Hydrogenation of Carbon Dioxide to Dimethyl Ether on CuO–ZnO/ZSM-5 Catalysts: Comparison of Powder and Electrospun Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalysts Synthesis
2.2.1. Synthesis of Sequential-Precipitated Catalyst
2.2.2. Synthesis of Sequential-Impregnated Catalyst
2.2.3. Fluorine-Assisted Single-Pot Synthesis of CuO–ZnO/ZSM-5 (FSP)
2.2.4. Preparation of the Spinnable Solutions
2.2.5. Fibrous Catalysts Fabrication
2.3. Characterization
2.4. Testing the Catalytic Activity
2.4.1. Description of the Reactor Setup
2.4.2. Criteria and the Performance Indicators for Catalytic CO2 Hydrogenation to DME
2.4.3. Design of Catalytic Tests
3. Results and Discussion
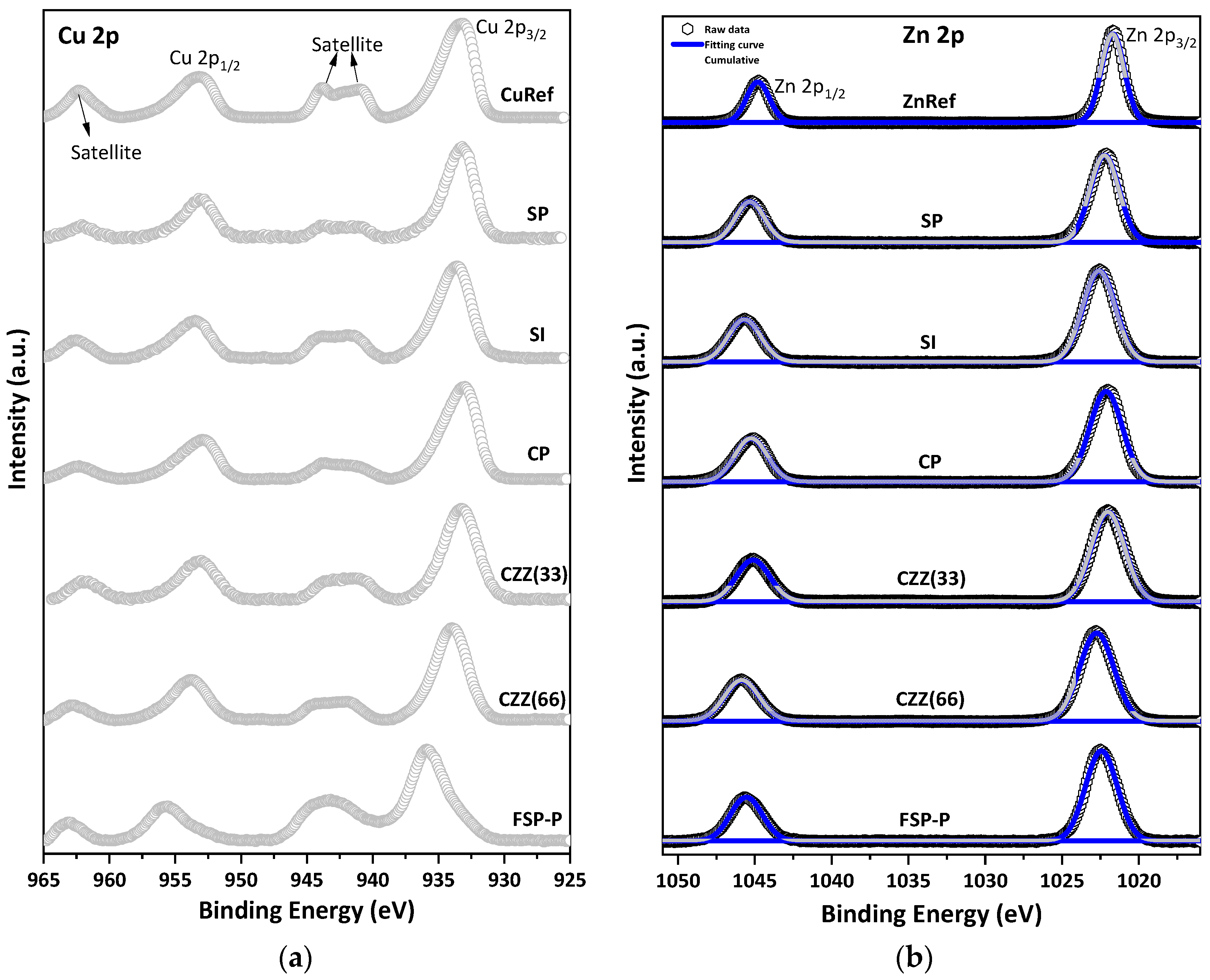
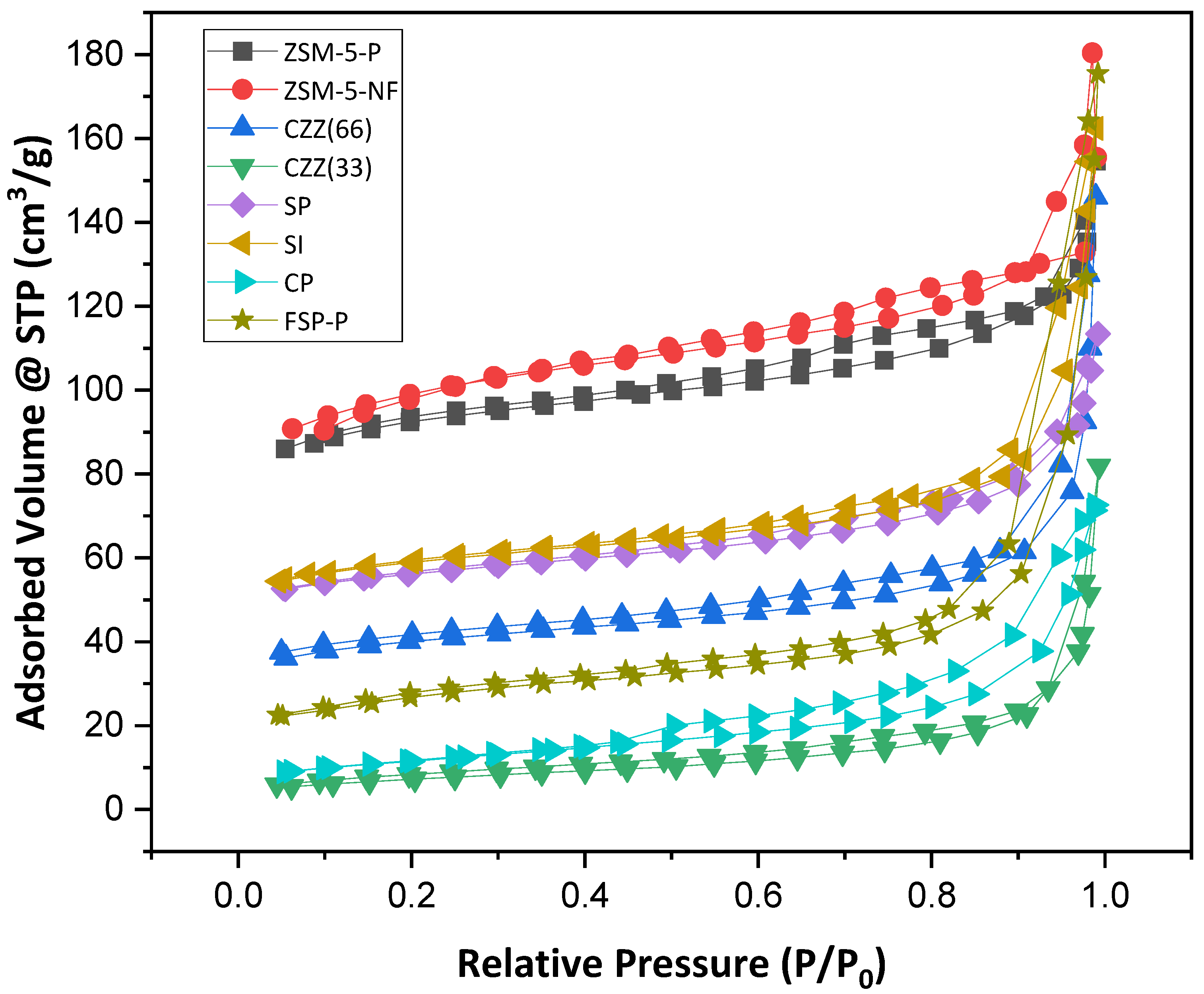
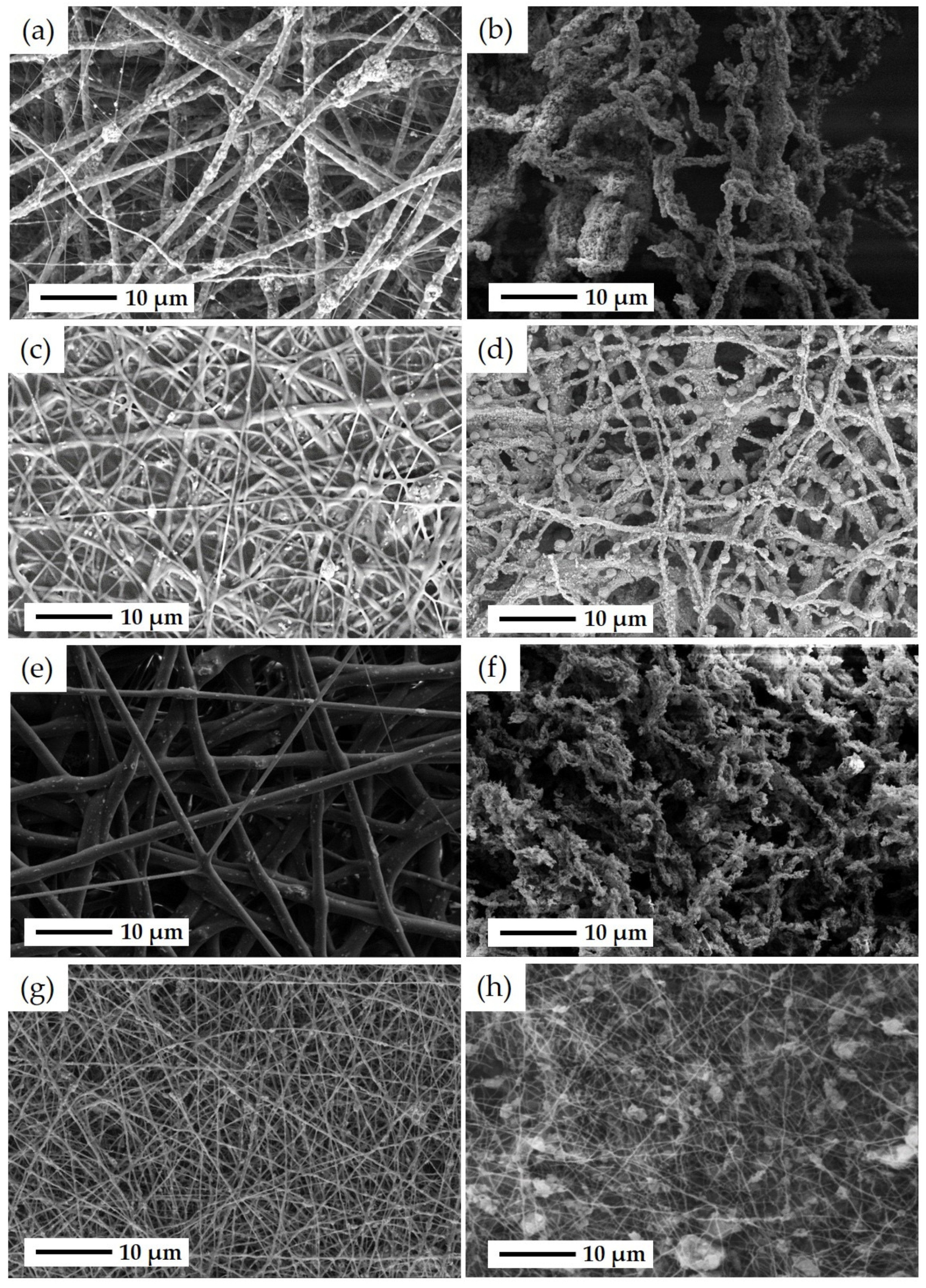
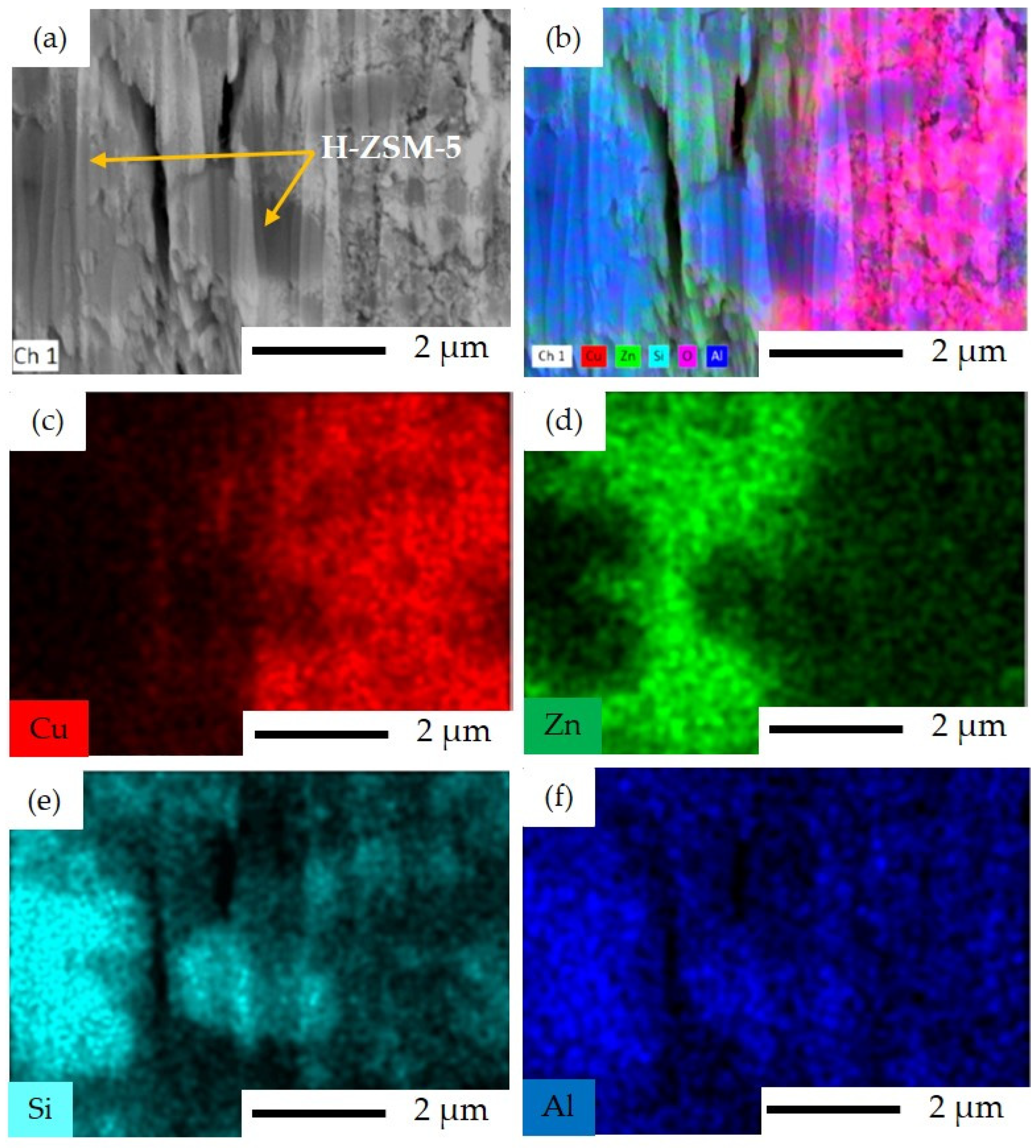
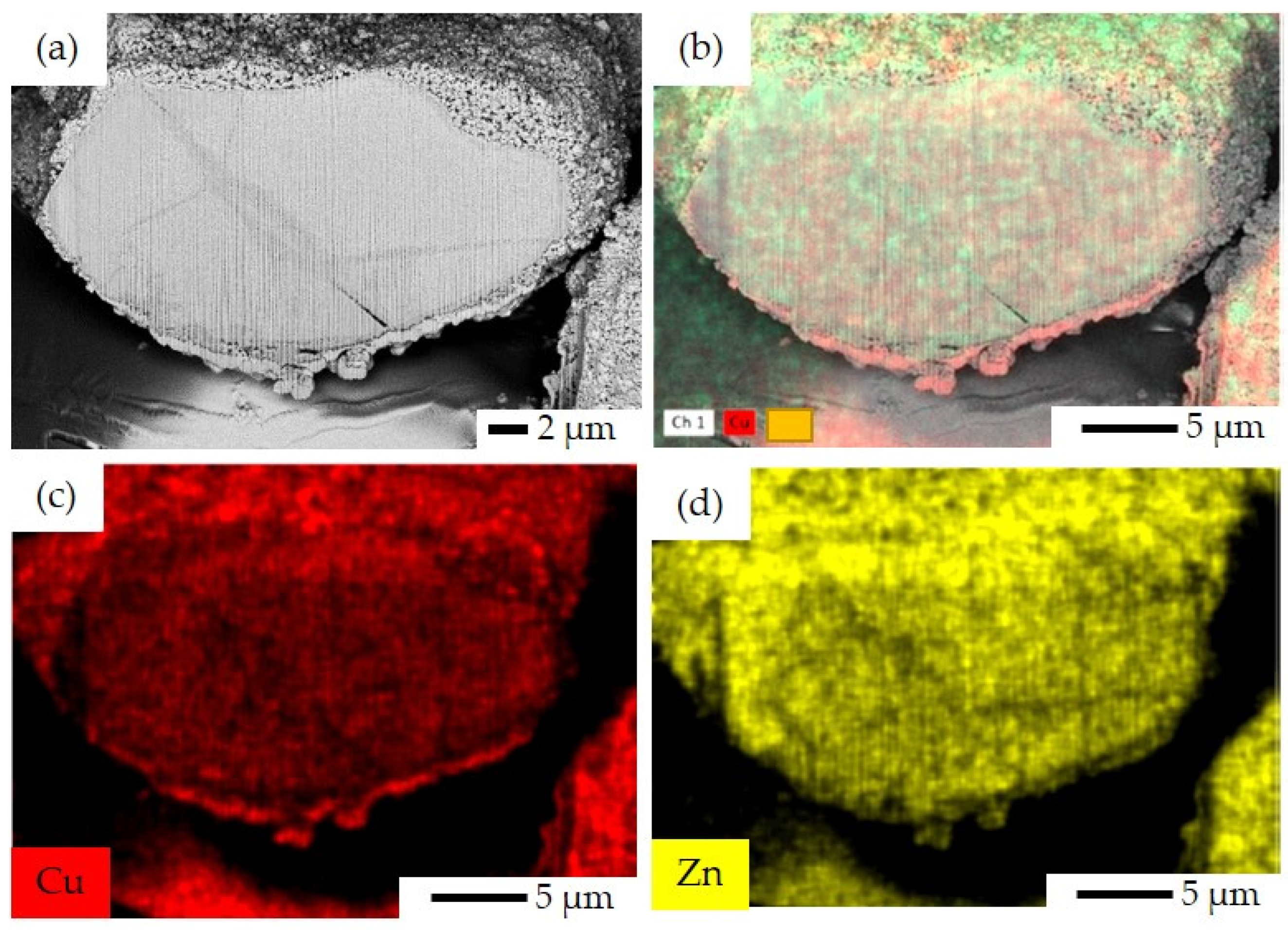
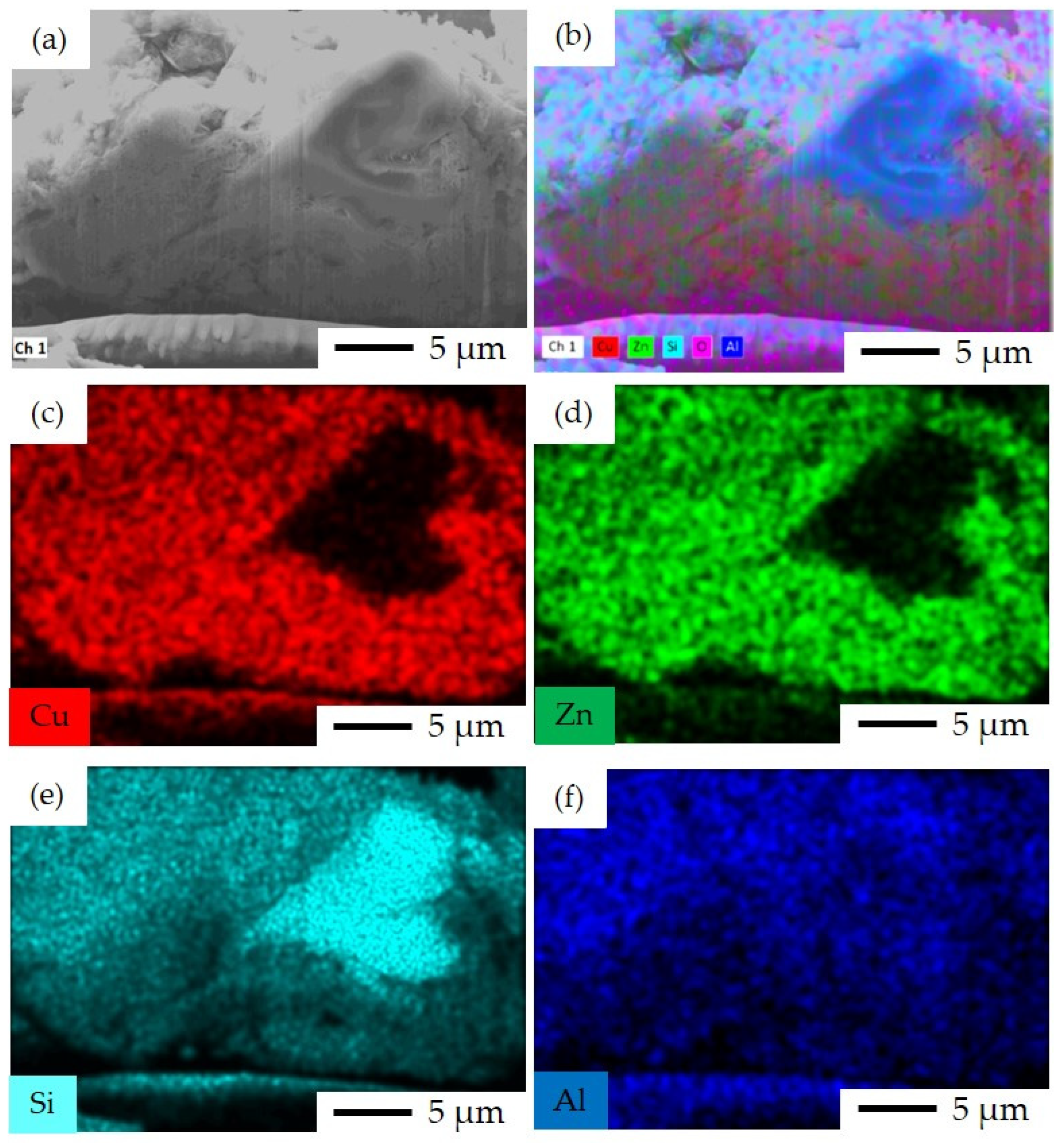
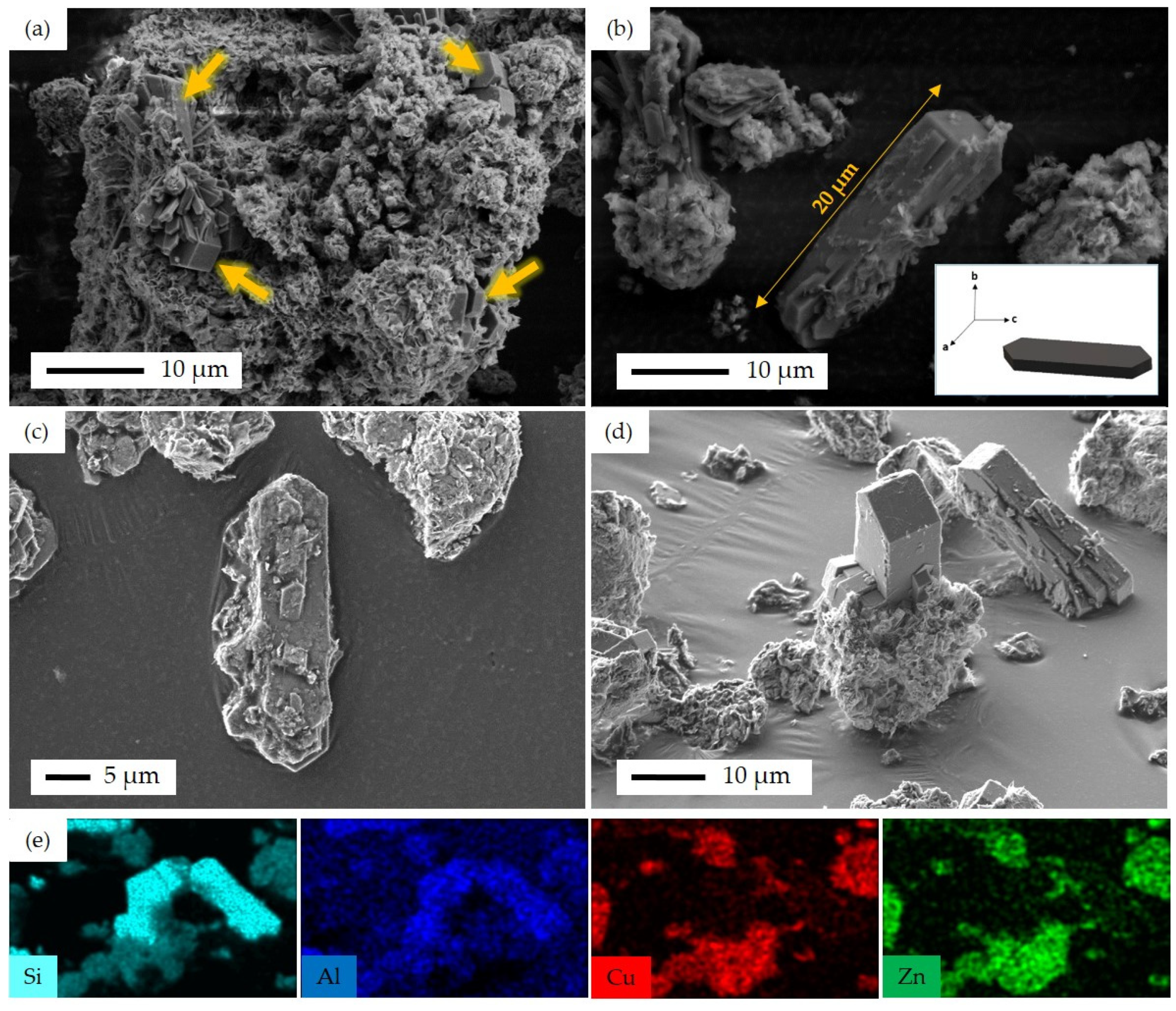
3.1. Characterization Results
3.2. Catalytic Performance
3.2.1. Effect of Reaction Temperature
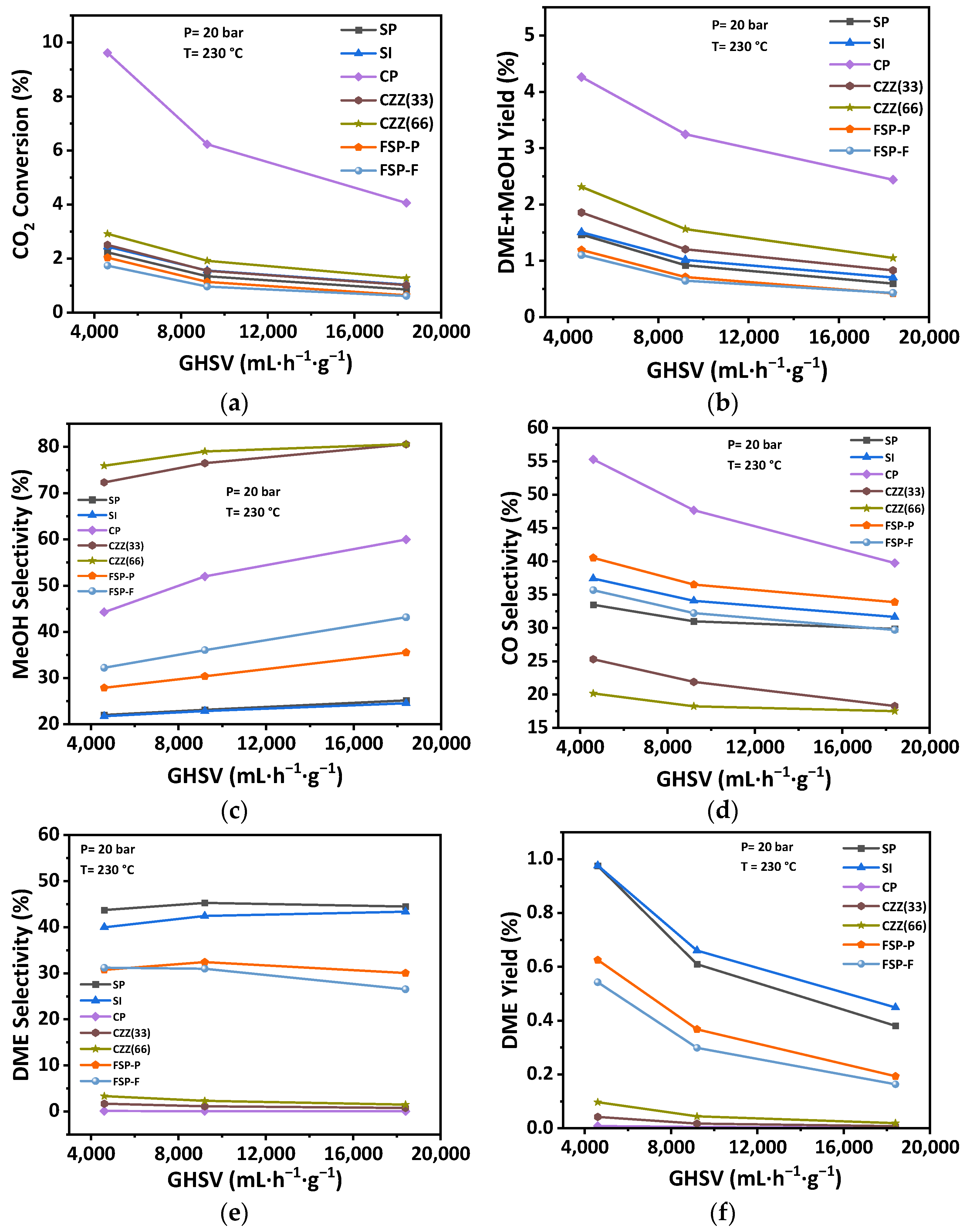
3.2.2. Effect of GHSV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Expression |
|---|---|
| BE | Binding Energy (the energy required to remove an electron from the sample) |
| BET | Brunauer–Emmett–Teller (a model for calculating the specific surface area) |
| BJH | Barrett–Joyner–Halenda (a model for obtaining pore-size distribution) |
| CP | Co-Precipitated Catalyst |
| CZZ | CuO–ZnO/ZSM-5 Electrospun Fibers |
| DI | De-Ionized (water) |
| DME | DiMethyl Ether (Methoxymethane) |
| DMF | N,N-Dimethylformamide |
| DOE | Design Of Experiment |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| ES | Electrospinning |
| F | Fiber |
| FIB | Focused Ion Beam |
| FSP | Fluorine-assisted Single-Pot CuO–ZnO/ZSM-5 |
| GC | Gas Chromatography |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectroscopy |
| MeOH | Methanol (CH3OH) |
| P | Powder |
| PVP | Polyvinylpyrrolidone |
| SEM | Scanning Electron Microscope |
| SI | Sequential Impregnated Catalyst |
| SP | Sequential Precipitated Catalyst |
| TPAOH | Tetra Propyl Ammonium Hydroxide |
| TPD | Temperature Programmed Desorption |
| XRD | X-Ray Diffraction |
| XPS | X-ray photoelectron spectroscopy |
| Symbols | Description | Unit |
|---|---|---|
| GHSV | Gas Hourly Space Velocity (under standard conditions) | |
| Mw | Molecular weight | |
| P | Pressure | bar |
| Q | Total flow rate | |
| S | Selectivity | |
| T | Temperature | °C |
| X | CO2 conversion | |
| x | Mole fraction | - |
| Y | Yield |
References
- Ralf Diemer, e.A. (Ed.) Role of eFuelsin Europe’s sustainable future. In Proceedings of the 9th International DME Conference, Dübendorf, Switzerland, 15–17 June 2022. [Google Scholar]
- SHV Energy. New Joint Venture: Advanced Thermal Conversion Technology for Renewable DME. Available online: https://www.shvenergy.com/news-stories/20210603-new-joint-venture-circular-fuels (accessed on 13 November 2023).
- Cocchi, S. (Ed.) Waste to rDME projects. In Proceedings of the 9th International DME Conference, Dübendorf, Switzerland, 15–17 June 2022. [Google Scholar]
- Velty, A.; Corma, A. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 2023, 52, 1773–1946. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Anise-Hicks, E. (Ed.) Making the DME solution relevant in a world searching for answers. In Proceedings of the 9th International DME Conference, Dübendorf, Switzerland, 15–17 June 2022. [Google Scholar]
- Wang, X.; Jeong, S.Y.; Jung, H.S.; Shen, D.; Ali, M.; Zafar, F.; Chung, C.-H.; Bae, J.W. Catalytic activity for direct CO2 hydrogenation to dimethyl ether with different proximity of bifunctional Cu-ZnO-Al2O3 and ferrierite. Appl. Catal. B Environ. 2023, 327, 122456. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Du, J.; Li, C.; Wang, K.; Liu, L.; Yu, X.; Wang, K.; Liu, N. The influence of composition on the functionality of hybrid CuO–ZnO–Al2O3/HZSM-5 for the synthesis of DME from CO2 hydrogenation. RSC Adv. 2018, 8, 30387–30395. [Google Scholar] [CrossRef]
- Lam, E.; Corral-Pérez, J.J.; Larmier, K.; Noh, G.; Wolf, P.; Comas-Vives, A.; Urakawa, A.; Copéret, C. CO2 hydrogenation on Cu/Al2O3: Role of the metal/support interface in driving activity and selectivity of a bifunctional catalyst. Angew. Chem. Int. Ed. 2019, 58, 13989–13996. [Google Scholar] [CrossRef]
- Wang, S.; Mao, D.; Guo, X.; Wu, G.; Lu, G. Dimethyl ether synthesis via CO2 hydrogenation over CuO–TiO2–ZrO2/HZSM-5 bifunctional catalysts. Catal. Commun. 2009, 10, 1367–1370. [Google Scholar] [CrossRef]
- Krim, K.; Sachse, A.; Le Valant, A.; Pouilloux, Y.; Hocine, S. One step dimethyl ether (DME) synthesis from CO2 hydrogenation over hybrid catalysts containing Cu/ZnO/Al2O3 and nano-sized hollow ZSM-5 zeolites. Catal. Lett. 2023, 153, 83–94. [Google Scholar] [CrossRef]
- Ren, S.; Shoemaker, W.R.; Wang, X.; Shang, Z.; Klinghoffer, N.; Li, S.; Yu, M.; He, X.; White, T.A.; Liang, X. Highly active and selective Cu-ZnO based catalyst for methanol and dimethyl ether synthesis via CO2 hydrogenation. Fuel 2019, 239, 1125–1133. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Ereña, J.; Sierra, I.; Olazar, M.; Bilbao, J. Deactivation and regeneration of hybrid catalysts in the single-step synthesis of dimethyl ether from syngas and CO2. Catal. Today 2005, 106, 265–270. [Google Scholar] [CrossRef]
- Abu-Dahrieh, J.; Rooney, D.; Goguet, A.; Saih, Y. Activity and deactivation studies for direct dimethyl ether synthesis using CuO–ZnO–Al2O3 with NH4ZSM-5, HZSM-5 or γ-Al2O3. Chem. Eng. J. 2012, 203, 201–211. [Google Scholar] [CrossRef]
- Sobczak, J.; Wysocka, I.; Murgrabia, S.; Rogala, A. A review on deactivation and regeneration of catalysts for dimethyl ether synthesis. Energies 2022, 15, 5420. [Google Scholar] [CrossRef]
- Xu, M.; Lunsford, J.H.; Goodman, D.; Bhattacharyya, A. Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts. Appl. Catal. A Gen. 1997, 149, 289–301. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Perspective of dimethyl ether as fuel: Part I. Catalysis. J. CO2 Util. 2019, 32, 299–320. [Google Scholar] [CrossRef]
- Atakan, A.; Mäkie, P.; Söderlind, F.; Keraudy, J.; Björk, E.M.; Odén, M. Synthesis of a Cu-infiltrated Zr-doped SBA-15 catalyst for CO2 hydrogenation into methanol and dimethyl ether. Phys. Chem. Chem. Phys. 2017, 19, 19139–19149. [Google Scholar] [CrossRef]
- Liu, R.; Qin, Z.; Ji, H.; Su, T. Synthesis of dimethyl ether from CO2 and H2 using a Cu–Fe–Zr/HZSM-5 catalyst system. Ind. Eng. Chem. Res. 2013, 52, 16648–16655. [Google Scholar] [CrossRef]
- Maximov, A.L.; Magomedova, M.V.; Galanova, E.G.; Afokin, M.I.; Ionin, D.A. Primary and secondary reactions in the synthesis of hydrocarbons from dimethyl ether over a Pd-Zn-HZSM-5/Al2O3 catalyst. Fuel Process. Technol. 2020, 199, 106281. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Mezzapica, A.; Spadaro, L.; Arena, F.; Frusteri, F. Catalytic behaviour of a bifunctional system for the one step synthesis of DME by CO2 hydrogenation. Catal. Today 2014, 228, 51–57. [Google Scholar] [CrossRef]
- Nejadsalim, A.; Bashiri, N.; Godini, H.R.; Oliveira, R.L.; Tufail Shah, A.; Bekheet, M.F.; Thomas, A.; Schomäcker, R.; Gurlo, A.; Görke, O. Core-Sheath Pt-CeO2/Mesoporous SiO2 Electrospun Nanofibers as Catalysts for the Reverse Water Gas Shift Reaction. Nanomaterials 2023, 13, 485. [Google Scholar] [CrossRef]
- Lee, C.-G.; Javed, H.; Zhang, D.; Kim, J.-H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous Electrospun Fibers Embedding TiO2 for Adsorption and Photocatalytic Degradation of Water Pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Wang, H.; Wang, C. Advanced electrospun nanomaterials for highly efficient electrocatalysis. Inorg. Chem. Front. 2019, 6, 3012–3040. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, R.; Liu, C.; Yuan, L.; Wang, J.; Zhao, Y.; Yu, C. High yield electrosynthesis of hydrogen peroxide from water using electrospun CaSnO3@ carbon fiber membrane catalysts with abundant oxygen vacancy. Adv. Funct. Mater. 2021, 31, 2100099. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid electrospinning for multi-drug delivery systems: Pros and cons, challenges, and future directions. Biomater. Sci. 2023, 11, 37–61. [Google Scholar] [CrossRef]
- Burrola Gándara, L.A.; Vázquez Zubiate, L.; Carrillo Flores, D.M.; Elizalde Galindo, J.T.; Ornelas, C.; Ramos, M. Tuning Magnetic Entropy Change and Relative Cooling Power in La0.7Ca0.23Sr0.07MnO3 Electrospun Nanofibers. Nanomaterials 2020, 10, 435. [Google Scholar] [CrossRef]
- Godini, H.R.; Kumar, S.R.; Tadikamalla, N.; Gallucci, F. Performance analysis of hybrid catalytic conversion of CO2 to DiMethyl ether. Int. J. Hydrog. Energy 2022, 47, 11341–11358. [Google Scholar] [CrossRef]
- Chizallet, C.; Bouchy, C.; Larmier, K.; Pirngruber, G. Molecular Views on Mechanisms of Brønsted Acid-Catalyzed Reactions in Zeolites. Chem. Rev. 2023, 123, 6107–6196. [Google Scholar] [CrossRef]
- Godini, H.R.; Khadivi, M.; Azadi, M.; Görke, O.; Jazayeri, S.M.; Thum, L.; Schomäcker, R.; Wozny, G.; Repke, J.-U. Multi-scale analysis of integrated C1 (CH4 and CO2) utilization catalytic processes: Impacts of catalysts characteristics up to industrial-scale process flowsheeting, part i: Experimental analysis of catalytic low-pressure CO2 to methanol conversion. Catalysts 2020, 10, 505. [Google Scholar] [CrossRef]
- Hudec, P.; Smiešková, A.; Schneider, P.; Ŝolcová, O. Determination of microporous structure of zeolites by t-plot method—State-of-the-art. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1587–1594. ISBN 0167-2991. [Google Scholar]
- Yuk, J.M.; Lee, J.-Y.; Jung, J.H.; Lee, D.U.; Kim, T.W.; Son, D.I.; Choi, W.K. Formation mechanism of ZnSiO3 nanoparticles embedded in an amorphous interfacial layer between a ZnO thin film and an n-Si (001) substrate due to thermal treatment. J. Appl. Phys. 2008, 103, 083520. [Google Scholar] [CrossRef]
- Saedy, S.; Hiemstra, N.; Benz, D.; van Bui, H.; Nolan, M.; van Ommen, J.R. Dual promotional effect of CuxO clusters grown with atomic layer deposition on TiO2 for photocatalytic hydrogen production. Catal. Sci. Technol. 2022, 12, 4511–4523. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Yong, K. CuO/ZnO heterostructured nanorods: Photochemical synthesis and the mechanism of H2S gas sensing. J. Phys. Chem. C 2012, 116, 15682–15691. [Google Scholar] [CrossRef]
- van der Laan, G.; Westra, C.; Haas, C.; Sawatzky, G.A. Satellite structure in photoelectron and Auger spectra of copper dihalides. Phys. Rev. B 1981, 23, 4369. [Google Scholar] [CrossRef]
- Okada, K.; Kotani, A. Multiplet structures of Cu 2p-XPS in La2CuO4, CuO and Cu halides. J. Phys. Soc. Jpn. 1989, 58, 2578–2585. [Google Scholar] [CrossRef]
- Bagus, P.S.; Ilton, E.S.; Nelin, C.J. The interpretation of XPS spectra: Insights into materials properties. Surf. Sci. Rep. 2013, 68, 273–304. [Google Scholar] [CrossRef]
- Rani, A.A.; Ernest, S. Structural, morphological, optical and compositional characterization of spray deposited Ga doped ZnO thin film for dye-sensitized solar cell application. Superlattices Microstruct. 2014, 75, 398–408. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, J. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.; Lu, J.; Feng, H. ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion. Phys. Chem. Chem. Phys. 2016, 18, 601–614. [Google Scholar] [CrossRef]
- Nylund, A.; Olefjord, I. Surface analysis of oxidized aluminium. 1. Hydration of Al2O3 and decomposition of Al(OH)3 in a vacuum as studied by ESCA. Surf. Interface Anal. 1994, 21, 283–289. [Google Scholar] [CrossRef]
- Barrera, A.; Tzompantzi, F.; Campa-Molina, J.; Casillas, J.E.; Pérez-Hernández, R.; Ulloa-Godinez, S.; Velásquez, C.; Arenas-Alatorre, J. Photocatalytic activity of Ag/Al2O3–Gd2O3 photocatalysts prepared by the sol–gel method in the degradation of 4-chlorophenol. RSC Adv. 2018, 8, 3108–3119. [Google Scholar] [CrossRef]
- Mel’gunova, E.A.; Shmakov, A.N.; Larichev, Y.V.; Mel’gunov, M.S. Effect of the concentration of aluminum on the adsorption, texture, and structure characteristics of a mesoporous mineral mesophase of the SBA-15 type. Kinet. Catal. 2009, 50, 456–460. [Google Scholar] [CrossRef]
- Sherwood, P.M.A. Introduction to studies of aluminum and its compounds by XPS. Surf. Sci. Spectra 1998, 5, 1–3. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Yang, F.; Zhou, S.; Kong, Y. High promoting of selective oxidation of ethylbenzene by Mn-ZSM-5 synthesized without organic template and calcination. Res. Chem. Intermed. 2020, 46, 2817–2832. [Google Scholar] [CrossRef]
- Aghaei, E.; Haghighi, M. High temperature synthesis of nanostructured Ce-SAPO-34 catalyst used in conversion of methanol to light olefins: Effect of temperature on physicochemical properties and catalytic performance. J. Porous Mater. 2015, 22, 187–200. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Y.; Cheng, C.; Wang, L.; Fung, K.K.; Wang, N. Correlation between the morphology and performance enhancement of ZnO hierarchical flower photoanodes in quasi-solid dye-sensitized solar cells. J. Nanomater. 2012, 2012, 4. [Google Scholar] [CrossRef]
- Previtali, D.; Longhi, M.; Galli, F.; Di Michele, A.; Manenti, F.; Signoretto, M.; Menegazzo, F.; Pirola, C. Low pressure conversion of CO2 to methanol over Cu/Zn/Al catalysts. The effect of Mg, Ca and Sr as basic promoters. Fuel 2020, 274, 117804. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, S.; Wang, K.; Wu, J. CO2 hydrogenation to dimethyl ether over CuO–ZnO–Al2O3/HZSM-5 prepared by combustion route. RSC Adv. 2014, 4, 16391–16396. [Google Scholar] [CrossRef]
- Takeguchi, T.; Yanagisawa, K.; Inui, T.; Inoue, M. Effect of the property of solid acid upon syngas-to-dimethyl ether conversion on the hybrid catalysts composed of Cu–Zn–Ga and solid acids. Appl. Catal. A Gen. 2000, 192, 201–209. [Google Scholar] [CrossRef]
- Poreddy, R.; Mossin, S.; Jensen, A.D.; Riisager, A. Promoting effect of copper loading and mesoporosity on Cu-MOR in the carbonylation of dimethyl ether to methyl acetate. Catalysts 2021, 11, 696. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2017, 23, 31. [Google Scholar] [CrossRef]
- Jia, G.; Tan, Y.; Han, Y. A comparative study on the thermodynamics of dimethyl ether synthesis from CO hydrogenation and CO2 hydrogenation. Ind. Eng. Chem. Res. 2006, 45, 1152–1159. [Google Scholar] [CrossRef]
- Zhang, Z.; You, R.; Huang, W. Cu2O nanocrystal model catalysts. Chin. J. Chem. 2022, 40, 846–855. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect. Appl. Catal. B Environ. 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Yano, T.; Ebizuka, M.; Shibata, S.; Yamane, M. Anomalous chemical shifts of Cu 2p and Cu LMM Auger spectra of silicate glasses. J. Electron Spectrosc. Relat. Phenom. 2003, 131–132, 133–144. [Google Scholar] [CrossRef]
- Hassanpour, S.; Taghizadeh, M.; Yaripour, F. Preparation, characterization, and activity evaluation of H-ZSM-5 catalysts in vapor-phase methanol dehydration to dimethyl ether. Ind. Eng. Chem. Res. 2010, 49, 4063–4069. [Google Scholar] [CrossRef]
- Niamnuy, C.; Prapaitrakul, P.; Panchan, N.; Seubsai, A.; Witoon, T.; Devahastin, S.; Chareonpanich, M. Synthesis of dimethyl ether via CO2 hydrogenation: Effect of the drying technique of alumina on properties and performance of alumina-supported copper catalysts. ACS Omega 2020, 5, 2334–2344. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; Yu, X.; Huang, Z.; Dai, W.; Yang, L. Improvement on the Catalytic Performance of MoO3 Nanobelts for NH3-SCR Reaction by SnO2-Modification: Enhancement of Acidity and Redox Property. Catal. Lett. 2022, 152, 480–488. [Google Scholar] [CrossRef]
- Kumar, A.; Belwal, M.; Maurya, R.R.; Mohan, V.; Vishwanathan, V. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by Au/AC nanocatalysts. Mater. Sci. Energy Technol. 2019, 2, 526–531. [Google Scholar] [CrossRef]






| Chemicals | Chemical Formula | Molecular Weight (g·mol−1) | Purity (%) | Supplier |
|---|---|---|---|---|
| PVP (Polyvinylpyrrolidone) | (C6H9NO)n | 1,300,000 | N/A 1 | Sigma-Aldrich, Saint Louis, MO, USA |
| PVP (Polyvinylpyrrolidone) | (C6H9NO)n | 40,000 | N/A | Sigma-Aldrich, Saint Louis, MO, USA |
| DMF (N,N-Dimethylformamide) | HCON(CH3)2 | 73.09 | 99.5 | Sigma-Aldrich, Saint Louis, MO, USA |
| Copper(II) nitrate trihydrate | Cu(NO3)2x3H2O | 241.60 | ≥99.5 | Merck, Darmstadt, Germany |
| Zinc nitrate hexahydrate | Zn(NO3)2x6H2O | 297.49 | ≥99.0 | Honeywell, Muskegon, MI, USA |
| H–ZSM-5 (SiO2/Al2O3 = 30/1) | (SiO2)30(Al2O3)H+ | N/A (3.4 μm av. Particle) | N/A | Alfa-Aesar, Kandel, Germany |
| Fumed silica | SiO2 | 60.08 | ≥99.0 | AEROPERL, Hanau, Germany |
| Aluminum nitrate nonahydrate | Al(NO3)3x9H2O | 375.13 | 98.5 | Merck, Darmstadt, Germany |
| TPAOH (Tetra Propyl Ammonium Hydroxide) | (CH3CH2CH2)4N(OH) (1 Molar in H2O) | 203.36 | N/A | Sigma-Aldrich, Saint Louis, MO, USA |
| Ammonium fluoride | NH4F | 37.04 | 95 | Merck, Darmstadt, Germany |
| Catalyst Code | Material System | Desired Structure | Composition (wt.%) | Preparation Method | Calcination Temperature (°C) |
|---|---|---|---|---|---|
| CP | CuO–ZnO | Powder | 50%CuO:50%ZnO | Co-precipitation | 360 |
| SP | H–ZSM-5/CuO–ZnO | Powder | 33.3%CuO:33.3%ZnO:33.3%H–ZSM-5 | Sequential precipitation | 360 |
| SI | H–ZSM-5/CuO–ZnO | Powder | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | Sequential impregnation | 360 |
| H–ZSM-5-P 2 | H–ZSM-5 | Powder | 100%H–ZSM-5-P | Commercial | N/A |
| H–ZSM-5-F 3 | H–ZSM-5 | Fiber | 100%H–ZSM-5-P | Electrospinning | 550 |
| CZZ(33%) | H–ZSM-5/CuO–ZnO | Fiber | 33.3%CuO:33.3%ZnO:33.3%H–ZSM-5 | Electrospinning | 550 |
| CZZ(66%) | H–ZSM-5/CuO–ZnO | Fiber | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | Electrospinning | 550 |
| FSP-P | Fluorine-ZSM-5/CuO–ZnO | Powder | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | Single-pot | 800 |
| FSP-F | Fluorine-ZSM-5/CuO–ZnO | Fiber | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | Electrospinning | 550 |
| Step | GHSV | Gas Flow Each Reactor (H2:CO2:N2) Total Flow | Temperature | Pressure (abs) |
|---|---|---|---|---|
| Units | Approx. mL·h−1·g−1 | NmL·min−1 | °C | Bar |
| 0 | - | Reduction step (50%H2:50%N2) 40 | 300 | 1 |
| 0 | - | Cooling under N2 40 | 260 | 1 |
| 1 | 4600 | (64%H2:21%CO2:15%N2) 25 | 260 | 20 |
| 2 | 4600 | (64%H2:21%CO2:15%N2) 25 | 260 | 10 |
| 3, repro 1 | 4600 | (64%H2:21%CO2:15%N2) 25 | 260 | 20 |
| 4 | 4600 | (64%H2:21%CO2:15%N2) 25 | 230 | 20 |
| 5 | 4600 | (64%H2:21%CO2:15%N2) 25 | 230 | 10 |
| 6 | 9200 | (64%H2:21%CO2:15%N2) 50 | 230 | 20 |
| 7 | 18,400 | (64%H2:21%CO2:15%N2) 100 | 230 | 20 |
| 8 | 4600 | (64%H2:21%CO2:15%N2) 25 | 200 | 10 |
| 9 | 4600 | (64%H2:21%CO2:15%N2) 25 | 200 | 20 |
| Sample | Composition (wt.%) 1 | XRD Phase Composition (wt.%) 2 | Crystallite Size (nm) 2 | Cu/Zn 3 | |||
|---|---|---|---|---|---|---|---|
| ZSM-5 | CuO | ZnO | ZSM-5 | CuO | |||
| H–ZSM-5-P | 100%H–ZSM-5-P | 100 | 0 | 0 | 57 | - | 0 |
| SP | 33.3%CuO:33.3%ZnO:33.3%H–ZSM-5 | 80.6 | 11 | 5.5 | 55 | 8 | 1.17 |
| SI | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | 74.7 | 20 | 5.2 | 57 | 7 | 1.01 |
| CZZ(33) | 33.3%CuO:33.3%ZnO:33.3%H–ZSM-5 | 32.8 | 35.8 | 31.5 | 40 | 28 | 1.12 |
| CZZ(66) | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | 67.4 | 16.7 | 15.9 | 36 | 12 | 1.04 |
| FSP-P | 16.7%CuO:16.7%ZnO:66.6%H–ZSM-5 | 63.4 | 3.9 | 1.6 | 44 | 22 | 1.5 |
| Catalyst/Sample | Composition (wt.%) | SBET (m2·g−1) 1 | Sexternal (m2·g−1) 2 | Smicropore (m2·g−1) 3 | Vmicropore (cm3·g−1) 4 | Vmesopore (cm3·g−1) 4 | Pore Size (nm) 5 |
|---|---|---|---|---|---|---|---|
| CP | 50% CuO:50% ZnO | 40.9 | 38.1 | 2.8 | 0.001 | 0.11 | 3.78 |
| SP | 33.3% CuO:33.3%ZnO:33.3% H–ZSM-5 | 183 | 41.1 | 141.9 | 0.07 | 0.10 | 1.76 |
| SI | 16.7% CuO:16.7% ZnO:66.6% H–ZSM-5 | 191.7 | 42.6 | 149.2 | 0.073 | 0.18 | 1.54 |
| CZZ(33) | 33.3% CuO:33.3% ZnO:33.3% H–ZSM-5 | 26.1 | 25.3 | 0.8 | 0.001 | 0.12 | 1.61 |
| CZZ(66) | 16.7% CuO:16.7% ZnO:66.6% H–ZSM-5 | 132 | 35.8 | 96.1 | 0.048 | 0.18 | 1.68 |
| FSP-P | 16.7% CuO:16.7% ZnO:66.6% H–ZSM-5 | 91.8 | 42.04 | 49.8 | 0.025 | 0.25 | 1.68 |
| FSP-F | 16.7% CuO:16.7% ZnO:66.6% H–ZSM-5 | 69. 6 | 51.1 | 18.5 | 0.01 | 0.19 | 1.61 |
| H–ZSM-5-P | 16.7% CuO:16.7% ZnO:66.6% H–ZSM-5 | 308.1 | 51.04 | 257.04 | 0.13 | 0.14 | 1.76 |
| H–ZSM-5-F | 16.7% CuO:16.7% ZnO:66.6% H–ZSM-5 | 324.6 | 71.5 | 253.1 | 0.12 | 0.15 | 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejadsalim, A.; Godini, H.R.; Ramesh Kumar, S.; Gallucci, F.; Kober, D.; Gurlo, A.; Görke, O. Hydrogenation of Carbon Dioxide to Dimethyl Ether on CuO–ZnO/ZSM-5 Catalysts: Comparison of Powder and Electrospun Structures. Materials 2023, 16, 7255. https://doi.org/10.3390/ma16237255
Nejadsalim A, Godini HR, Ramesh Kumar S, Gallucci F, Kober D, Gurlo A, Görke O. Hydrogenation of Carbon Dioxide to Dimethyl Ether on CuO–ZnO/ZSM-5 Catalysts: Comparison of Powder and Electrospun Structures. Materials. 2023; 16(23):7255. https://doi.org/10.3390/ma16237255
Chicago/Turabian StyleNejadsalim, Aidin, Hamid Reza Godini, Sanjay Ramesh Kumar, Fausto Gallucci, Delf Kober, Aleksander Gurlo, and Oliver Görke. 2023. "Hydrogenation of Carbon Dioxide to Dimethyl Ether on CuO–ZnO/ZSM-5 Catalysts: Comparison of Powder and Electrospun Structures" Materials 16, no. 23: 7255. https://doi.org/10.3390/ma16237255
APA StyleNejadsalim, A., Godini, H. R., Ramesh Kumar, S., Gallucci, F., Kober, D., Gurlo, A., & Görke, O. (2023). Hydrogenation of Carbon Dioxide to Dimethyl Ether on CuO–ZnO/ZSM-5 Catalysts: Comparison of Powder and Electrospun Structures. Materials, 16(23), 7255. https://doi.org/10.3390/ma16237255



_CHAINOK.jpg)





