifications with a Library of Functional Diols: Chemical and Physical Effects. Polymer 2021, 228, 123912. [Google Scholar] [CrossRef]
- DrugBank Online DrugBank Online|Database for Drug and Drug Target Info. Available online: https://go.drugbank.com/ (accessed on 27 September 2022).
- PubChem-National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 6 April 2023).
- Yin, G.Z.; Díaz Palencia, J.L.; Wang, D.Y. Fully Bio-Based Poly (Glycerol-Itaconic Acid) as Supporter for PEG Based form Stable Phase Change Materials. Compos. Commun. 2021, 27, 100893. [Google Scholar] [CrossRef]
- Yin, G.Z.; Yang, X.M.; Hobson, J.; López, A.M.; Wang, D.Y. Bio-Based Poly (Glycerol-Itaconic Acid)/PEG/APP as form Stable and Flame-Retardant Phase Change Materials. Compos. Commun. 2022, 30, 101057. [Google Scholar] [CrossRef]
- Metzger, K.; Dannenberger, D.; Tuchscherer, A.; Ponsuksili, S.; Kalbe, C. Effects of Temperature on Proliferation of Myoblasts from Donor Piglets with Different Thermoregulatory Maturities. BMC Mol. Cell Biol. 2021, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.L.; Halevy, O.; Yahav, S.; Velleman, S.G. The Effect of Temperature on Proliferation and Differentiation of Chicken Skeletal Muscle Satellite Cells Isolated from Different Muscle Types. Physiol. Rep. 2016, 4, e12770. [Google Scholar] [CrossRef] [PubMed]
- Feith, R. Side Effects of Acrylic Cement Implanted into Bone. A Histological (Micro)Angiographic, Fluorescence Microscopic and Autoradiographic Study in the Rabbit Femur. Acta Orthop. Scand. 1975, 46, 14–30. [Google Scholar] [CrossRef]
- Shridhar, P.; Chen, Y.; Khalil, R.; Plakseychuk, A.; Cho, S.K.; Tillman, B.; Kumta, P.N.; Chun, Y.J. A Review of PMMA Bone Cement and Intra-Cardiac Embolism. Materials 2016, 9, 821. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater. 2020, 9, 2000349. [Google Scholar] [CrossRef]
- Gao, C.; Liu, H.; Yang, H.; Yang, L. Fabrication and Characterization of Injectable Calcium Phosphate-Based Cements for Kyphoplasty. Mater. Technol. 2015, 30, B256–B263. [Google Scholar] [CrossRef]
- Montelongo, S.A.; Guda, T.; Ong, J. Bone Grafts for Craniofacial Applications. In Translating Biomaterials for Bone Graft: Bench-Top to Clinical Applications; Routledge: London, UK, 2017; p. 131. [Google Scholar]


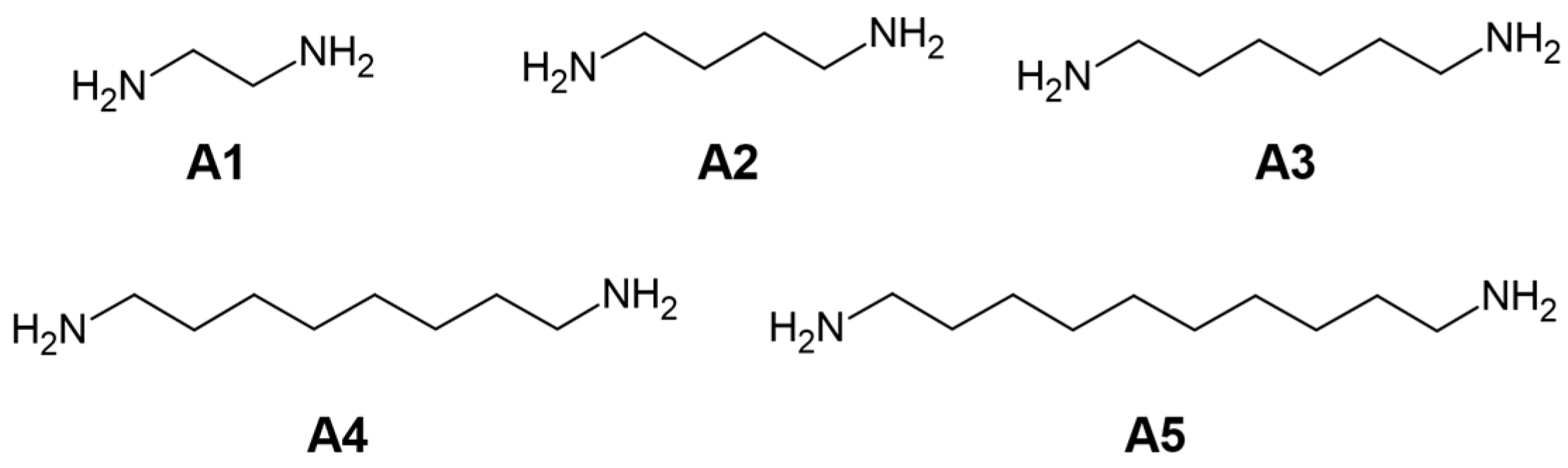


| Signature | Amine | Amine Weight [g] | Amine Volume [mL] | Functionality (Proton of the Amine Group: C=C Bond) |
|---|---|---|---|---|
| A1 | 1,2-EDA | 0.05 | 0.06 | 1:2 |
| A2 | 0.11 | 0.12 | 1:1 | |
| A3 | 0.21 | 0.24 | 2:1 | |
| A4 | 0.85 | 0.95 | 8:1 | |
| B1 | 1,4-BDA | 0.08 | 0.08 | 1:2 |
| B2 | 0.16 | 0.18 | 1:1 | |
| B3 | 0.31 | 0.36 | 2:1 | |
| B4 | 1.25 | 1.43 | 8:1 | |
| C1 | 1,6-HDA | 0.10 | 0.11 | 1:2 |
| C2 | 0.21 | 0.22 | 1:1 | |
| C3 | 0.41 | 0.44 | 2:1 | |
| C4 | 1.65 | 1.77 | 8:1 | |
| D1 | 1,8-ODA | 0.13 | 0.13 | 1:2 |
| D2 | 0.26 | 0.26 | 1:1 | |
| D3 | 0.51 | 0.52 | 2:1 | |
| - | 1.02 | 1.04 | 4:1 | |
| D4 | 2.05 | 2.09 | 8:1 | |
| E1 | 1,10-DDA | 0.15 | 0.18 | 1:2 |
| E2 | 0.31 | 0.36 | 1:1 | |
| E3 | 0.61 | 0.71 | 2:1 | |
| E4 | 2.45 | 2.85 | 8:1 |
| No. | Molar Ratio (G:AcItc) | Temperature (T) [°C] | Time (t) [min] | AN [mgKOH/gsample] | EN [mgKOH/gsample] | EDtitr [%] |
|---|---|---|---|---|---|---|
| 1 ** | 2:3 | 120 | 60 | 79 | 442 | 15.2 |
| 2 ** | 180 | n. s. | n. s. | - | ||
| 3 | 150 | 60 | ||||
| 4 | 120 | |||||
| 5 * | 165 | 20 | 385 | 176 | 31.4 | |
| 6 * | 45 | 338 | 245 | 42.0 | ||
| 7 * | 60 | 315 | 197 | 38.5 | ||
| 8 * | 120 | n. s. | n. s. | - | ||
| 9 * | 1:3 | 20 | 526 | 135 | 20.5 | |
| 10 * | 45 | 374 | 187 | 33.3 | ||
| 11 * | 1:1 | 20 | 308 | 187 | 37.7 | |
| 12 * | 45 | 265 | 241 | 47.7 | ||
| 13 * | 2:3 | 180 | 30 | 310 | 294 | 48.7 |
| 14 * | 45 | 298 | n. s. | - | ||
| 15 * | 60 | n. s. | ||||
| 16 * | 120 | |||||
| 17 * | 1:3 | 45 | 441 | 529 | 54.5 | |
| 18 * | 60 | n. s. | n. s. | - | ||
| 19 * | 1:1 | 45 | 222 | |||
| 20 * | 60 | n. s. |
| No. | Molar Ratio (G:AcItc) | Temperature [°C] | Time [min] | Amount of Catalyst [%wa.] | AN [mgKOH/gsample] | EN [mgKOH/gsample] | EDtitr [%] |
|---|---|---|---|---|---|---|---|
| 1 ** | 2:3 | 100 | 30 | 0.1 | n. s. | n. s. | - |
| 2 ** | 120 | 20 | 2.0 | ||||
| 3 ** | 30 | 0.25 | |||||
| 4 ** | 0.1 | ||||||
| 5 ** | 0.5 | ||||||
| 6 ** | 1.0 | ||||||
| 7 ** | 2.0 | ||||||
| 8 | 135 | 0.25 | |||||
| 9 | 150 | 0.05 | |||||
| 10 | 0.25 | ||||||
| 11 | 0.5 | ||||||
| 12 | 1.0 | ||||||
| 13 | 45 | 1.0 | |||||
| 14 | 60 | 1.0 | |||||
| 15 | 2.0 |
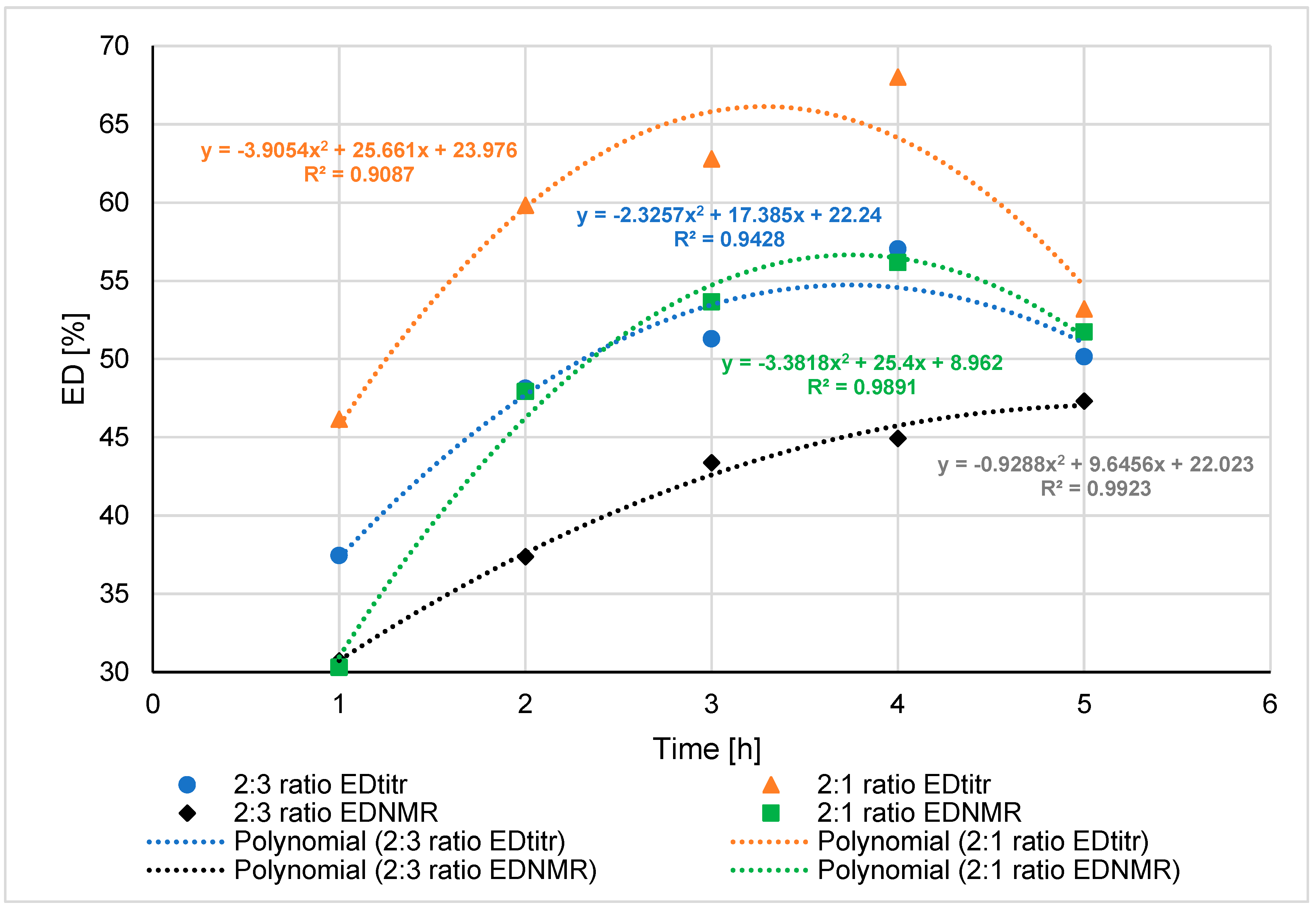
| No. | Molar Ratio (G:AcItc) | Temperature [°C] | Time (t) [h] | AN [mgKOH/gsample] | EN [mgKOH/gsample] | EDtitr [%] | EDNMR [%] | %IzMes [%] | %Ord [%] | %X13CNMR [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 ** | 2:3 | 120 | 2 | 406 | n. s. | - | 22.5 | 0.3 | 7.2 | - |
| 2 | 150 | 1 | 365 | 219 | 37.5 | 30.7 | 0.7 | 7.2 | 70.4 | |
| 3 | 2 | 333 | 309 | 48.1 | 37.4 | 0.8 | 9.8 | 70.8 | ||
| 4 | 3 | 315 | 332 | 51.3 | 43.4 | 1.0 | 8.2 | 77.4 | ||
| 5 | 4 | 301 | 400 | 57.0 | 44.9 | 1.1 | 9.2 | 76.4 | ||
| 6 | 5 | 281 | 282 | 50.2 | 47,3 | 1,3 | 12.1 | 77.0 | ||
| 7 ** | 2:1 | 120 | 2 | 111 | n. s. | - | 22.5 | 0.3 | 7.3 | - |
| 8 | 150 | 1 | 157 | 135 | 46.2 | 30.3 | 0.7 | 9.9 | 77.8 | |
| 9 | 2 | 135 | 201 | 59.8 | 47.9 | 0.9 | 10.8 | 89.4 | ||
| 10 | 3 | 116 | 195 | 62.8 | 53.7 | 1.3 | 11.1 | 92.6 | ||
| 11 *** | 4 | 104 | 221 | 68.0 | 56.2 | 1.3 | 14.0 | 89.9 | ||
| 12 | 5 | 93 | 106 | 53.2 | 51.7 | 1.5 | 14.1 | 65.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miętus, M.; Kolankowski, K.; Gołofit, T.; Ruśkowski, P.; Mąkosa-Szczygieł, M.; Gadomska-Gajadhur, A. Poly(glycerol itaconate) Crosslinking via the aza-Michael Reaction—A Preliminary Research. Materials 2023, 16, 7319. https://doi.org/10.3390/ma16237319
Miętus M, Kolankowski K, Gołofit T, Ruśkowski P, Mąkosa-Szczygieł M, Gadomska-Gajadhur A. Poly(glycerol itaconate) Crosslinking via the aza-Michael Reaction—A Preliminary Research. Materials. 2023; 16(23):7319. https://doi.org/10.3390/ma16237319
Chicago/Turabian StyleMiętus, Magdalena, Krzysztof Kolankowski, Tomasz Gołofit, Paweł Ruśkowski, Marcin Mąkosa-Szczygieł, and Agnieszka Gadomska-Gajadhur. 2023. "Poly(glycerol itaconate) Crosslinking via the aza-Michael Reaction—A Preliminary Research" Materials 16, no. 23: 7319. https://doi.org/10.3390/ma16237319
APA StyleMiętus, M., Kolankowski, K., Gołofit, T., Ruśkowski, P., Mąkosa-Szczygieł, M., & Gadomska-Gajadhur, A. (2023). Poly(glycerol itaconate) Crosslinking via the aza-Michael Reaction—A Preliminary Research. Materials, 16(23), 7319. https://doi.org/10.3390/ma16237319










