Biological Performance of Titanium Surfaces with Different Hydrophilic and Nanotopographical Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Modifications
2.2. Pre-Treatment of Samples
2.3. Surface Characterization
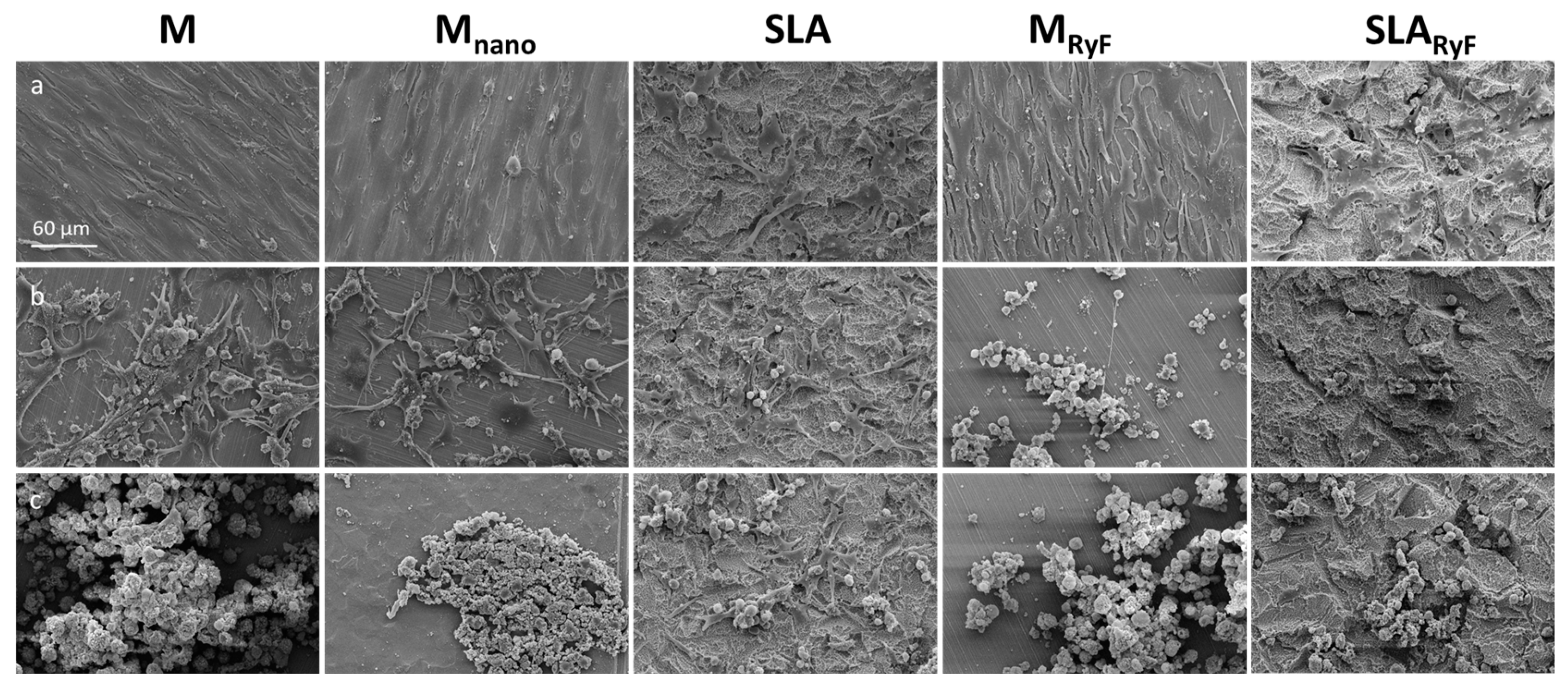
2.3.1. Scanning Electron Microscopy (SEM) of the Different Surfaces
2.3.2. Roughness
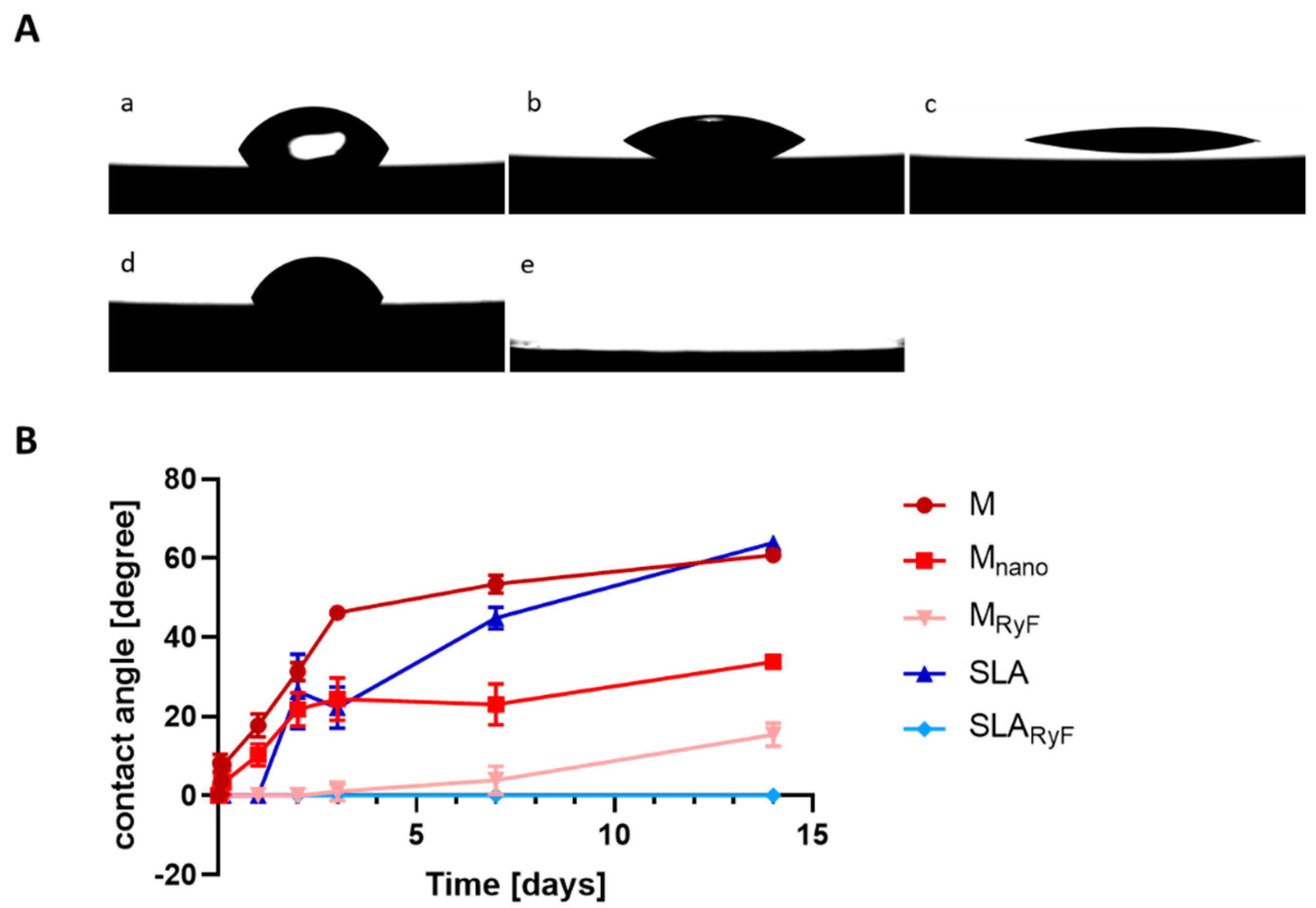
2.3.3. Surface Wettability
2.3.4. Biological Tests
2.3.5. Cultivation of Cells
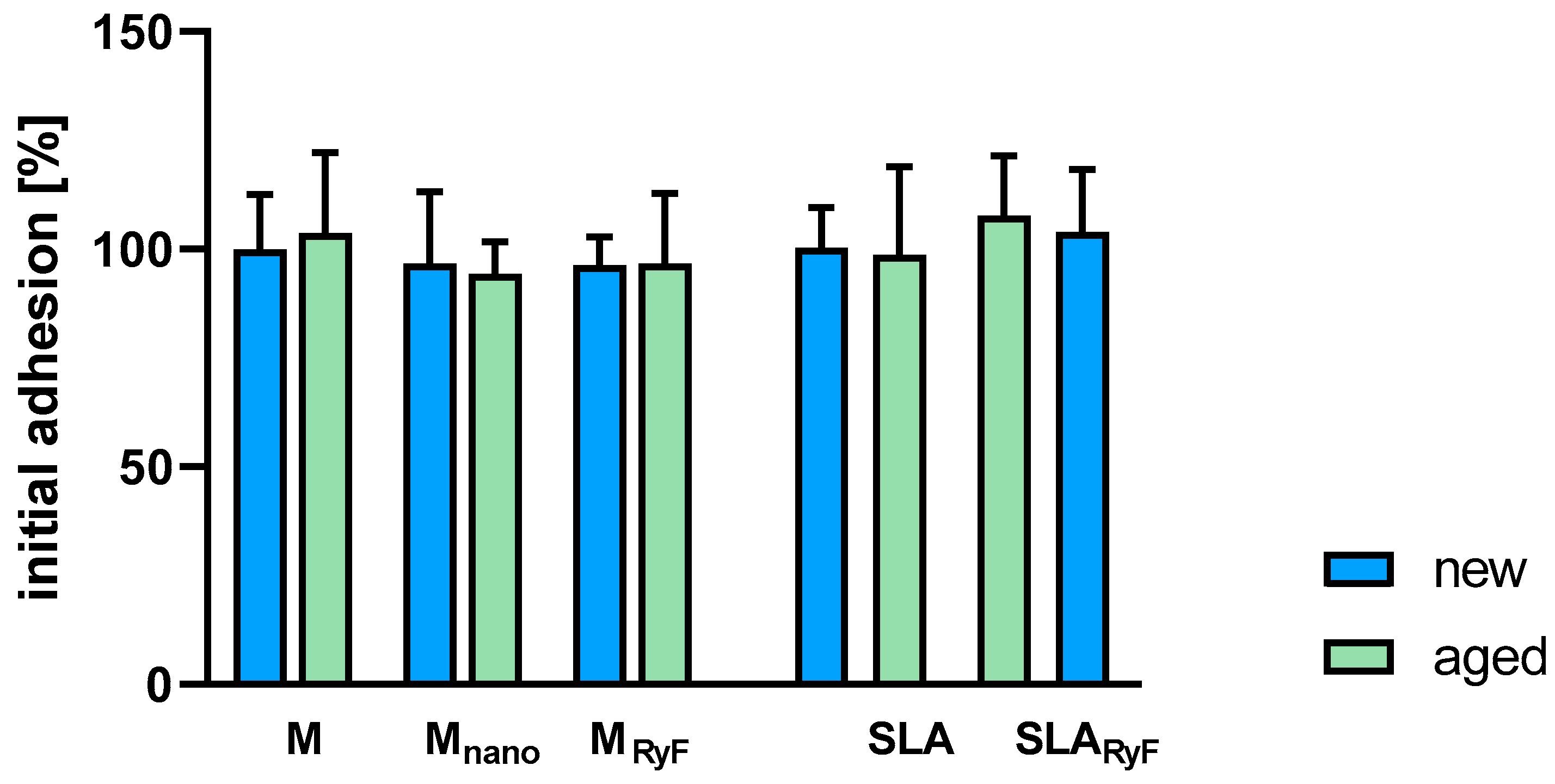
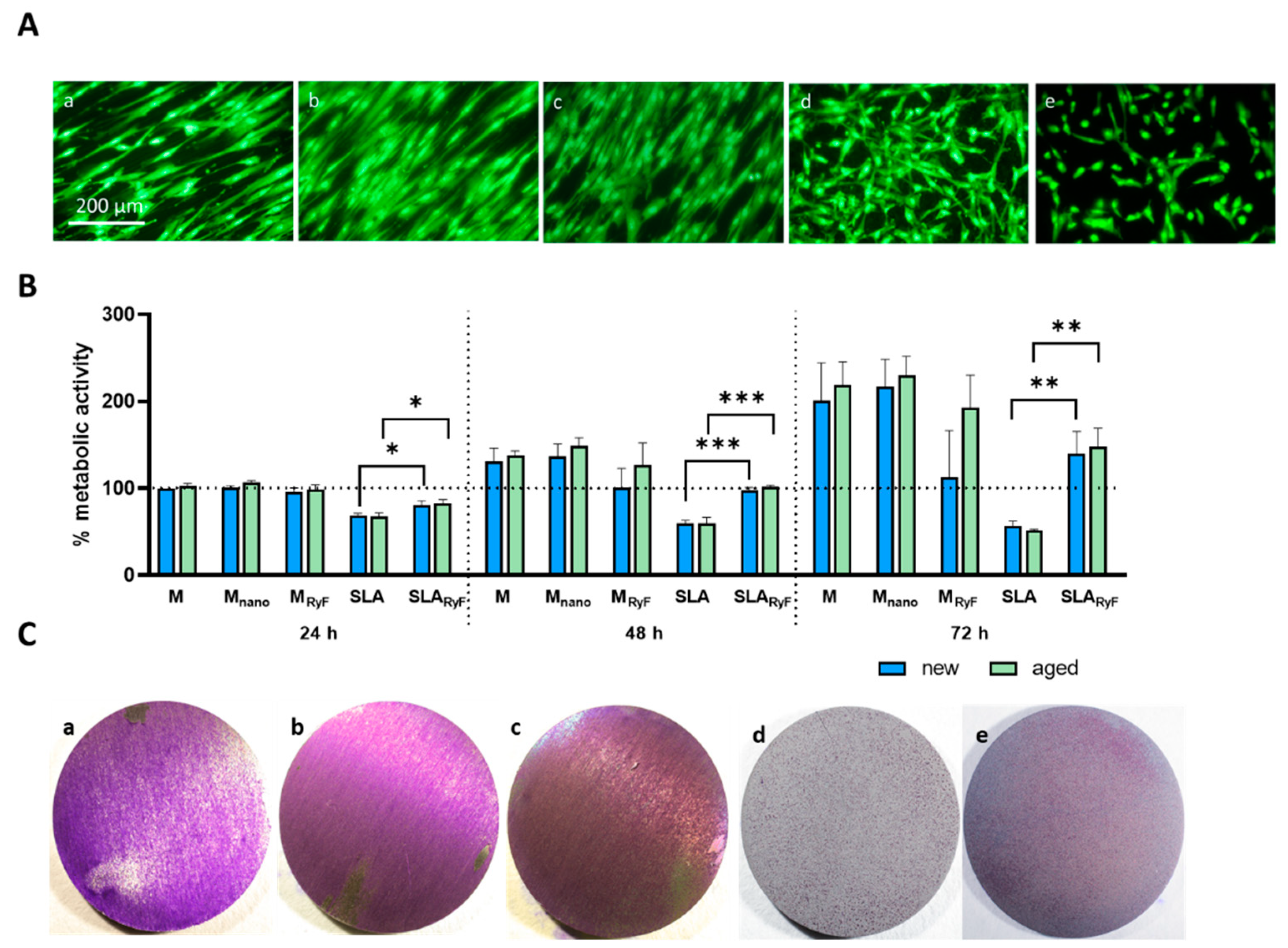
2.3.6. Adhesion, Viability, and Proliferation Assay
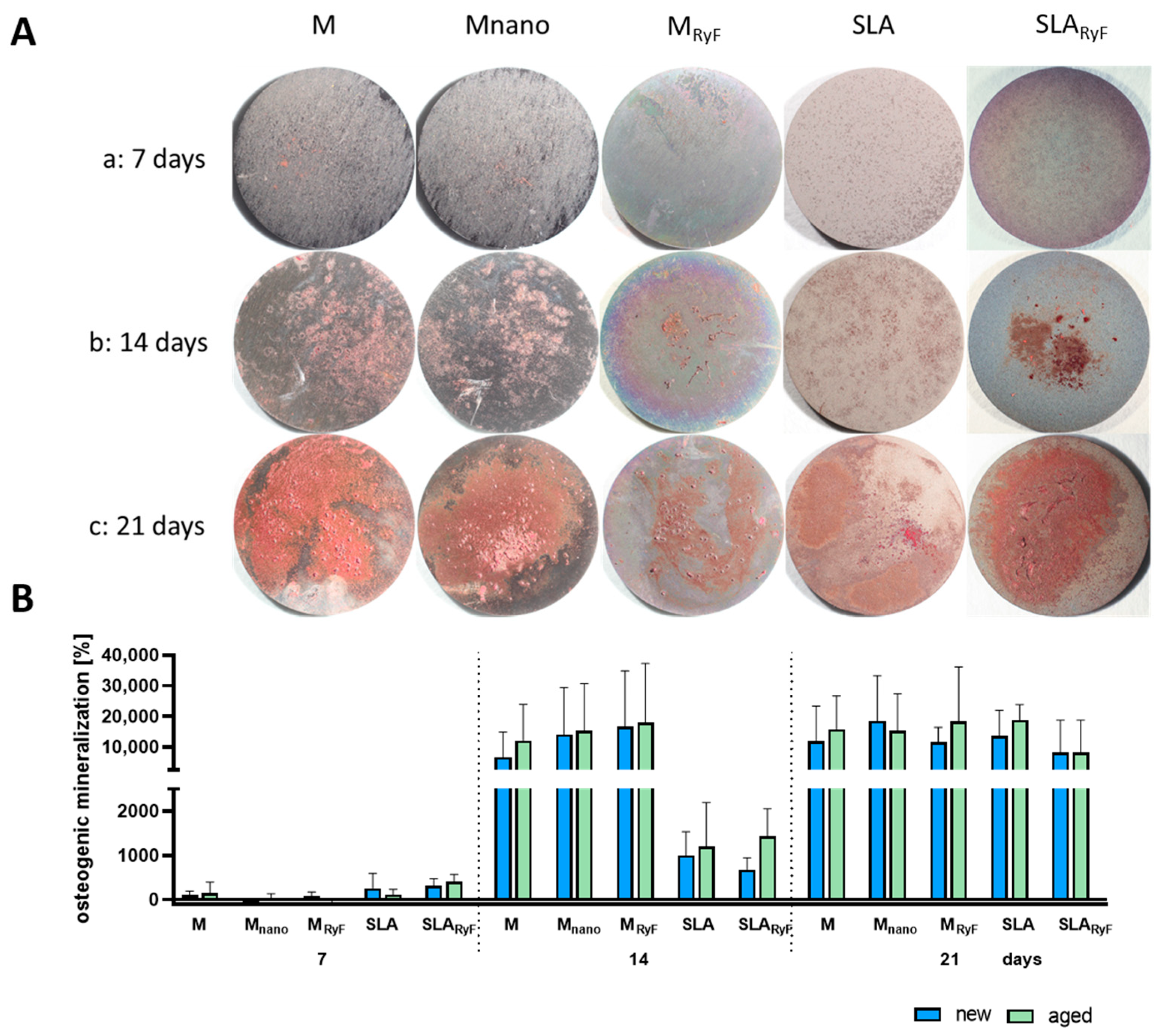
2.3.7. Differentiation of SAOS-2
2.3.8. Statistical Analysis
3. Results
3.1. Surface Characteristics
3.2. Fibroblast Attachment and Proliferation
3.3. Osteoblast Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amoroso, P.F.; Adams, R.J.; Waters, M.G.J.; Williams, D.W. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 633–637. [Google Scholar]
- Palmquist, A.; Johansson, A.; Suska, F.; Brånemark, R.; Thomsen, P. Acute inflammatory response to laser-induced micro- and nano-sized titanium surface features. Clin. Implant. Dent. Relat. Res. 2013, 15, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11, S123–S136. [Google Scholar] [PubMed]
- Brånemark, P.-I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-Osseous Anchorage of Dental Prostheses: I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Kim, J.-J.; Lee, J.-H.; Kim, J.C.; Lee, J.-B.; Yeo, I.-S.L. Biological Responses to the Transitional Area of Dental Implants: Material- and Structure-Dependent Responses of Peri-Implant Tissue to Abutments. Materials 2019, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, C.; Wang, D.; Wang, M.; Song, D.; Xu, X.; Zhang, D. The burden of diabetes on the soft tissue seal surrounding the dental implants. Front. Physiol. 2023, 14, 1136973. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Schwarz, F.; Wieland, M.; Schwartz, Z.; Zhao, G.; Rupp, F.; Geis-Gerstorfer, J.; Schedle, A.; Broggini, N.; Bornstein, M.M.; Buser, D.; et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 544–557. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Toffoli, A.; Parisi, L.; Tatti, R.; Lorenzi, A.; Verucchi, R.; Manfredi, E.; Lumetti, S.; Macaluso, G.M. Thermal-induced hydrophilicity enhancement of titanium dental implant surfaces. J. Oral Sci. 2020, 62, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular Responses Evoked by Different Surface Characteristics of Intraosseous Titanium Implants. BioMed Res. Int. 2015, 2015, 171945. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jung, U.W.; Lee, I.S.; Kim, C.S.; Lee, Y.K.; Choi, S.H. Resolution of surgically created three-wall intrabony defects in implants using three different biomaterials: An in vivo study. Clin. Oral Implant. Res. 2011, 22, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef]
- Liang, J.; Xu, S.; Shen, M.; Cheng, B.; Li, Y.; Liu, X.; Qin, D.; Bellare, A.; Kong, L. Osteogenic activity of titanium surfaces with hierarchical micro-/nano-structures obtained by hydrofluoric acid treatment. Int. J. Nanomed. 2017, 2017, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, R.; Mao, Z.; Fang, J.; Ren, F. Topographical biointerface regulating cellular functions for bone tissue engineering. Biosurface Biotribology 2022, 8, 165–187. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Zuo, J.; Li, J.; Wei, Q.; Yu, Z.; Tang, Z. Cell responses to titanium treated by a sandblast-free method for implant applications. Mater. Sci. Eng. C 2017, 78, 1187–1194. [Google Scholar] [CrossRef]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Ivanoff, C.J.; Hallgren, C.; Widmark, G.; Sennerby, L.; Wennerberg, A. Histologic evaluation of the bone integration of TiO2 blasted and turned titanium microimplants in humans. Clin. Oral Implant. Res. 2001, 12, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.W.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, TODENTJ-8-50. [Google Scholar] [CrossRef] [PubMed]
- Ostrikov, K.; Neyts, E.C.; Meyyappan, M. Plasma Nanoscience: From Nano-Solids in Plasmas to Nano-Plasmas in Solids. Adv. Phys. 2013, 62, 113–224. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of Surface Wettability and Topography on the Adhesion of Osteosarcoma Cells on Plasma-modified Polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, A. Atomic-Scale Etching Mechanism of Aluminum with Fluorine-Based Plasma. J. Phys. Chem. C 2022, 126, 14180–14186. [Google Scholar] [CrossRef]
- Clainche, T.L.; Linklater, D.; Wong, S.; Le, P.; Juodkazis, S.; Guével, X.L.; Coll, J.-L.; Ivanova, E.P.; Martel-Frachet, V. Mechano-Bactericidal Titanium Surfaces for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 48272–48283. [Google Scholar] [CrossRef]
- Liang, L.C.; Krieg, P.; Rupp, F.; Kimmerle-Muller, E.; Spintzyk, S.; Richter, M.; Richter, G.; Killinger, A.; Geis-Gerstorfer, J.; Scheideler, L. Osteoblast Response to Different UVA-Activated Anatase Implant Coatings. Adv. Mater. Interfaces 2019, 6, 1801720. [Google Scholar] [CrossRef]
- Engstrand, T.; Kihlström, L.; Lundgren, K.; Trobos, M.; Engqvist, H.; Thomsen, P. Bioceramic implant induces bone healing of cranial defects. Plast. Reconstr. Surg.—Glob. Open 2015, 3, e491. [Google Scholar] [CrossRef]
- Omar, O.; Lennerås, M.; Svensson, S.; Suska, F.; Emanuelsson, L.; Hall, J.; Nannmark, U.; Thomsen, P. Integrin and chemokine receptor gene expression in implant-adherent cells during early osseointegration. J. Mater. Sci. Mater. Med. 2010, 21, 969–980. [Google Scholar] [CrossRef]
- Taylor, S.R.; Gibbons, D.F. Effect of surface texture on the soft tissue response to polymer implants. J. Biomed. Mater. Res. 1983, 17, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Stanford, C.M. Surface modification of biomedical and dental implants and the processes of inflammation, wound healing and bone formation. Int. J. Mol. Sci. 2010, 11, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S. Reality of dental implant surface modification: A short literature review. Open Biomed. Eng. J. 2014, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Zhou, L.; Li, S.; Lin, X.; Wen, B.; Lai, C.; Ding, X. Phosphoric acid and sodium fluoride: A novel etching combination on titanium. Biomed. Mater. 2014, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Choi, H.; An, H.W.; Kwon, T.Y.; Hong, M.H. Effects of Surface Treatment Method Forming New Nano/Micro Hierarchical Structures on Attachment and Proliferation of Osteoblast-like Cells. Materials 2023, 16, 5717. [Google Scholar] [CrossRef]
- Cruz, M.B.; Silva, N.; Marques, J.F.; Mata, A.; Silva, F.S.; Caramês, J. Biomimetic implant surfaces and their role in biological integration—A concise review. Biomimetics 2022, 7, 74. [Google Scholar] [CrossRef]
- Kauzmann, W. Some factors in the interpretation of protein denaturation. In Advances in Protein Chemistry; Academic Press: Cambridge, MA, USA, 1959; Volume 14, pp. 1–63. [Google Scholar]
- Tengvall, P. Protein Interactions with Biomaterials. Compr. Biomater. 2017, 4, 63–73. [Google Scholar]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Landin, M.A.; Tiainen, H.; Reseland, J.E.; Ellingsen, J.E.; Haugen, H.J. The effect of hydrofluoric acid treatment of titanium and titanium dioxide surface on primary human osteoblasts. Clin. Oral Implant. Res. 2014, 25, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lamolle, S.F.; Monjo, M.; Rubert, M.; Haugen, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials 2009, 30, 736–742. [Google Scholar] [CrossRef]
- Ellingsen, J.E.; Johansson, C.B.; Wennerberg, A.; Holmen, A. Improved retention and bone-tolmplant contact with fluoride-modified titanium implants. Int. J. Oral Maxillofac. Implant. 2004, 19, 659–666. [Google Scholar]
- Collaert, B.; Wijnen, L.; De Bruyn, H. A 2-year prospective study on immediate loading with fluoride-modified implants in the edentulous mandible. Clin. Oral. Implants Res. 2011, 22, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. 2008, 1, 234–242. [Google Scholar] [CrossRef]

| Group | Surface Modification |
|---|---|
| M | Machined surface without further surface treatment |
| Mnano | Plasma cleaning followed by hydrothermal treatment with sodium chloride |
| MRyF | Plasma etching of machined surface with 2,3,3,3-tetrafluoropropene |
| SLA | Blasted with large grits of 0.25–0.50 mm corundum and acid-etched in a mixture of HCl and H2SO4. |
| SLARyF | Plasma etching of SLA surface with 2,3,3,3-tetrafluoropropene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illing, B.; Mohammadnejad, L.; Theurer, A.; Schultheiss, J.; Kimmerle-Mueller, E.; Rupp, F.; Krajewski, S. Biological Performance of Titanium Surfaces with Different Hydrophilic and Nanotopographical Features. Materials 2023, 16, 7307. https://doi.org/10.3390/ma16237307
Illing B, Mohammadnejad L, Theurer A, Schultheiss J, Kimmerle-Mueller E, Rupp F, Krajewski S. Biological Performance of Titanium Surfaces with Different Hydrophilic and Nanotopographical Features. Materials. 2023; 16(23):7307. https://doi.org/10.3390/ma16237307
Chicago/Turabian StyleIlling, Barbara, Leila Mohammadnejad, Antonia Theurer, Jacob Schultheiss, Evi Kimmerle-Mueller, Frank Rupp, and Stefanie Krajewski. 2023. "Biological Performance of Titanium Surfaces with Different Hydrophilic and Nanotopographical Features" Materials 16, no. 23: 7307. https://doi.org/10.3390/ma16237307
APA StyleIlling, B., Mohammadnejad, L., Theurer, A., Schultheiss, J., Kimmerle-Mueller, E., Rupp, F., & Krajewski, S. (2023). Biological Performance of Titanium Surfaces with Different Hydrophilic and Nanotopographical Features. Materials, 16(23), 7307. https://doi.org/10.3390/ma16237307






