UV-Vis Sintering Process for Fabrication of Conductive Coatings Based on Ni-Ag Core–Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
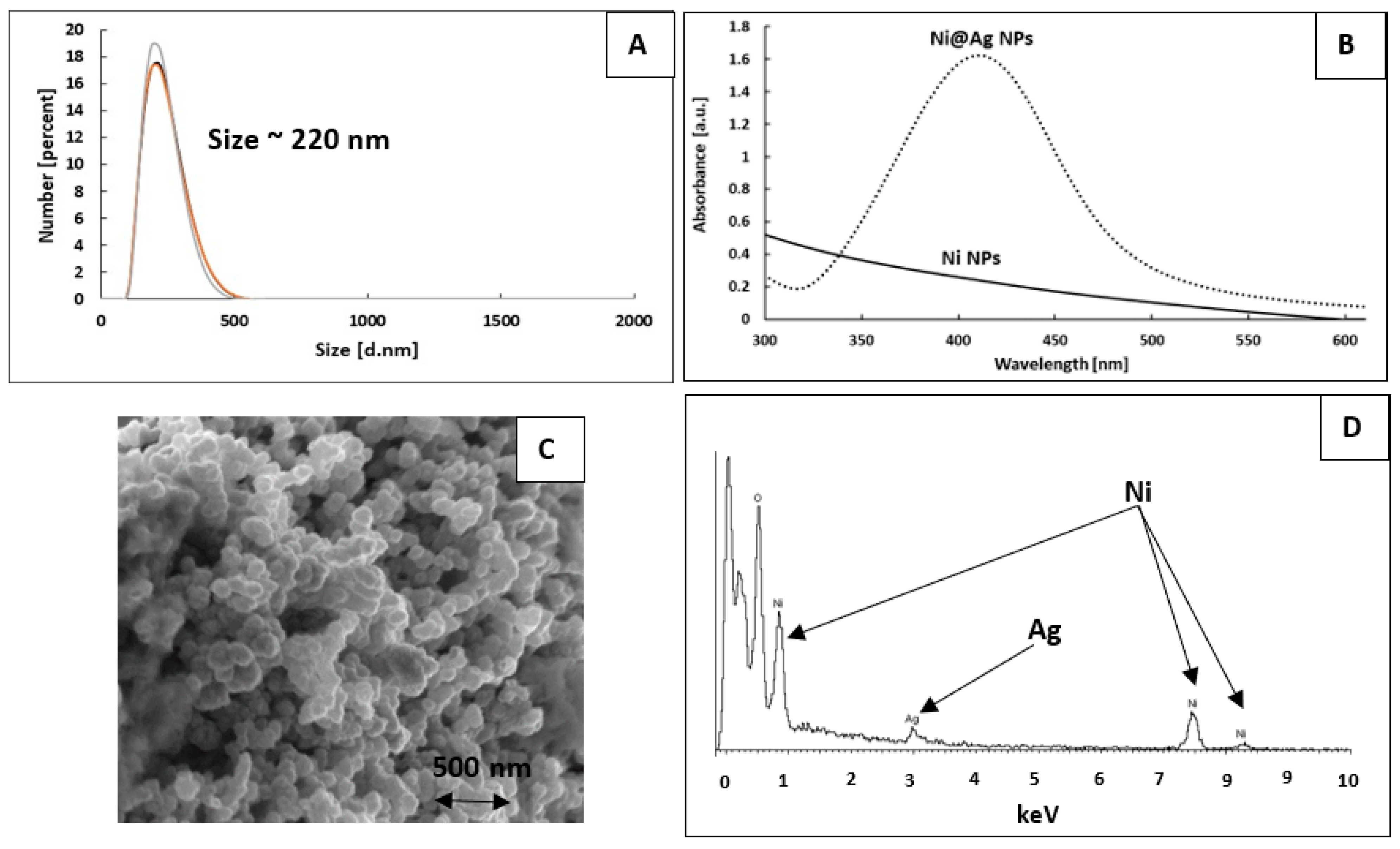
2.2. Fabrication of Metallic Ink
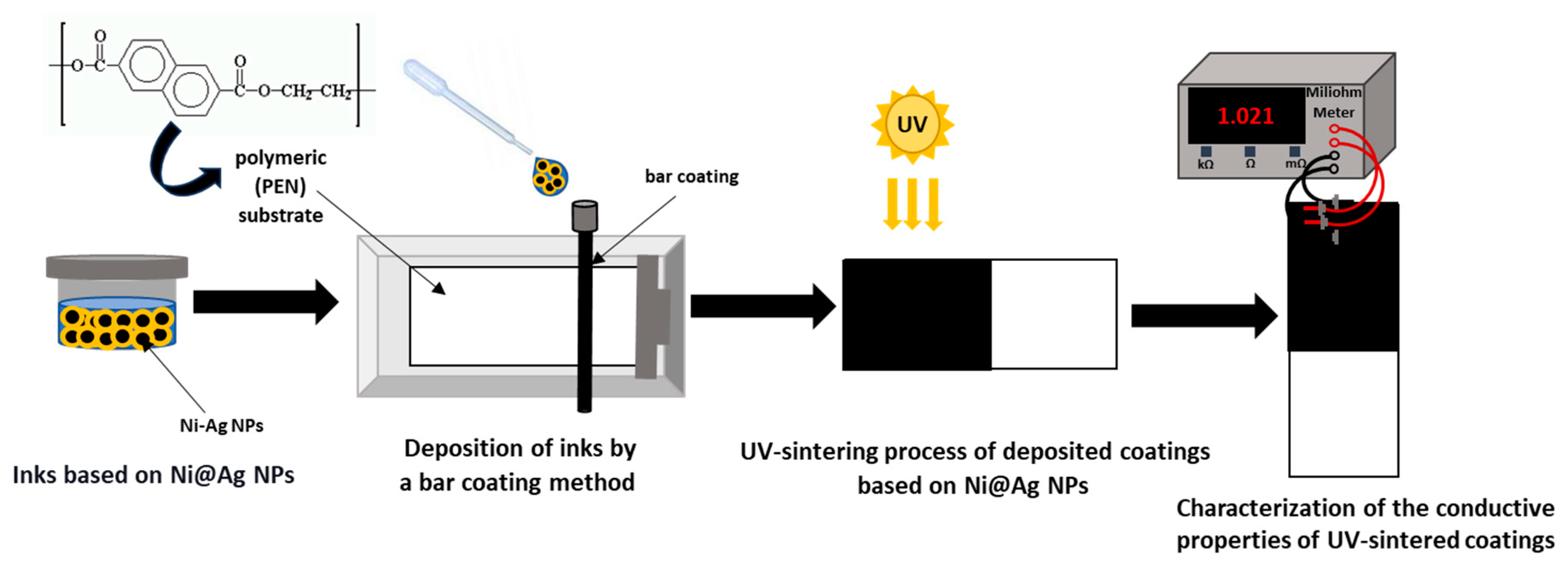
2.3. Preparation of Conductive Coatings
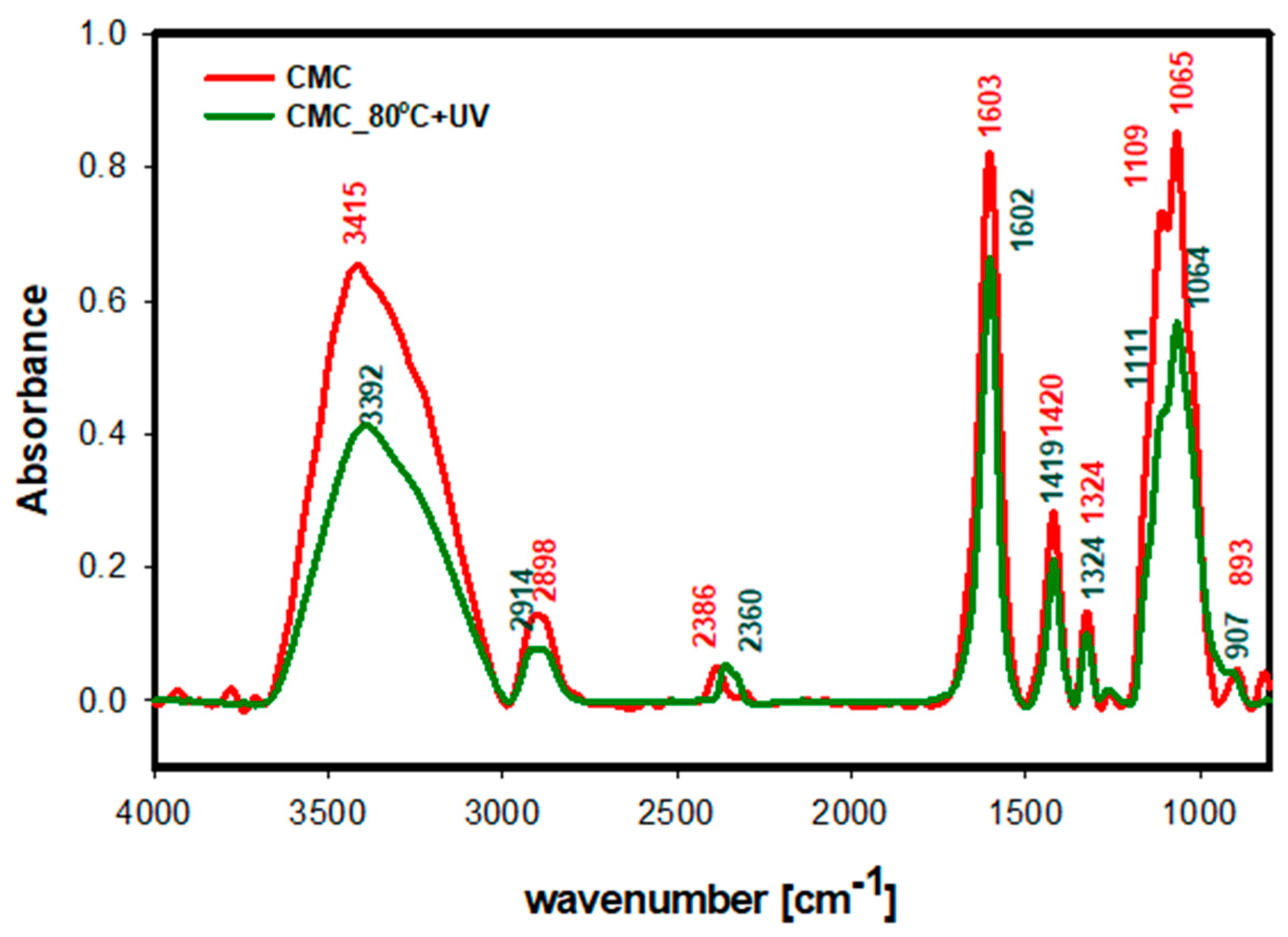
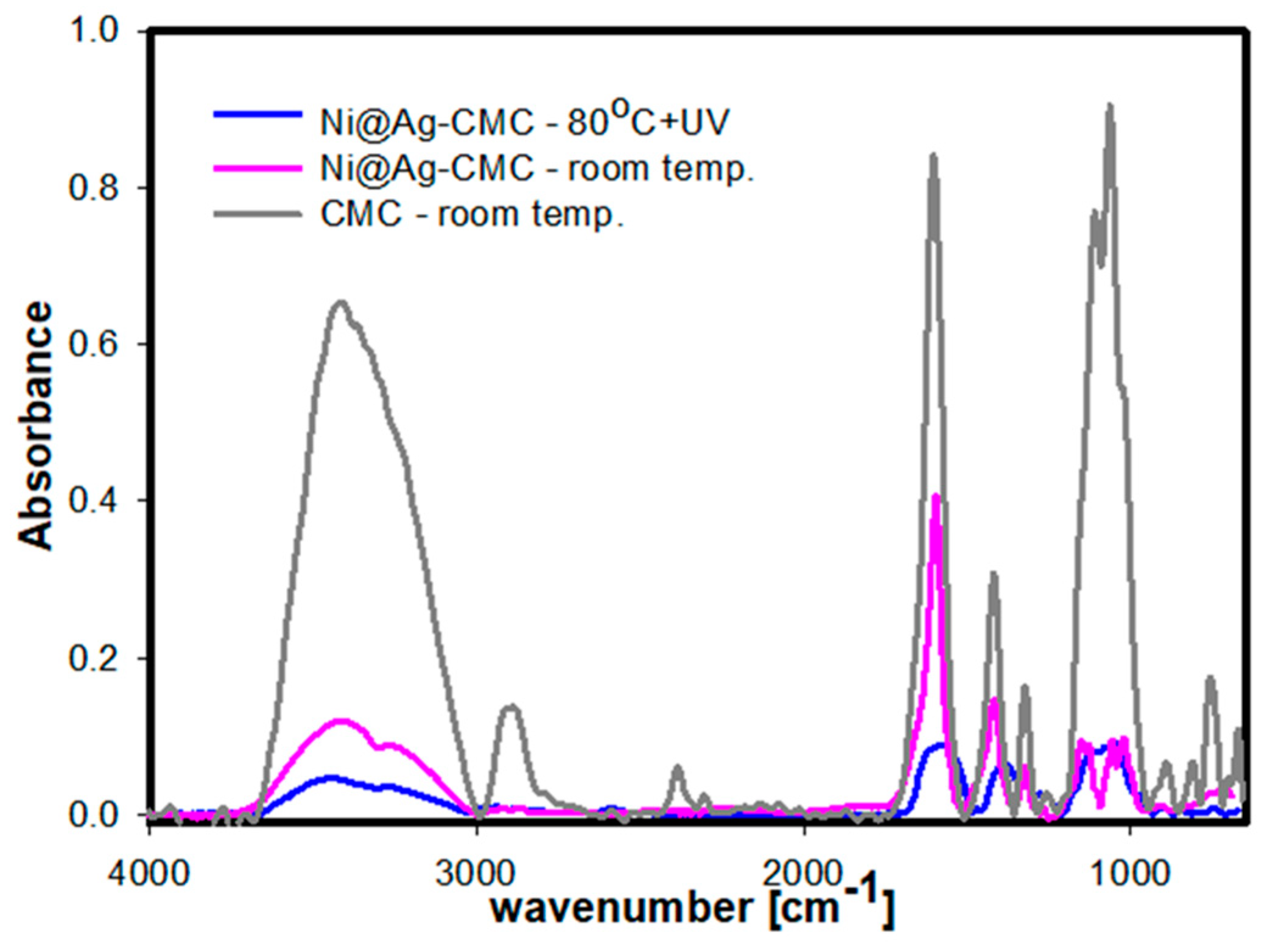
2.4. Characterization
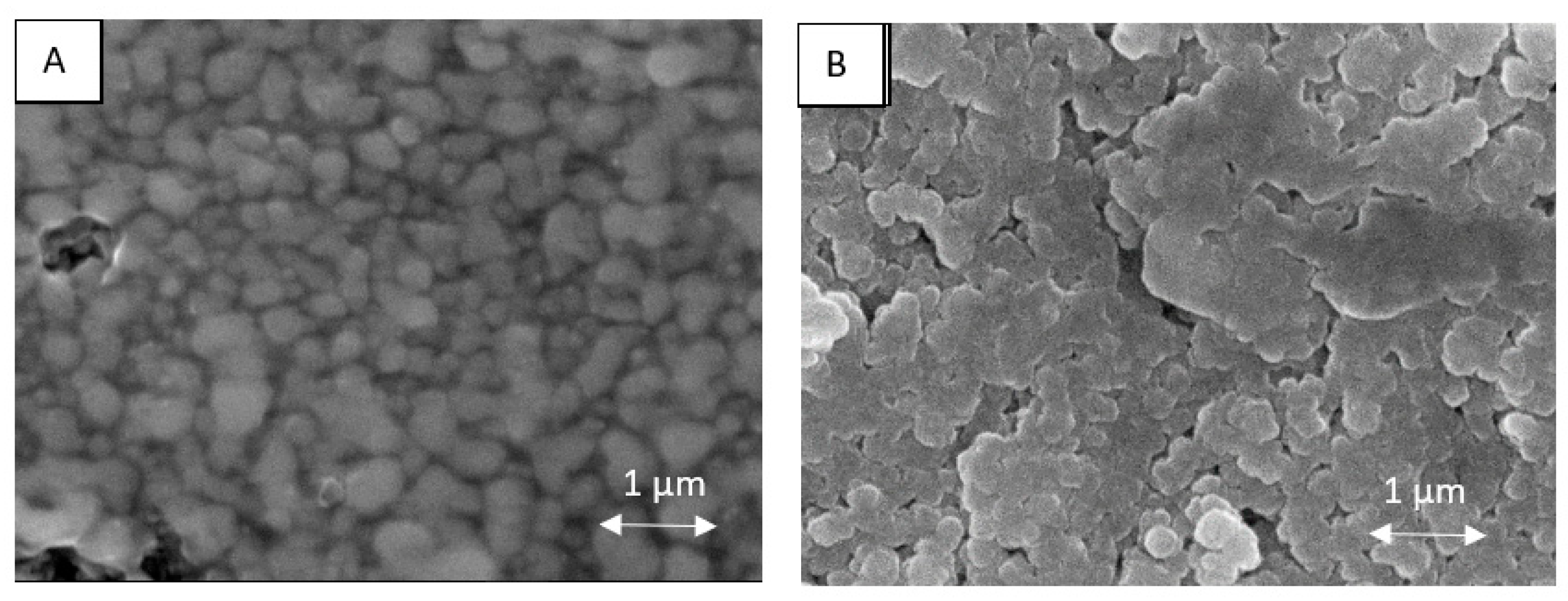
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for 2D and 3D printed flexible electronics. Chem. Soc. Rev. 2018, 48, 1712–1740. [Google Scholar] [CrossRef]
- Verboven, I.; Deferme, W. Printing of flexible light emitting devices: A review on different technologies and devices, printing technologies and state-of-the-art applications and future prospects. Prog. Mater. Sci. 2020, 118, 100760. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Yang, T.-Y.; Suhonen, R.; Kemppainen, A.; Hwang, K.; Jeon, N.J.; Seo, J. Roll-to-roll gravure-printed flexible per-ovskite solar cells using eco-friendly antisolvent bathing with wide processing window. Nat. Commun. 2020, 11, 5146. [Google Scholar] [CrossRef]
- Reinhold, I. Inkjet printing of functional materials and post-processing. In Nanomaterials for 2D and 3D Printing; Kamyshny, A., Magdassi, S., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 27–49. [Google Scholar]
- Liao, Y.; Zhang, R.; Wang, H.; Ye, S.; Zhou, Y.; Ma, T.; Zhu, J.; Pfefferle, L.D.; Qian, J. Highly conductive carbon-based aqueous inks toward electroluminescent devices, printed capacitive sensors and flexible wearable electronics. RSC Adv. 2019, 9, 15184–15189. [Google Scholar] [CrossRef]
- Mavuri, A.; Mayes, A.G.; Alexander, M.S. Inkjet Printing of Polyacrylic Acid-Coated Silver Nanoparticle Ink onto Paper with Sub-100 Micron Pixel Size. Materials 2019, 12, 2277. [Google Scholar] [CrossRef]
- Tomotoshi, D.; Kawasaki, H. Surface and Interface Designs in Copper-Based Conductive Inks for Printed/Flexible Electronics. Nanomaterials 2020, 10, 1689. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, T.; Li, K.; Huang, F.; Tang, C. Preparation of Bimodal Silver Nanoparticle Ink Based on Liquid Phase Reduction Method. Nanomaterials 2022, 12, 560. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Matallic nanoinks for inkjet printing of conductive 2D and 3D structures. In Nanomaterials for 2D and 3D Printing; Kamyshny, A., Magdassi, S., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 119–160. [Google Scholar]
- Sohn, J.H.; Pham, L.Q.; Kang, H.S.; Park, J.H.; Lee, B.C.; Kang, Y.S. Preparation of conducting silver paste with Ag nanoparticles prepared by e-beam irradiation. Radiat. Phys. Chem. 2010, 79, 1149–1153. [Google Scholar] [CrossRef]
- Greer, J.R.; Street, R.A. Thermal cure effects on electrical performance of nanoparticle silver inks. Acta Mater. 2007, 55, 6345–6349. [Google Scholar] [CrossRef]
- Buffat, P.; Borel, J.-P. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Berezin, O.; Kamyshny, A. Triggering the Sintering of Silver Nanoparticles at Room Temperature. ACS Nano 2010, 4, 1943–1948. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Mihailescu, C.F.; Anghel, D.F.; Magdassi, S. Conductive Inks with a “Built-In” Mechanism that Enables Sintering at Room Temperature. ACS Nano 2011, 5, 3354–3359. [Google Scholar] [CrossRef]
- Corsino, D.C.; Balela, M.D.L. Room temperature sintering of printer silver nanoparticle conductive ink. Mater. Sci. Eng. 2017, 264, 012020. [Google Scholar] [CrossRef]
- Layani, M.; Magdassi, S. Flexible transparent conductive coatings by combining self-assembly with sintering of silver nano-particles performed at room temperature. J. Mater. Chem. 2011, 21, 15378. [Google Scholar] [CrossRef]
- Kwon, J.; Cho, H.; Eom, H.; Lee, H.; Suh, Y.D.; Moon, H.; Shin, J.; Hong, S.; Ko, S.H. Low-Temperature Oxidation-Free Se-lective Laser Sintering of Cu Nanoparticle Paste on a Polymer Substrate for the Flexible Touch Panel Applications. ACS Appl. Mater. Interfaces 2016, 8, 11575–11582. [Google Scholar] [CrossRef]
- Noha, J.; Hab, J.; Kima, D. Femtosecond and nanosecond laser sintering of silver nanoparticles on a flexible substrate. Appl. Surf. Sci. 2020, 511, 145574. [Google Scholar] [CrossRef]
- Hernandez-Castaneda, J.C.; Lok, B.K.; Zheng, H. Laser sintering of Cu nanoparticles on PET polymer substrate for printed electronics at different wavelengths and process conditions. Front. Mech. Eng. 2020, 15, 303–318. [Google Scholar] [CrossRef]
- Nam, V.B.; Shin, J.; Choi, A.; Choi, H.; Ko, S.H.; Lee, D. High-temperature, thin, flexible and transparent Ni-based heaters patterned by laser-induced reductive sintering on colorless polyimide. J. Mater. Chem. C 2021, 9, 5652–5661. [Google Scholar] [CrossRef]
- Bischoff, K.; Esen, C.; Hellmann, R. Preparation of Dispersed Copper(II) Oxide Nanosuspensions as Precursor for Femtosecond Reductive Laser Sintering by High-Energy Ball Milling. Nanomaterials 2023, 13, 2693. [Google Scholar] [CrossRef]
- Tumkin, I.I.; Khairullina, E.M.; Panov, M.S.; Yoshidomi, K.; Mizoshiri, M. Copper and Nickel Microsensors Produced by Selective Laser Reductive Sintering for Non-Enzymatic Glucose Detection. Materials 2021, 14, 2493. [Google Scholar] [CrossRef]
- Polzinger, B.; Schoen, F.; Matic, V.; Keck, J.; Willeck, H.; Eberhardt, W.; Kueck, H. UV-sintering of inkjet-printed conductive silver tracks. In Proceedings of the 2011 11th IEEE International Conference on Nanotechnology, Portland, OR, USA, 15–19 August 2011; pp. 201–204. [Google Scholar] [CrossRef]
- Saleh, E.; Zhang, F.; He, Y.; Vaithilingam, J.; Fernandez, J.L.; Wildman, R.; Ashcroft, I.; Hague, R.; Dickens, P.; Tuck, C. 3D Inkjet Printing of Electronics Using UV Conversion. Adv. Mater. Technol. 2017, 2, 1700134. [Google Scholar] [CrossRef]
- Li, Y.; Verbiest, T.; Strobbe, R.; Vankelecom, I.F.J. Silver nanoparticles as localized “nano-heaters” under LED light irradiation to improve membrane performance. J. Mater. Chem. A 2013, 2, 3182–3189. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.-S. Flash light sintering of nickel nanoparticles for printed electronics. Thin Solid Films 2014, 550, 575–581. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Szendera, F.; Pawłowski, R.; Szczepanowicz, K. Nanocomposite Inks Based on Nickel–Silver Core–Shell and Silver Nanoparticles for Fabrication Conductive Coatings at Low-Temperature Sintering. Colloids Interfaces 2021, 5, 15. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Staśko, D.; Pawłowski, R.; Mordarski, G.; Kamyshny, A.; Szczepanowicz, K. Polydispersity vs. Monodispersity. How the Properties of Ni-Ag Core-Shell Nanoparticles Affect the Conductivity of Ink Coatings. Materials 2021, 14, 2304. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Pawłowski, R.; Sobik, P.; Kamyshny, A.; Szczepanowicz, K. Effect of Oxalic Acid Treatment on Conductive Coatings Formed by Ni@Ag Core–Shell Nanoparticles. Materials 2022, 15, 305. [Google Scholar] [CrossRef]
- K Hand Coater, Pre-Press Equipment, RK Print Coat Instruments. Available online: https://www.rkprint.com/products/khand-coater/ (accessed on 25 October 2022).
- Extech Instruments, Milliohm Meters. Available online: http://www.extech.com/products/resources/380560_380562_UM-en.pdf (accessed on 25 October 2022).
- Kamyshny, A.; Steinke, J.; Magdassi, S. Metal-based inkjet inks for printed electronics. Open Appl. Phys. J. 2011, 4, 19–36. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Gold and silver nanoparticles: A class of chromophores with colors tunable in the range from 400 to 750 nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Peng, P.; Hu, A.; Duley, W.W.; Zhou, Y. Controlled joining of Ag nanoparticles with femtosecond laser radiation. J. Appl. Phys. 2012, 112, 123519. [Google Scholar] [CrossRef]
- Wünscher, S.; Abbel, R.; Perelaer, J.; Schubert, U.S. Progress of alternative sintering approaches of inkjet-printed metal inks and their application for manufacturing of flexible electronic devices. J. Mater. Chem. C 2014, 2, 10232–10261. [Google Scholar] [CrossRef]
- Kim, M.; Jee, H.; Lee, J. Photo-sintered silver thin films by a high-power UV-LED module for flexible electronic applications. Nanomaterials 2021, 11, 2840. [Google Scholar] [CrossRef]
- Altay, B.N.; Turkani, V.S.; Pekarovicova, A.; Fleming, P.D.; Atashbar, M.Z.; Bolduc, M.; Cloutier, S.G. One step photonic curing of screen printed conductive Ni fake electrodes for use in fexible electronics. Sci. Rep. 2021, 11, 3393. [Google Scholar] [CrossRef]
- Nam, V.B.; Shin, J.; Yoon, Y.; Giang, T.T.; Kwon, J.; Suh, Y.D.; Yeo, J.; Hong, S.; Ko, S.H.; Lee, D. Highly Stable Ni-Based Flexible Transparent Conducting Panels Fabricated by Laser Digital Patterning. Adv. Funct. Mater. 2019, 29, 1806895. [Google Scholar] [CrossRef]
- Wang, J.; Somasundaran, P. Adsorption and conformation of CMC at solid–liquid interfaces using spectroscopic, AFM and allied techniques. J. Colloid Interface Sci. 2005, 291, 75–83. [Google Scholar] [CrossRef]
- Basta, A.H.; El-Saied, H.; Hasanin, M.S.; El-Deftar, M.M. Green carboxymethyl cellulose-silver complex versus cellulose origins in biological activity applications. Int. J. Biol. Macromol. 2018, 107, 1364–1372. [Google Scholar] [CrossRef]
- Donati, I.; Travan, A.; Pelillo, C.; Scarpa, T.; Coslovi, A.; Bonifacio, A.; Sergo, V.; Paoletti, S. Polyol Synthesis of Silver Nanoparticles: Mechanism of Reduction by Alditol Bearing Polysaccharides. Biomacromolecules 2009, 10, 210–213. [Google Scholar] [CrossRef]
- Basuny, M.; Ali, O.I.; Abd El-Gawad, A.; Bakr, F.M.; Salama, M.T. A fast green synthesis of Ag nanoparticles in carboxymethyl cellulose (CMC) through UV irradiation technique for antibacterial applications. Sol.-Gel. Sci. Technol. 2015, 75, 530–540. [Google Scholar] [CrossRef]
- Prajapat, A.L.; Gogate, P.R. Depolymerization of carboxymethyl cellulose using hydrodynamic cavitation combined with ultraviolet irradiation and potassium persulfate. Ultrason. Sonochemistry 2019, 51, 258–263. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajor-Świerzy, A.; Szyk-Warszyńska, L.; Duraczyńska, D.; Szczepanowicz, K. UV-Vis Sintering Process for Fabrication of Conductive Coatings Based on Ni-Ag Core–Shell Nanoparticles. Materials 2023, 16, 7218. https://doi.org/10.3390/ma16227218
Pajor-Świerzy A, Szyk-Warszyńska L, Duraczyńska D, Szczepanowicz K. UV-Vis Sintering Process for Fabrication of Conductive Coatings Based on Ni-Ag Core–Shell Nanoparticles. Materials. 2023; 16(22):7218. https://doi.org/10.3390/ma16227218
Chicago/Turabian StylePajor-Świerzy, Anna, Lilianna Szyk-Warszyńska, Dorota Duraczyńska, and Krzysztof Szczepanowicz. 2023. "UV-Vis Sintering Process for Fabrication of Conductive Coatings Based on Ni-Ag Core–Shell Nanoparticles" Materials 16, no. 22: 7218. https://doi.org/10.3390/ma16227218
APA StylePajor-Świerzy, A., Szyk-Warszyńska, L., Duraczyńska, D., & Szczepanowicz, K. (2023). UV-Vis Sintering Process for Fabrication of Conductive Coatings Based on Ni-Ag Core–Shell Nanoparticles. Materials, 16(22), 7218. https://doi.org/10.3390/ma16227218





