Dissipative Particle Dynamic Simulation on Self-Assembly of Symmetric CBABC Pentablock Terpolymers in Solution
Abstract
:1. Introduction
2. Simulation Methods
2.1. Dissipative Particle Dynamic Method
2.2. Polymer Model: Simulation Conditions
3. Results and Discussion
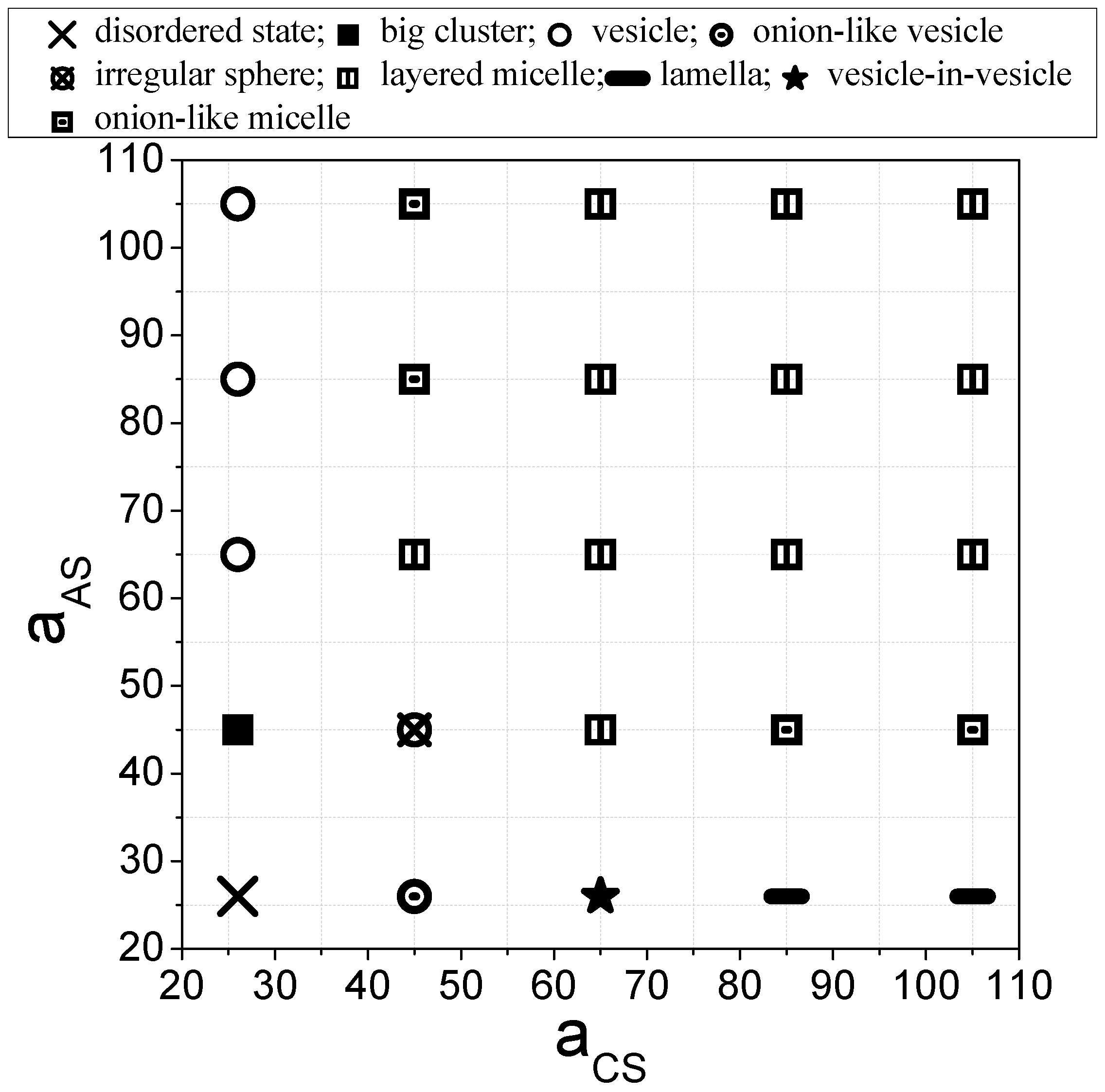
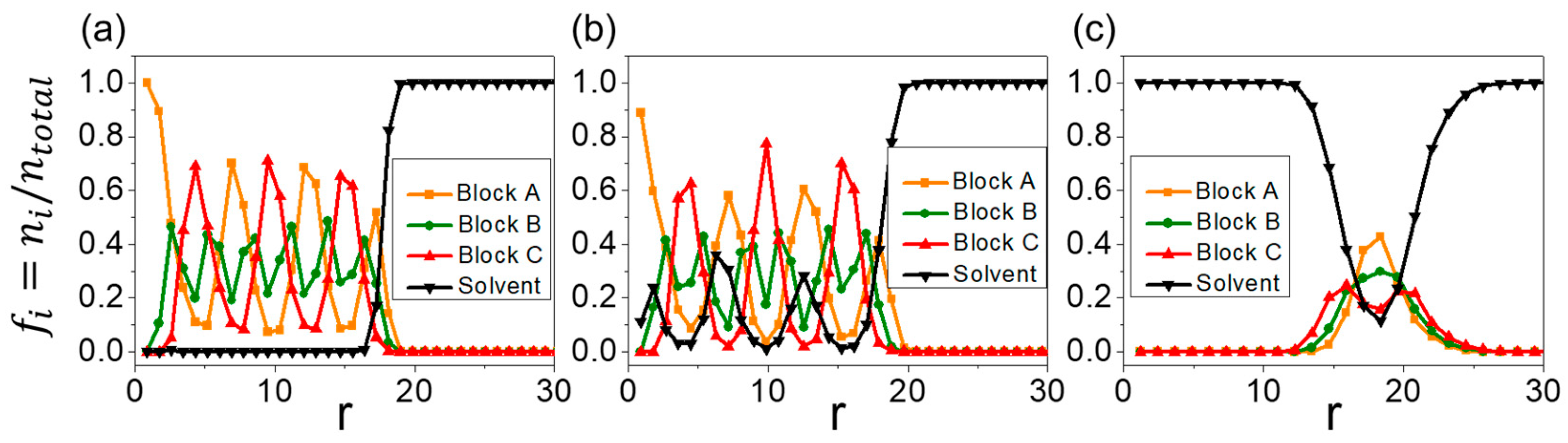
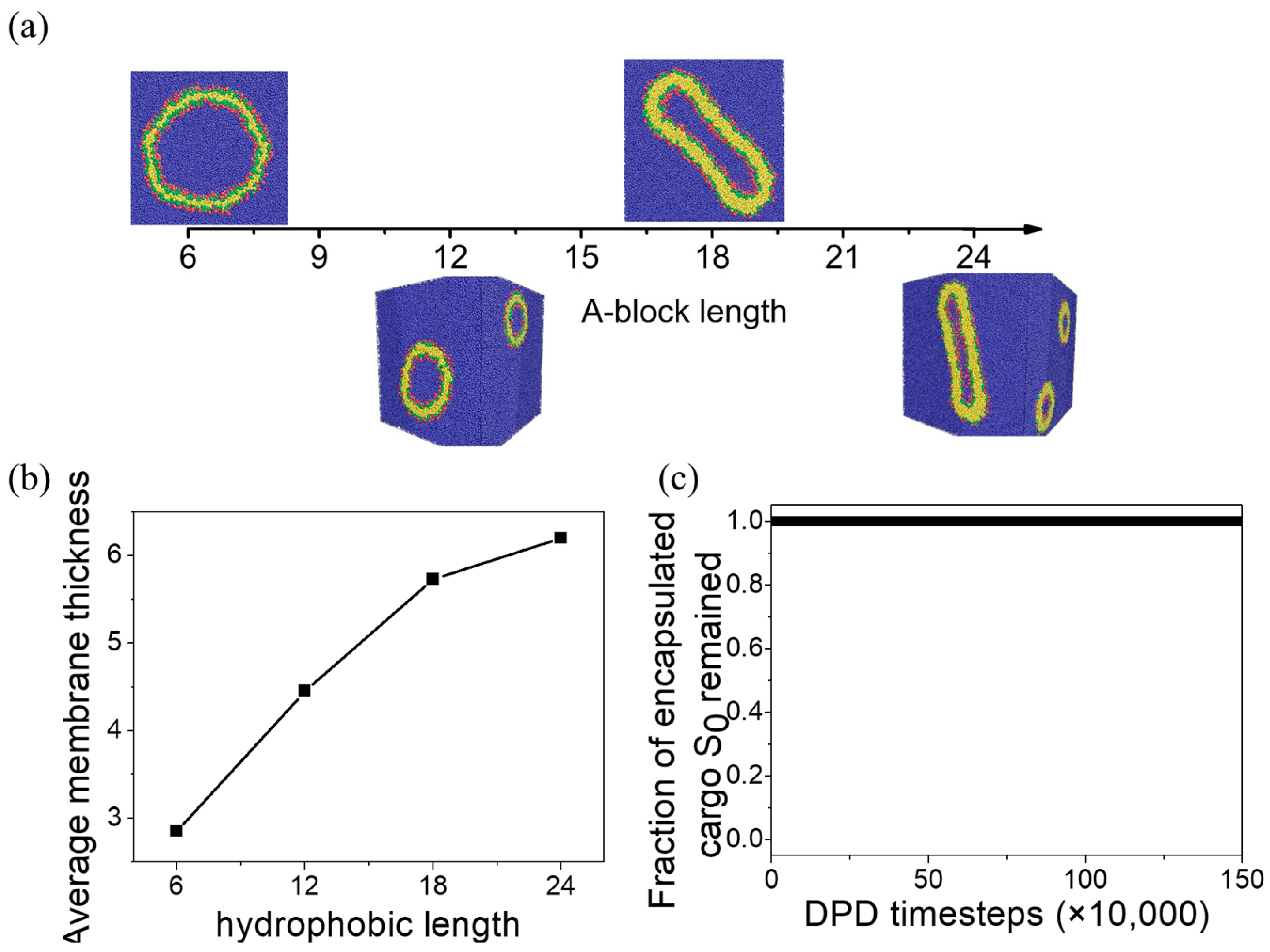
3.1. State Diagram and General Description of Equilibrium Aggregate Structure
3.2. Formation Mechanism of Onion-like Aggregates
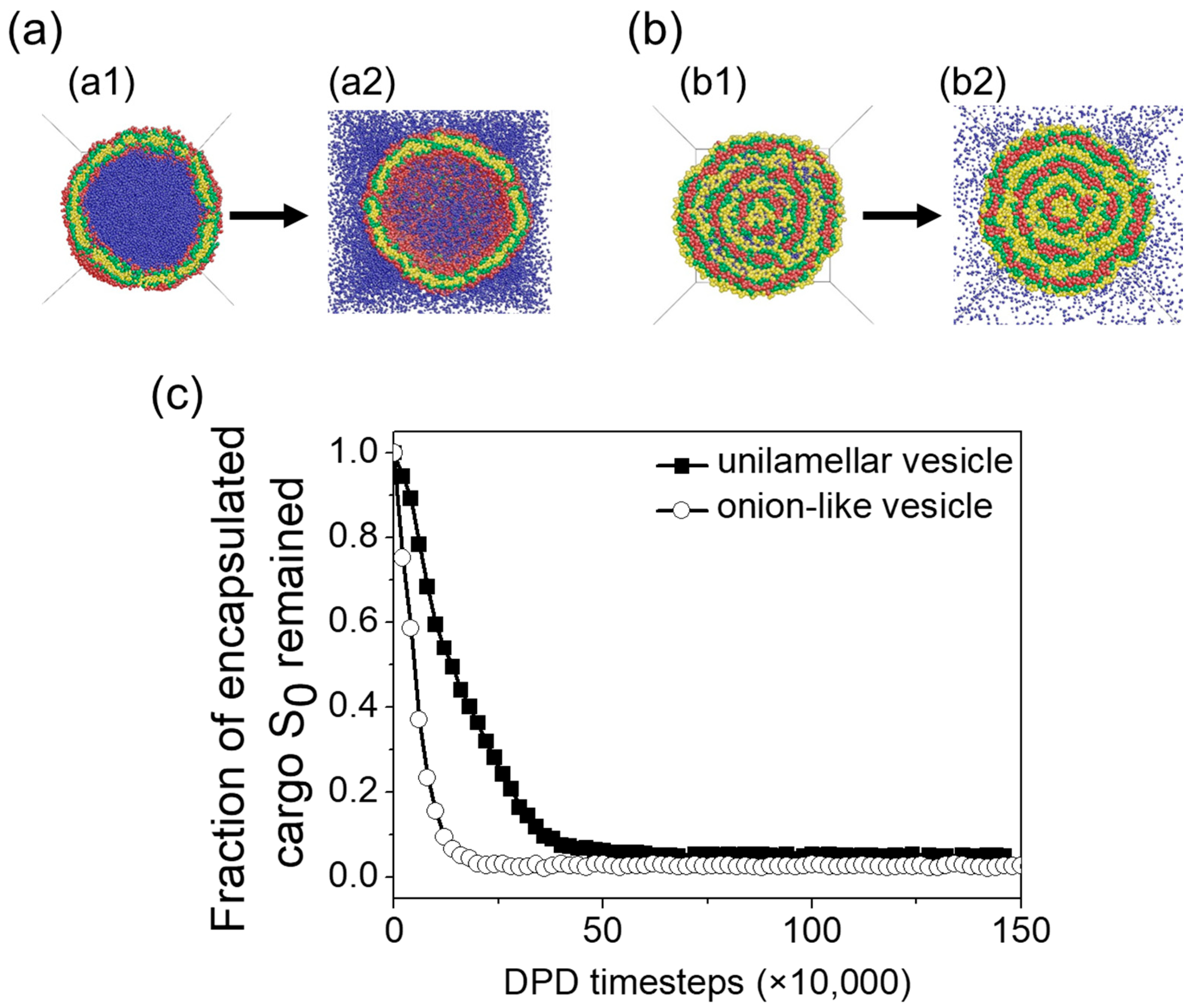
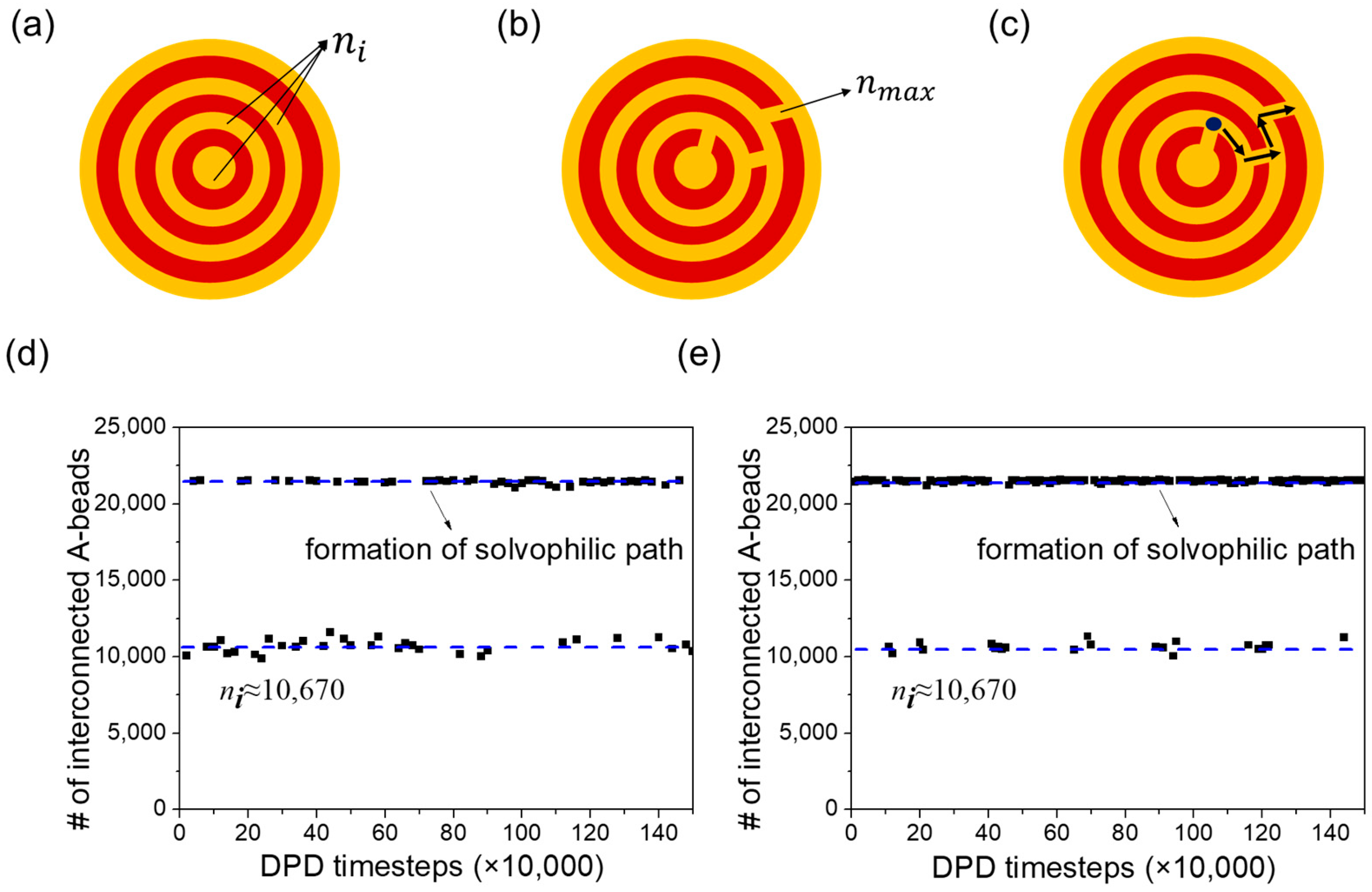
3.3. Vesicle Membrane Permeability
3.4. Effect of Bulk Shear on Vesicle Behaviour
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dashtimoghadam, E.; Salimi-Kenari, H.; Forooqi Motlaq, V.; Hasani-Sadrabadi, M.M.; Mirzadeh, H.; Zhu, K.; Knudsen, K.D.; Nyström, B. Synthesis and temperature-induced self-assembly of a positively charged symmetrical pentablock terpolymer in aqueous solutions. Eur. Polym. J. 2017, 97, 158–168. [Google Scholar] [CrossRef]
- You, C.-C.; Miranda, O.R.; Gider, B.; Ghosh, P.S.; Kim, I.-B.; Erdogan, B.; Krovi, S.A.; Bunz, U.H.F.; Rotello, V.M. Detection and identification of proteins using nanoparticle–fluorescent polymer ‘chemical nose’ sensors. Nat. Nanotechnol. 2007, 2, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.; Taresco, V.; Penelle, J.; Couturaud, B. Polymerisation-induced self-assembly (PISA) as a straightforward formulation strategy for stimuli-responsive drug delivery systems and biomaterials: Recent advances. Biomater. Sci. 2021, 9, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Qiu, Y.; Zhang, M.; Yan, K.; Zhang, P.; Lu, S.; Liu, Z.; Shi, X.; Zhao, X. Aqueous Self-Assembly of Block Copolymers to Form Manganese Oxide-Based Polymeric Vesicles for Tumor Microenvironment-Activated Drug Delivery. Nano-Micro Lett. 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.G.; Ko, J.; Choi, H.J.; Kim, D.-H.; Han, K.H.; Kim, J.H.; Kim, M.H.; Park, C.; Jin, H.M.; Kim, I.-D.; et al. Multilevel Self-Assembly of Block Copolymers and Polymer Colloids for a Transparent and Sensitive Gas Sensor Platform. ACS Nano 2022, 16, 18767–18776. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, K.H.; Yoon, H. Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers 2018, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Gao, J.; An, K.; Nie, J.; Xu, J.; Du, B. Self-assembly of the Thermosensitive and pH-Sensitive Pentablock Copolymer PNIPAMx-b-P(tBA-co-AA)90-b-PPO36-b-P(tBA-co-AA)90-b-PNIPAMx in Dilute Aqueous Solutions. Macromolecules 2021, 54, 6489–6501. [Google Scholar] [CrossRef]
- Suzuki, M.; Orido, T.; Takano, A.; Matsushita, Y. The Largest Quasicrystalline Tiling with Dodecagonal Symmetry from a Single Pentablock Quarterpolymer of the AB1CB2D Type. ACS Nano 2022, 16, 6111–6117. [Google Scholar] [CrossRef]
- Xie, N.; Liu, M.; Deng, H.; Li, W.; Qiu, F.; Shi, A.-C. Macromolecular Metallurgy of Binary Mesocrystals via Designed Multiblock Terpolymers. J. Am. Chem. Soc. 2014, 136, 2974–2977. [Google Scholar] [CrossRef]
- Guo, L.; Xu, J.; Du, B. Self-assembly of ABCBA Linear Pentablock Terpolymers. Polymer Rev. 2023, 1–35. [Google Scholar] [CrossRef]
- Beheshti, N.; Zhu, K.; Kjøniksen, A.-L.; Knudsen, K.D.; Nyström, B. Characterization of temperature-induced association in aqueous solutions of charged ABCBA-type pentablock tercopolymers. Soft Matter 2011, 7, 1168–1175. [Google Scholar] [CrossRef]
- Gao, J.; An, K.; Lv, C.; Nie, J.; Xu, J.; Du, B. Self-Assembly of Linear Amphiphilic Pentablock Terpolymer PAAx-PS48-PEO46-PS48-PAAxin Dilute Aqueous Solution. Polymers 2020, 12, 2183. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.-T.; Korogiannaki, M.; Marikou, K.; Tsitsilianis, C. CBABC terpolymer-based nanostructured vesicles with tunable membrane permeability as potential hydrophilic drug nanocarriers. Polymer 2014, 55, 2943–2951. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Hur, S.-M.; Ramírez-Hernández, A. Effect of Block Sequence on the Solution Self-Assembly of Symmetric ABCBA Pentablock Polymers in a Selective Solvent. J. Phys. Chem. B 2023, 127, 2575–2586. [Google Scholar] [CrossRef] [PubMed]
- Mineart, K.P.; Ryan, J.J.; Appavou, M.-S.; Lee, B.; Gradzielski, M.; Spontak, R.J. Self-Assembly of a Midblock-Sulfonated Pentablock Copolymer in Mixed Organic Solvents: A Combined SAXS and SANS Analysis. Langmuir 2019, 35, 1032–1039. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Z.; Gao, J.; Xue, J.; Li, J.; Nie, J.; Xu, J.; Du, B. Self-Assembly of Thermosensitive Amphiphilic Pentablock Terpolymer PNIPAMx-b-PtBA90-b-PPO36-b-PtBA90-b-PNIPAMx in Dilute Aqueous Solution. Macromolecules 2018, 51, 10136–10149. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Zhang, G.; Liu, S. Regulating vesicle bilayer permeability and selectivity via stimuli-triggered polymersome-to-PICsome transition. Nat. Commun. 2020, 11, 1524. [Google Scholar] [CrossRef]
- Qi, L.; Qiao, J. Design of Switchable Enzyme Carriers Based on Stimuli-Responsive Porous Polymer Membranes for Bioapplications. ACS Appl. Bio. Mater. 2021, 4, 4706–4719. [Google Scholar] [CrossRef]
- Machtakova, M.; Han, S.; Yangazoglu, Y.; Lieberwirth, I.; Thérien-Aubin, H.; Landfester, K. Self-sustaining enzyme nanocapsules perform on-site chemical reactions. Nanoscale 2021, 13, 4051–4059. [Google Scholar] [CrossRef]
- Sobotta, F.H.; Kuchenbrod, M.T.; Gruschwitz, F.V.; Festag, G.; Bellstedt, P.; Hoeppener, S.; Brendel, J.C. Tuneable Time Delay in the Burst Release from Oxidation-Sensitive Polymersomes Made by PISA. Angew. Chem. Int. Ed. 2021, 60, 24716–24723. [Google Scholar] [CrossRef]
- Rodríguez-García, R.; Mell, M.; López-Montero, I.; Netzel, J.; Hellweg, T.; Monroy, F. Polymersomes: Smart vesicles of tunable rigidity and permeability. Soft Matter 2011, 7, 1532–1542. [Google Scholar] [CrossRef]
- Nishimura, T.; Sumi, N.; Koda, Y.; Sasaki, Y.; Akiyoshi, K. Intrinsically permeable polymer vesicles based on carbohydrate-conjugated poly(2-oxazoline)s synthesized using a carbohydrate-based initiator system. Polym. Chem. 2019, 10, 691–697. [Google Scholar] [CrossRef]
- Liu, F.; Kozlovskaya, V.; Medipelli, S.; Xue, B.; Ahmad, F.; Saeed, M.; Cropek, D.; Kharlampieva, E. Temperature-Sensitive Polymersomes for Controlled Delivery of Anticancer Drugs. Chem. Mater. 2015, 27, 7945–7956. [Google Scholar] [CrossRef]
- Hocine, S.; Cui, D.; Rager, M.N.; Di Cicco, A.; Liu, J.M.; Wdzieczak-Bakala, J.; Brûlet, A.; Li, M.H. Polymersomes with PEG corona: Structural changes and controlled release induced by temperature variation. Langmuir 2013, 29, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Morse, C.; Derry, M.; Thompson, K.; Fielding, L.; Mykhaylyk, O.; Armes, S. Time-Resolved SAXS Studies of the Kinetics of Thermally Triggered Release of Encapsulated Silica Nanoparticles from Block Copolymer Vesicles. Macromolecules 2017, 50, 4465–4473. [Google Scholar] [CrossRef]
- Mable, C.J.; Gibson, R.R.; Prevost, S.; McKenzie, B.E.; Mykhaylyk, O.O.; Armes, S.P. Loading of Silica Nanoparticles in Block Copolymer Vesicles during Polymerization-Induced Self-Assembly: Encapsulation Efficiency and Thermally Triggered Release. J. Am. Chem. Soc. 2015, 137, 16098–16108. [Google Scholar] [CrossRef]
- Borchert, U.; Lipprandt, U.; Bilang, M.; Kimpfler, A.; Rank, A.; Süss, R.; Schubert, R.; Lindner, P.; Förster, S. pH-induced release from P2VP-PEO block copolymer vesicles. Langmuir ACS J. Surf. Colloids 2006, 22, 5843–5847. [Google Scholar] [CrossRef]
- Chen, W.; Du, J. Ultrasound and pH Dually Responsive Polymer Vesicles for Anticancer Drug Delivery. Sci. Rep. 2013, 3, 2162. [Google Scholar] [CrossRef]
- Scherer, M.; Kappel, C.; Mohr, N.; Fischer, K.; Heller, P.; Forst, R.; Depoix, F.; Bros, M.; Zentel, R. Functionalization of Active Ester-Based Polymersomes for Enhanced Cell Uptake and Stimuli-Responsive Cargo Release. Biomacromolecules 2016, 17, 3305–3317. [Google Scholar] [CrossRef]
- Gaitzsch, J.; Appelhans, D.; Wang, L.; Battaglia, G.; Voit, B. Synthetic Bio-nanoreactor: Mechanical and Chemical Control of Polymersome Membrane Permeability. Angew. Chem. Int. Ed. 2012, 51, 4448–4451. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, G.; Tian, J.; Wang, H.; Gong, M.; Liu, S. Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions. J. Am. Chem. Soc. 2015, 137, 15262–15275. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, E.; Cuvelier, D.; Brochard-Wyart, F.; Nassoy, P.; Li, M.H. Bursting of sensitive polymersomes induced by curling. Proc. Natl. Acad. Sci. USA 2009, 106, 7294–7298. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Pérez-Andrés, E.; Thevenot, J.; Sandre, O.; Berra, E.; Lecommandoux, S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. J. Control Release 2013, 169, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.-L.; Ziegler, A.; et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat. Nanotechnol. 2012, 7, 536–543. [Google Scholar] [CrossRef]
- Poschenrieder, S.T.; Schiebel, S.K.; Castiglione, K. Stability of polymersomes with focus on their use as nanoreactors. Eng. Life Sci. 2018, 18, 101–113. [Google Scholar] [CrossRef]
- Guo, Y.; Di Mare, L.; Li, R.K.Y.; Wong, J.S.S. Cargo Release from Polymeric Vesicles under Shear. Polymers 2018, 10, 336. [Google Scholar] [CrossRef]
- Rifaie-Graham, O.; Galensowske, N.F.B. Shear Stress-Responsive Polymersome Nanoreactors Inspired by the Marine Bioluminescence of Dinoflagellates. Angew. Chem. Int. Ed. 2021, 60, 904–909. [Google Scholar] [CrossRef]
- Meuler, A.J.; Fleury, G.; Hillmyer, M.A.; Bates, F.S. Structure and Mechanical Properties of an O70 (Fddd) Network-Forming Pentablock Terpolymer. Macromolecules 2008, 41, 5809–5817. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J.M.V.A. Simulating Microscopic Hydrodynamic Phenomena with Dissipative Particle Dynamics. Europhys. Lett. 1992, 19, 155. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Herranz, M.; Santiago, M.; Foteinopoulou, K.; Karayiannis, N.C.; Laso, M. Crystal, Fivefold and Glass Formation in Clusters of Polymers Interacting with the Square Well Potential. Polymers 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]






| Interaction Energy, (in DPD Units) | (Water) | |||
|---|---|---|---|---|
| 25 | ||||
| 40 | 25 | |||
| 40 | 40 | 25 | ||
| (water) | 26–105 | 45 | 26–105 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y. Dissipative Particle Dynamic Simulation on Self-Assembly of Symmetric CBABC Pentablock Terpolymers in Solution. Materials 2023, 16, 7273. https://doi.org/10.3390/ma16237273
Guo Y. Dissipative Particle Dynamic Simulation on Self-Assembly of Symmetric CBABC Pentablock Terpolymers in Solution. Materials. 2023; 16(23):7273. https://doi.org/10.3390/ma16237273
Chicago/Turabian StyleGuo, Yingying. 2023. "Dissipative Particle Dynamic Simulation on Self-Assembly of Symmetric CBABC Pentablock Terpolymers in Solution" Materials 16, no. 23: 7273. https://doi.org/10.3390/ma16237273
APA StyleGuo, Y. (2023). Dissipative Particle Dynamic Simulation on Self-Assembly of Symmetric CBABC Pentablock Terpolymers in Solution. Materials, 16(23), 7273. https://doi.org/10.3390/ma16237273






