Review on Synthesis and Properties of Lithium Lanthanum Titanate
Abstract
:1. Introduction
| Type of Material | Composition | Ionic Conductivity (S/cm) | Ref. |
|---|---|---|---|
| polymer—inert material | LiAlO2—PEO 1—LiClO4 | 10−4 (at 60 °C) | [12] |
| TiO2—PEO—LiClO4 | 10−5 (at 30 °C) | [13] | |
| Al2O3—PEO—LiClO4 | 10−2 (60 °C) | [14] | |
| SiO2—Al2O3—PVDF-HFP 2-LiPF3(CF3CF2)3 | 10−3 (25 °C) | [15] | |
| super ionic conductor—polymer | Li5La3Zr2O12—PEO—LiClO4 | 4.42 × 10−4 (55 °C) | [16] |
| Li2.5Al0.5Ge1.5(PO4)3—PEO—LiClO4 | 2.6 × 10−4 (55 °C) | [17] | |
| Li10GeP2S12—PEO—LiTFSI 3 | 10−5 (25 °C) | [18] | |
| Li6.2Ga0.3La2.95Rb0.05Zr2O12—PVDF—LiTFSI | 1.62 × 10−3 (25 °C) | [19] | |
| perovskite—polymer | Li0.33La0.557TiO3 nanowires—PAN-LiClO4 | 2.4 × 10−4 (25 °C) | [20] |
| solid polymer—ionic liquid | LiTFSI—EMImTFSI 4—PEO | 10−2 | [21] |
| metal-organic framework | Zn4O(BDC)3 5—PEO | 3.16 × 10−5 | [22] |
| solid polymer—cellulose | PEO—LiClO4—cellulose | 10−4 (25 °C) | [23] |
1.1. Applications
1.1.1. Li Extraction
1.1.2. Battery Electrode
1.1.3. Sensors
1.1.4. Electronic Devices
1.2. Introduction to Perovskites
2. Synthesis
2.1. Sol-Gel Synthesis
2.2. Solid State Reaction
2.3. Thin Films
2.4. Electrospinning
2.5. Comparison between the Typical Methods of Synthesis for Bulk LLTO Samples
3. Structural Properties of LLTO
4. Conduction Mechanism
4.1. Charge Carrier Concentration
4.2. Grain Boundaries
4.3. Domain Boundaries
4.4. Conductive Paths and Relaxation Mechanisms
5. Effects of Substitutions
5.1. A Site Substitution
5.2. B-Site Substitution
5.3. Oxygen Site Substitution and Other Substitutions
6. Composite Electrolyte
6.1. LLNO Nanowires—Polymer Composite Electrolytes
6.2. Oxid—Oxide Hybrid Electrolytes
6.3. Dendrite Growth Suppression and Chemical Stability
6.4. Organic Matrix—LLTO Composites
7. Thin Films and Lamellar Structures
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le, H.T.; Ngo, D.T.; Kim, Y.-J.; Park, C.-N.; Park, C.-J. A perovskite-structured aluminium-substituted lithium lanthanum titanate as a potential artificial solid-electrolyte interface for aqueous rechargeable lithium-metal-based batteries. Electrochim. Acta 2017, 248, 232–242. [Google Scholar] [CrossRef]
- Li, B.; Su, Q.; Yu, L.; Dong, S.; Zhang, M.; Ding, S.; Du, G.; Xu, B. Ultrathin, flexible, and sandwiched structure composite polymer electrolyte membrane for solid-state lithium batteries. J. Membr. Sci. 2021, 618, 118734. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Yang, X.; Xiao, J.; Li, Y.; Sun, L.; Ren, X.; Zhang, P.; Mi, H. Fluoroethylene carbonate-Li-ion enabling composite solid-state electrolyte and lithium metal interface self-healing for dendrite-free lithium deposition. Chem. Eng. J. 2021, 408, 127254. [Google Scholar] [CrossRef]
- Liu, K.; Wu, M.; Jiang, H.; Lin, Y.; Xu, J.; Zhao, T. A Janus-faced, perovskite nanofiber framework reinforced composite electrolyte for high-voltage solid lithium-metal batteries. J. Power Sources 2022, 526, 231172. [Google Scholar] [CrossRef]
- Okumura, T.; Yokoo, K.; Fukutsuka, T.; Uchimoto, Y.; Saito, M.; Amezawa, K. Improvement of Li-ion conductivity in A-site disordering lithium-lanthanum-titanate perovskite oxides by adding LiF in synthesis. J. Power Sources 2009, 189, 536–538. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Guo, J.; Du, Z.; Wang, J.; Świerczek, K. Characterization of Sr-doped lithium lanthanum titanate with improved transport properties. Solid State Ion. 2019, 336, 39–46. [Google Scholar] [CrossRef]
- Shui, F.; Li, Z.; Zhao, X. Effect of annealing temperature on the transparent lithium strontium lanthanum titanate thin films. Chem. Phys. Lett. 2020, 750, 137496. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Tsurumaki, A.; Di Donato, G.; Adenusi, H.; Navarra, M.A.; Passerini, S. Quasi-solid-state electrolytes—Strategy towards stabilising Li|inorganic solid electrolyte interfaces in solid-state Li metal batteries. Energy Mater. 2023, 3, 300019. [Google Scholar] [CrossRef]
- Ding, W.-Q.; Lv, F.; Xu, N.; Wu, M.-T.; Liu, J.; Gao, X.-P. Polyethylene oxide-based solid-state composite polymer electrolytes for rechargeable lithium batteries. ACS Appl. Energy Mater. 2021, 4, 4581–46901. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-type solid state electrolytes: Materials, interfaces and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Lin, Y.; Li, J.; Lai, Y.; Yuan, C.; Zhang, J.; Zhu, K. All-solid-state lithium-ion battery: Research and industrial prospects. Acta Chim. Sin. 2013, 71, 869–878. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.W.; Wang, S.F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Liu, W.; Lee, S.W.; Lin, D.; Shi, F.; Wang, S.; Sendek, A.D. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439. [Google Scholar] [CrossRef]
- Shi, Q.X.; Xia, Q.; Xiang, X.; Ye, Y.S.; Peng, H.Y.; Xue, Z.G.; Xie, X.L.; Mai, Y.-W. Self-assembled polymeric ionic liquid-functionalized cellulose nanocrystals: Constructing 3D ion-conducting channels within ionic liquid based composite polymer electrolytes. Chem. Eur. J. 2017, 23, 11881–11890. [Google Scholar] [CrossRef] [PubMed]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novak, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Subianto, S.; Mistry, M.K.; Choudhury, N.R.; Dutta, N.K.; Knout, R. Composite polymer electrolyte containing ionic liquid and functionalized polyhedral oligomeric silsesquioxanes for anhydrous PEM applications. ACS Appl. Mater. Interfaces 2009, 1, 1173–1182. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Rhee, H.W.; Reddy, P.A.; Gupta, D.; Mitu, L.; Polu, A.R. Ionic liquid incorporated nanocomposite polymer electrolytes for rechargeable lithium-ion battery: A way to achieve improved electrochemical and interfacial properties. J. Ind. Eng. Chem. 2016, 40, 168–176. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Han, P.; Lai, Y.; Zhang, Z.; Liu, J. Enhanced electrochemical performance of poly(ethylene oxide) based composite polymer electrolyte by incorporation of nano-sized metal-organic framework. J. Power Sources 2013, 240, 653–658. [Google Scholar] [CrossRef]
- Asghar, A.; Samad, Y.A.; Lalia, B.S.; Hashaikeh, R. PEG based quasisolid polymer electrolyte: Mechanically supported by networked cellulose. J. Membr. Sci. 2012, 421, 85–90. [Google Scholar] [CrossRef]
- Wu, W.; Liang, Y.; Li, D.; Bo, Y.; Wu, D.; Ci, L.; Li, M.; Zhang, J. A Competitive Solvation of Ternary Eutectic Electrolytes Tailoring the Electrode/Electrolyte Interphase for Lithium Metal Batteries. ACS Nano 2022, 16, 14558–14568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, J.; Yang, M.; Wang, S.; Zeng, G.; Liu, H.; Cheng, J.; Li, D.; Wei, Y.; Si, P.; et al. Carbon Microstructure Dependent Li-Ion Storage Behaviors in SiOx/C Anodes. Small 2023, 19, 2300759. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Q.; Cheng, J.; Zhang, H.; Xu, X.; Li, Y.; Zeng, Z.; Zhao, Y.; Li, D.; Lu, J.; et al. Stable operation of polymer electrolyte-solid-state batteries via lone-pair electron fillers. Nano Res. 2023, 16, 1–11. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, X.; Xiong, X.; Zhang, Q.; Peng, B.; Chen, Y.; Wang, Z.; Fu, L.; Wu, Y. A Solid Electrolyte Based on Electrochemical Active Li4Ti5O12 with PVDF for Solid State Lithium Metal Battery. Adv. Energy Mater. 2022, 12, 2201991. [Google Scholar] [CrossRef]
- Peng, B.; Liu, Z.; Zhou, Q.; Xiong, X.; Xia, S.; Yuan, X.; Wang, F.; Ozoemena, K.I.; Liu, L.; Fu, L.; et al. A Solid-State Electrolyte Based on Li0.95Na0.05FePO4 for Lithium Metal Batteries. Adv. Mater. 2023. accepted for publication. [Google Scholar] [CrossRef]
- Morita, K.; Matsumoto, T.; Hoshino, T. Efficient lithium extraction via electrodialysis using acid-processed lithium-adsorbing lithium lanthanum titanate. Desalination 2022, 543, 116117. [Google Scholar] [CrossRef]
- Kežionis, A.; Kazakevičius, E.; Kazlauskas, S.; Žalga, A. Metal-like temperature dependent conductivity in fast Li+ ionic conductor Lithium Lanthanum Titanate. Solid State Ion. 2019, 342, 115060. [Google Scholar] [CrossRef]
- Hua, C.; Fang, X.; Wang, Z.; Chen, L. Lithium storage in perovskite lithium lanthanum titanate. Electrochem. Commun. 2013, 32, 5–8. [Google Scholar] [CrossRef]
- Bohnke, C.; Regrag, B.; Leberre, F.; Fourquet, J.-L.; Randrianantoandro, N. Comparison of pH sensitivity of lithium lanthanum titanate obtained by sol–gel synthesis and solid-state chemistry. Solid State Ion. 2005, 176, 73–80. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, X.; Wang, J.; Yuan, L.; Fan, J. Construction of Li0.5La0.5TiO3 (LLTO)-In2O3 n-n step scheme heterostructure nanorods for drastically heightening the sensing behavior in H2S gas. Mater. Chem. Phys. 2023, 295, 127085. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Ban, J.; He, S.; Zheng, N.; Shao, G.; Cao, G. Mechanism of enhanced H2S sensor ability based on emerging Li0.5La0.5TiO3-SnO2 core-shell structure. Sens. Actuators B Chem. 2022, 352, 131054. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, X.; Zhang, L.; Du, F.; Liu, Q.; Chen, J.; Meng, K.; Wu, Y.; Yang, M.; Jiang, Y. Lithium incorporation enhanced resistive switching behaviors in lithium lanthanum titanium oxide-based heterostructure. J. Mater. Sci. Technol. 2022, 128, 142–147. [Google Scholar] [CrossRef]
- Hua, Q.; Ren, P.; Wang, X.; Zhao, H.; Yang, J.; Lu, X.; Zhao, G. Optical and electrical properties of Li0.5La0.5TiO3 modified potassium sodium niobate-based ceramics. Ceram. Int. 2022, 48, 14633–14639. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, H.; Wang, C.; Chen, S.; Zhang, L.; Hao, C.; Lei, Q. Electrical properties of semi-conductive composites with different Li0.5La0.5TiO3 content. Mater. Lett. 2020, 281, 128664. [Google Scholar] [CrossRef]
- Ciobota, C.F.; Piticescu, R.M.; Neagoe, C.; Tudor, I.A.; Matei, A.; Dragut, D.V.; Sobetkii, A.; Anghel, E.M.; Stanoiu, A.; Simion, C.E.; et al. Nanostructured Cobalt Doped Barium Strontium Titanate Thin Films with Potential in CO2 Detection. Materials 2020, 13, 4797. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, P.; Sasani, A.; Bousquet, E.; Guennou, M.; Moreira, J.A. Lattice dynamics and Raman spectrum of supertetragonal PbVO3. J. Phys. Chem. Solids 2023, 173, 111092. [Google Scholar] [CrossRef]
- Okos, A.; Colin, C.; Darie, C.; Raita, O.; Bordet, P.; Pop, A. Structure and magnetic properties of the layered perovskite PbVO3. J. Alloys Compd. 2014, 602, 265–268. [Google Scholar] [CrossRef]
- Darie, C.; Goujon, C.; Bacia, M.; Klein, H.; Toulemonde, P.; Bordet, P.; Suard, E. Magnetic and crystal structures of BiCrO3. Solid State Sci. 2010, 12, 660–664. [Google Scholar] [CrossRef]
- Toulemonde, P.; Darie, C.; Goujon, C.; Legendre, M.; Mendonca, T.; Álvarez-Murga, M.; Simonet, V.; Bordet, P.; Bouvier, P.; Kreisel, J.; et al. Single crystal growth of BiMnO3 under high pressure–high temperature. High Press. Res. 2009, 29, 600–604. [Google Scholar] [CrossRef]
- Guennou, M.; Bouvier, P.; Toulemonde, P.; Darie, C.; Goujon, C.; Bordet, P.; Hanfland, M.; Kreisel, J. Jahn-Teller, Polarity, and Insulator-to-Metal Transition in BiMnO3 at High Pressure. Phys. Rev. Lett. 2014, 112, 075501. [Google Scholar] [CrossRef] [PubMed]
- Goujon, C.; Darie, C.; Bacia, M.; Klein, H.; Ortega, L.; Bordet, P. High pressure synthesis of BiCrO3, a candidate for multiferroism. J. Phys. Conf. Ser. 2008, 121, 022009. [Google Scholar] [CrossRef]
- Chouhan, P.; Dwivedi, R. Comparative studies of La and Co modified BiFeO3 (0.05) prepared by sol–gel versus conventional solid state reaction method. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Jin, N.; Sun, L.; Yang, X.; Liu, Y. High-capacity anode materials for lithium-ion batteries: Perovskite strontium vanadate with crystalline-amorphous dual-phase structure. Mater. Today Chem. 2023, 30, 101542. [Google Scholar] [CrossRef]
- Huang, J.; Hu, L.; Yang, Z.; Li, J.; Wang, P.; Wei, Y.; Sun, P. Hollow spherical LaFeO3 perovskite as anode material for Lithium-ion battery. Inorg. Chem. Commun. 2023, 150, 110458. [Google Scholar] [CrossRef]
- Joshi, A.; Bandyopadhyay, S.; Gupta, A.; Srivastava, R.K.; Nandan, B. Perovskite lead zirconate titanate (PbZr0.52Ti0.48O3) nanofibers for inhibiting polysulfide shuttle effect in lithium-sulfur batteries. J. Alloys Compd. 2023, 948, 169769. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Feng, X. Perovskite strontium vanadate nanoparticle as an ideal fast charging anode material in lithium-ion batteries. Mater. Lett. 2022, 314, 131859. [Google Scholar] [CrossRef]
- Yue, B.; Hu, Q.; Yu, W.; Ma, Q.; Bao, Y.; Li, L.; Wang, J.; Liu, G.; Dong, X.; Liu, J. Perovskite-type RMnO3 (R = La, Nd, Eu) nanofibers with fast Li+ transport properties as anode for lithium-ion batteries. Mater. Today Commun. 2023, 36, 106459. [Google Scholar] [CrossRef]
- Diktanaitė, A.; Gaidamavičienė, G.; Kazakevičius, E.; Kežionis, A.; Žalga, A. Aqueous sol-gel synthesis, structural, thermoanalytical studies, and conductivity properties of lithium lanthanum titanate. Thermochim. Acta 2022, 715, 179268. [Google Scholar] [CrossRef]
- Fernandes, S.L.; Gasparotto, G.; Teixeira, G.F.; Cebim, M.A.; Longo, E.; Zaghete, M.A. Lithium lanthanum titanate perovskite ionic conductor: Influence of europium doping on structural and optical properties. Ceram. Int. 2018, 44, 21578–21584. [Google Scholar] [CrossRef]
- Le, H.T.; Kalubarme, R.S.; Ngo, D.T.; Jang, S.Y.; Jung, K.N.; Shin, K.H.; Park, C.J. Citrate gel synthesis of aluminum-doped lithium lanthanum titanate solid electrolyte for application in organic-type lithi-um-oxygen batteries. J. Power Sources 2015, 274, 1188–1199. [Google Scholar] [CrossRef]
- Liang, Y.; Ji, L.; Guo, B.; Lin, Z.; Yao, Y.; Li, Y.; Alcoutlabi, M.; Qiu, Y.; Zhang, X. Preparation and electrochemical characterization of ionic-conducting lithium lanthanum titanate oxide/polyacrylonitrile submicron composite fiber-based lithium-ion battery separators. J. Power Sources 2011, 196, 436–441. [Google Scholar] [CrossRef]
- Abhilash, K.; Selvin, P.C.; Nalini, B.; Somasundaram, K.; Sivaraj, P.; Bose, A.C. Study of the temperature dependent transport properties in nanocrystalline lithium lanthanum titanate for lithium ion batteries. J. Phys. Chem. Solids 2016, 91, 114–121. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Chan, C.K. Enhanced lithium ion conductivity in lithium lanthanum titanate solid electrolyte nanowires prepared by electrospinning. J. Power Sources 2015, 287, 164–169. [Google Scholar] [CrossRef]
- Borštnar, P.; Žuntar, J.; Spreitzer, M.; Dražič, G.; Daneu, N. Exaggerated grain growth and the development of coarse-grained microstructures in lithium lanthanum titanate perovskite ceramics. J. Eur. Ceram. Soc. 2023, 43, 1017–1027. [Google Scholar] [CrossRef]
- Lalena, J.N.; Cleary, D.A.; Carpenter, E.E.; Dean, N.F. Solid-solid reactions. In Inorganic Materials Synthesis and Fabrication; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 183–209. [Google Scholar]
- Schröckert, F.; Schiffmann, N.; Bucharsky, E.C.; Schell, K.G.; Hoffmann, M.J. Tape casted thin films of solid electrolyte Lithium-Lanthanum-Titanate. Solid State Ion. 2018, 328, 25–29. [Google Scholar] [CrossRef]
- Takatori, K.; Saura, K.; Orum, A.; Kadoura, H.; Tani, T. Textured lithium lanthanum titanate polycrystals prepared by a reactive-templated grain growth method. J. Eur. Ceram. Soc. 2016, 36, 551–558. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, C.; Wang, D.; Peng, S.; Huang, L.; Yang, W.; Zhang, W.-H.; Gao, X. Revealing the dominant factor of domain boundary resistance on bulk conductivity in lanthanum lithium titanates. J. Energy Chem. 2022, 73, 354–359. [Google Scholar] [CrossRef]
- Inaguma, Y.; Nakashima, M. A rechargeable lithium–air battery using a lithium ion-conducting lanthanum lithium titanate ceramics as an electrolyte separator. J. Power Sources 2013, 228, 250–255. [Google Scholar] [CrossRef]
- Furusawa, S.-I.; Tabuchi, H.; Sugiyama, T.; Tao, S.; Irvine, J.T. Ionic conductivity of amorphous lithium lanthanum titanate thin film. Solid State Ion. 2005, 176, 553–558. [Google Scholar] [CrossRef]
- Mori, K.; Tomihira, S.; Iwase, K.; Fukunaga, T. Visualization of conduction pathways in a lanthanum lithium titanate superionic conductor synthesized by rapid cooling. Solid State Ion. 2014, 268, 76–81. [Google Scholar] [CrossRef]
- Tang, H.; Zan, L.; Zhu, J.; Ma, Y.; Zhao, N.; Tang, Z. High rate capacity nanocomposite lanthanum oxide coated lithium zinc titanate anode for rechargeable lithium-ion battery. J. Alloys Compd. 2016, 667, 82–90. [Google Scholar] [CrossRef]
- Li, C.-L.; Zhang, B.; Fu, Z.-W. Physical and electrochemical characterization of amorphous lithium lanthanum titanate solid electrolyte thin-film fabricated by e-beam evaporation. Thin Solid Films 2006, 515, 1886–1892. [Google Scholar] [CrossRef]
- Reda, A. Effect of ZnO on sintering and microwave dielectric properties of 0.5CaTiO3-0.5 (Li0.5La0.5) TiO3 ceramics. J. Indian Chem. Soc. 2023, 100, 100901. [Google Scholar] [CrossRef]
- V’yunov, O.; Plutenko, T.; Fedorchuk, O.; Belous, A.; Lobko, Y. Synthesis and dielectric properties in the lithium-ion conducting material La0.5Li0.5−xNaxTiO3. J. Alloys Compd. 2021, 889, 161556. [Google Scholar] [CrossRef]
- Salami, T.J.; Imanieh, S.H.; Lawrence, J.G.; Martin, I. Amorphous glass-perovskite composite as solid electrolyte for lithium-ion battery. Mater. Lett. 2019, 254, 294–296. [Google Scholar] [CrossRef]
- Maqueda, O.; Sauvage, F.; Laffont, L.; Martínez-Sarrión, M.; Mestres, L.; Baudrin, E. Structural, microstructural and transport properties study of lanthanum lithium titanium perovskite thin films grown by Pulsed Laser Deposition. Thin Solid Films 2008, 516, 1651–1655. [Google Scholar] [CrossRef]
- Diaz-Moreno, C.A.; Ding, Y.; Portelles, J.; Heiras, J.; Macias, A.H.; Syeed, A.; Paez, A.; Li, C.; López, J.; Wicker, R. Optical properties of ferroelectric lanthanum lithium niobate. Ceram. Int. 2018, 44, 4727–4733. [Google Scholar] [CrossRef]
- Inaguma, Y.; Chen, L.; Itoh, M.; Nakamura, T. Candidate Compounds with Perovskite Structure for High Lithium Ionic conductivity. Solid State Ion. 1994, 70–71, 196–202. [Google Scholar] [CrossRef]
- Li, J.; Wen, Z.; Xu, X.; Zhu, X. Lithium-ion conduction in the anion substituted La2/3–xLi3x–yTiO3–yFy electrolyte with perovskite-type structure. Solid State Ion. 2005, 176, 2269–2273. [Google Scholar] [CrossRef]
- Bohnke, O.; Lorant, S.; Roffat, M.; Berger, P. Fast H+/Li+ ion exchange in Li0.30La0.57TiO3 nanopowder and films in water and in ambient air. Solid State Ion. 2014, 262, 563–567. [Google Scholar] [CrossRef]
- Abhilash, K.; Sivaraj, P.; Selvin, P.; Nalini, B.; Somasundaram, K. Investigation on spin coated LLTO thin film nano-electrolytes for rechargeable lithium ion batteries. Ceram. Int. 2015, 41, 13823–13829. [Google Scholar] [CrossRef]
- Aguesse, F.; Roddatis, V.; Roqueta, J.; García, P.; Pergolesi, D.; Santiso, J.; Kilner, J.A. Microstructure and ionic conductivity of LLTO thin films: Influence of different substrates and excess lithium in the target. Solid State Ion. 2015, 272, 1–8. [Google Scholar] [CrossRef]
- Xiong, Y.; Tao, H.; Zhao, J.; Cheng, H.; Zhao, X. Effects of annealing temperature on structure and opt-electric properties of ion-conducting LLTO thin films prepared by RF magnetron sputtering. J. Alloys Compd. 2011, 509, 1910–1914. [Google Scholar] [CrossRef]
- Na Lee, Y.; Yoon, Y.S. Cycle stability increase by insertion of Li–La–Ta–O thin-film electrolyte between cathode and solid electrolyte for all-solid-state battery. Thin Solid Films 2015, 579, 75–80. [Google Scholar] [CrossRef]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings—Science, Applications and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–902. [Google Scholar]
- Ohring, M. Materials Science of Thin Films, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–781. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Pham, Q.N.; Bohnke, C. Lithium lanthanum titanate ceramic as sensitive material for pH sensor: Influence of synthesis methods and powder grains size. J. Eur. Ceram. Soc. 2005, 25, 2973–2976. [Google Scholar] [CrossRef]
- Oguni, M.; Inaguma, Y.; Itoh, M.; Nakamura, T. Calorimetric and Electrical Studies on the Positional Disorder of Lithium Ions in Lithium Lanthanum Titanate. Solid State Commun. 1994, 91, 627–630. [Google Scholar] [CrossRef]
- Latie, L.; Villeneuve, G.; Conte, D.; LeFlem, G. Ionic Conductivity of Oxides with General Formula LixLn1/3Nb1−xTixO3 (Ln = La, Nd). J. Solid-State Chem. 1984, 51, 293–299. [Google Scholar] [CrossRef]
- Belous, A.G.; Novitskaya, G.N.; Polyanetskaya, S.V.; Gornikov, Y.I. Crystallochemical and electrical properties of complex oxides Ln2/3−xM3xTiO3. Zhurnal Neorg. Khimii 1987, 32, 283–286. [Google Scholar]
- Gao, X.; Fisher, C.A.J.; Kimura, T.; Ikuhara, Y.H.; Moriwake, H.; Kuwabara, A.; Oki, H.; Tojigamori, T.; Huang, R.; Ikuhara, Y. Lithium Atom and A-Site Vacancy Distributions in Lanthanum Lithium Titanate. Chem. Mater. 2013, 25, 1607–1614. [Google Scholar] [CrossRef]
- Mei, A.; Wang, X.-L.; Lan, J.-L.; Feng, Y.-C.; Geng, H.-X.; Lin, Y.-H.; Nan, C.-W. Role of amorphous boundary layer in enhancing ionic conductivity of lithium–lanthanum–titanate electrolyte. Electrochim. Acta 2010, 55, 2958–2963. [Google Scholar] [CrossRef]
- Abhilash, K.; Selvin, P.C.; Nalini, B.; Jose, R.; Hui, X.; Elim, H.I.; Reddy, M. Correlation study on temperature dependent conductivity and line profile along the LLTO/LFP-C cross section for all solid-state Lithium-ion batteries. Solid State Ion. 2019, 341, 115032. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium Lanthanum Titanates: A Review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Hu, Y.; Luo, L. Structural origin of low Li-ion conductivity in perovskite solid-state electrolyte. Nano Energy 2022, 92, 106758. [Google Scholar] [CrossRef]
- Inaguma, Y.; Katsumata, T.; Itoh, M.; Morii, Y.; Tsurui, T. Structural investigations of migration pathways in lithium ion-conducting La2/3−xLi3xTiO3 perovskites. Solid State Ion. 2006, 177, 3037–3044. [Google Scholar] [CrossRef]
- Inaguma, Y.; Matsui, Y.; Shan, Y.-J.; Itoh, M.; Nakamura, T. Lithium ion conductivity in the perovskite-type LiTaO3-SrTiO3 solid solution. Solid State Ion. 1995, 79, 91–97. [Google Scholar] [CrossRef]
- Šalkus, T.; Kazakevičius, E.; Kežionis, A.; Orliukas, A.; Badot, J.; Bohnke, O. Determination of the non-Arrhenius behaviour of the bulk conductivity of fast ionic conductors LLTO at high temperature. Solid State Ion. 2011, 188, 69–72. [Google Scholar] [CrossRef]
- Bohnke, O. The fast lithium-ion conducting oxides Li3xLa2/3−xTiO3 from fundamentals to application. Solid State Ion. 2008, 179, 9–15. [Google Scholar] [CrossRef]
- Emery, J.; Bohnké, O.; Fourquet, J.L.; Buzaré, J.Y.; Florian, P.; Massiot, D. Nuclear magnetic resonance investigation of Li+-ion dynamics in the perovskite fast-ion conductor Li3xLa2/3-x□1/3-2xTiO3. J. Phys. Condens. Matter 2002, 14, 523–539. [Google Scholar] [CrossRef]
- Bohnke, O.; Emery, J.; Fourquet, J.-L. Anomalies in Li+ ion dynamics observed by impedance spectroscopy and 7Li NMR in the perovskite fast ion conductor (Li3xLa2/3−x□1/3−2x)TiO3. Solid State Ion. 2003, 158, 119–132. [Google Scholar] [CrossRef]
- Mei, A.; Wang, X.-L.; Feng, Y.-C.; Zhao, S.-J.; Li, G.-J.; Geng, H.-X.; Lin, Y.-H.; Nan, C.-W. Enhanced ionic transport in lithium lanthanum titanium oxide solid state electrolyte by introducing silica. Solid State Ion. 2008, 179, 2255–2259. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Gupta, R.K. Review Superionic solids: Composite electrolyte phase—An overview. J. Mater. Sci. 1999, 34, 1131–1162. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Qi, Y.; Liu, V. On the La2/3−xLi3xTiO3/Al2O3 composite solid-electrolyte for Li-ion conduction. J. Alloys Compd. 2013, 577, 57–63. [Google Scholar] [CrossRef]
- Moriwake, H.; Gao, X.; Kuwabara, A.; Fisher, C.A.; Kimura, T.; Ikuhara, Y.H.; Kohama, K.; Tojigamori, T.; Ikuhara, Y. Domain boundaries and their influence on Li migration in solid-state electrolyte (La,Li)TiO3. J. Power Sources 2015, 276, 203–207. [Google Scholar] [CrossRef]
- Gao, X.; Fisher, C.A.J.; Kimura, T.; Ikuhara, Y.H.; Kuwabara, A.; Moriwake, H.; Oki, H.; Tojigamori, T.; Kohama, K.; Ikuhara, Y. Domain boundary structures in lanthanum lithium titanates. J. Mater. Chem. A 2014, 2, 843–852. [Google Scholar] [CrossRef]
- Yashima, M.; Itoh, M.; Inaguma, Y.; Morii, Y. Crystal Structure and Diffusion Path in the Fast Lithium-Ion Conductor La0.62Li0.16TiO3. J. Am. Chem. Soc. 2005, 127, 3491–3495. [Google Scholar] [CrossRef]
- Abhilash, K.; Selvin, P.; Nalini, B.; Nithyadharseni, P.; Pillai, B. Investigations on pure and Ag doped lithium lanthanum titanate (LLTO) nanocrystalline ceramic electrolytes for rechargeable lithium-ion batteries. Ceram. Int. 2013, 39, 947–952. [Google Scholar] [CrossRef]
- Hu, X.; Fisher, C.A.; Kobayashi, S.; Ikuhara, Y.H.; Fujiwara, Y.; Hoshikawa, K.; Moriwake, H.; Kohama, K.; Iba, H.; Ikuhara, Y. Atomic scale imaging of structural changes in solid electrolyte lanthanum lithium niobate upon annealing. Acta Mater. 2017, 127, 211–219. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Huang, R.; Hitosugi, T.; Ikuhara, Y.H.; Oki, H.; Ikuhara, Y. First-principles calculations of lithium-ion migration at a coherent grain boundary in a cathode material, LiCoO2. Adv. Mater. 2013, 25, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, K.; Liang, C.; Nan, C.-W.; Ishikawa, R.; More, K.; Chi, M. Atomic-scale origin of the large grain-boundary resistance in perovskite Li-ion-conducting solid electrolytes. Energy Environ. Sci. 2014, 7, 1638–1642. [Google Scholar] [CrossRef]
- Li, B.; Su, Q.; Yu, L.; Liu, W.; Dong, S.; Ding, S.; Zhang, M.; Du, G.; Xu, B. Biomimetic PVDF/LLTO composite polymer electrolyte enables excellent interface contact and enhanced ionic conductivity. Appl. Surf. Sci. 2021, 541, 148434. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim. Acta 2018, 292, 718–726. [Google Scholar] [CrossRef]
- Nourisabet, T.; Aval, H.J.; Shidpour, R.; Naji, L. Fabrication of a PEO-PVDF blend based polymer composite electrolyte with extremely high ionic conductivity via the addition of LLTO nanowires. Solid State Ion. 2022, 377, 115885. [Google Scholar] [CrossRef]
- Kim, D.-W.; Park, J.-K.; Rhee, H.-W. Conductivity and thermal studies of solid polymer electrolytes prepared by blending poly(ethylene oxide), poly(oligo[oxyethylene]oxysebacoyl) and lithium perchlorate. Solid State Ion. 1996, 83, 49–56. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Zhou, J.; Fedkiw, P.S. Ionic conductivity of composite electrolytes based on oligo(ethylene oxide) and fumed oxides. Solid State Ion. 2004, 166, 275–293. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Croce, F.; Sacchetti, S.; Scrosati, B. Advanced, lithium batteries based on high-performance composite polymer electrolytes. J. Power Sources 2006, 162, 685–689. [Google Scholar] [CrossRef]
- Yang, H.; Abdullah, M.; Bright, J.; Hu, W.; Kittilstved, K.; Xu, Y.; Wang, C.; Zhang, X.; Wu, N. Polymer-ceramic composite electrolytes for all-solid-state lithium batteries: Ionic conductivity and chemical interaction enhanced by oxygen vacancy in ceramic nanofibers. J. Power Sources 2021, 495, 229796. [Google Scholar] [CrossRef]
- Zhu, L.; Xie, H.; Zheng, W.; Zhang, K. Multi-component solid PVDF-HFP/PPC/LLTO-nanorods composite electrolyte enabling advanced solid-state lithium metal batteries. Electrochim. Acta 2022, 435, 141384. [Google Scholar] [CrossRef]
- Song, F.; Yamamoto, T.; Yabutsuka, T.; Yao, T.; Takai, S. Synthesis and Characterization of LAGP-Based Lithium Ion-Conductive Composites with an LLTO Additive. J. Alloys Compd. 2021, 853, 157089. [Google Scholar] [CrossRef]
- Yi, J.; He, P.; Liu, H.; Ni, H.; Bai, Z.; Fan, L.-Z. Manipulating interfacial stability of LiNi0.5Co0.3Mn0.2O2 cathode with sulfide electrolyte by nanosized LLTO coating to achieve high-performance all-solid-state lithium batterie. J. Energy Chem. 2021, 52, 202–209. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhao, T.; Kou, W.; Hua, Q.; Ren, W.; Wu, W. Overcoming the trade-off between ion conduction and stability using thin composite solid electrolyte for high performance all-solid-state lithium battery. Electrochim. Acta 2022, 432, 141226. [Google Scholar] [CrossRef]
- Kou, W.; Wang, J.; Li, W.; Lv, R.; Peng, N.; Wu, W.; Wang, J. Asymmetry-structure electrolyte with rapid Li+ transfer pathway towards high-performance all-solid-state lithium–sulfur battery. J. Membr. Sci. 2021, 634, 119432. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Cai, S.; Hu, C.; Chen, S.; Liu, X.; Liu, J.; Liu, Z. Li0.35La0.55TiO3 nanofibers filled poly (ethylene carbonate) composite electrolyte with enhanced ion conduction and electrochemical stability. Thin Solid Films 2021, 734, 138835. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Yang, T.; Chu, X.; Xie, Y.; Wang, Q.; Xia, Y.; Yang, W. Physicochemically dendrite-suppressed three-dimensional fluoridation solid-state electrolyte for high-rate lithium metal battery. Cell Rep. Phys. Sci. 2021, 2, 100644. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, M.; Gao, L.; He, Z.; Chen, K.; Li, Z.; He, H.; Lin, Y. Novel fast ionic conductor ceramic composite separator for high-performance safe Li-ion power batteries. J. Mater. 2022, 8, 1184–1190. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, Y.; Zhu, J.; Lv, R.; Li, Z.; Wu, W.; Li, W.; Wang, J. MOF lamellar membrane-derived LLTO solid state electrolyte for high lithium ion conduction. J. Membr. Sci. 2022, 663, 121041. [Google Scholar] [CrossRef]
- Prasad, N.K.; Kumar, V. Microstructure and magnetic properties of equiatomic FeNiCo alloy synthesized by mechanical alloying. J. Mater. Sci. Mater. Electron. 2015, 26, 10109–10118. [Google Scholar] [CrossRef]
- Wang, G.X.; Yao, P.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. Structure characteristics and lithium ionic conductivity of La(0.57−2x/3)SrxLi0.3TiO3 perovskites. J. Mater. Sci. 2000, 35, 4289–4291. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Wang, J.; Kober, D.; Li, S.; Wang, X.; Shen, X.; Bekheet, M.F.; Gurlo, A. Polymer-Derived SiOC Integrated with a Graphene Aerogel As a Highly Stable Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2020, 12, 46045–46056. [Google Scholar] [CrossRef]
- Liang, J.; Wu, T.; Wang, Z.; Yu, Y.; Hu, L.; Li, H.; Zhang, X.; Zhu, X.; Zhao, Y. Accelerating perovskite materials discovery and correlated energy applications through artificial intelligence. Energy Mater. 2022, 2, 200016. [Google Scholar] [CrossRef]
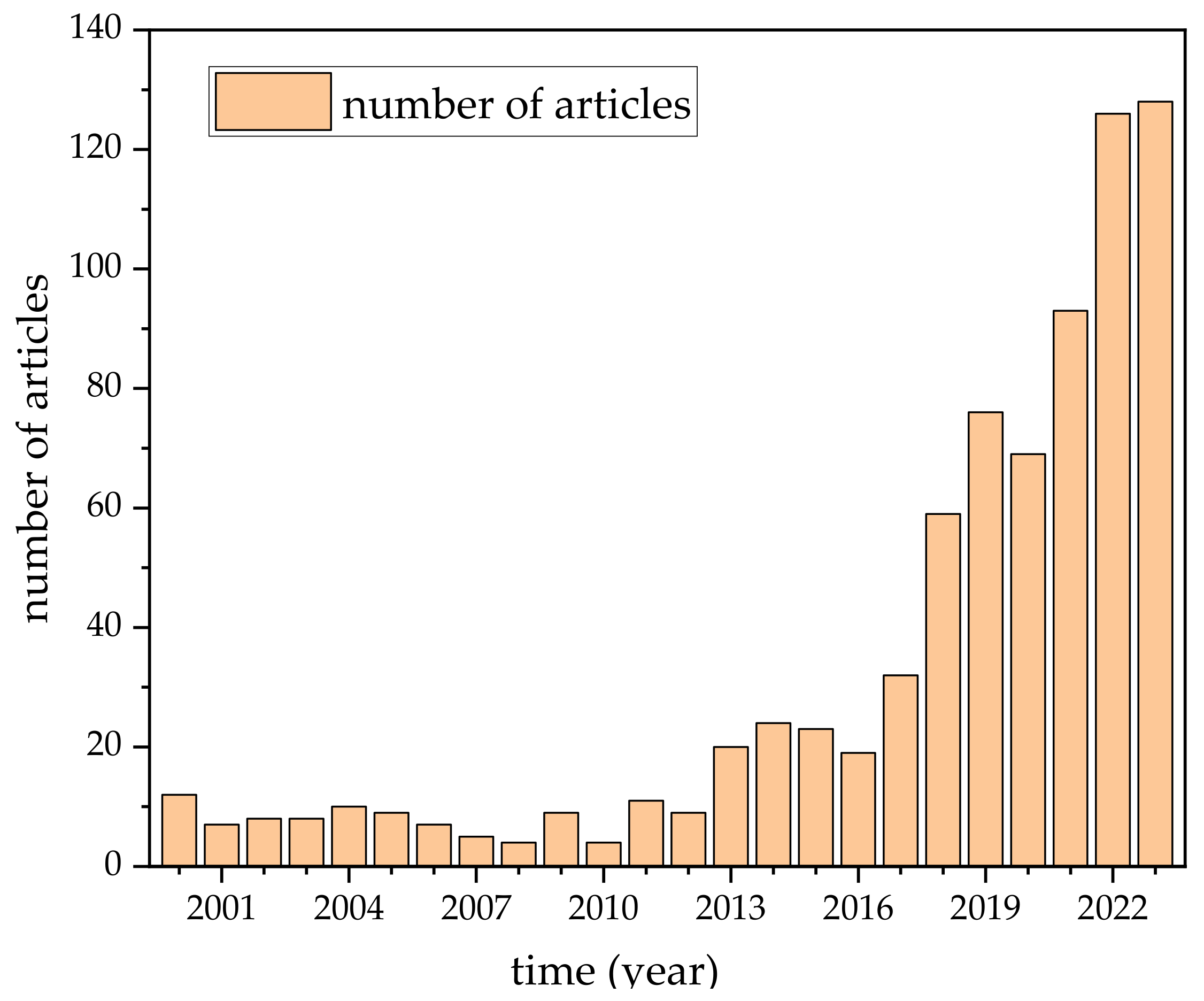


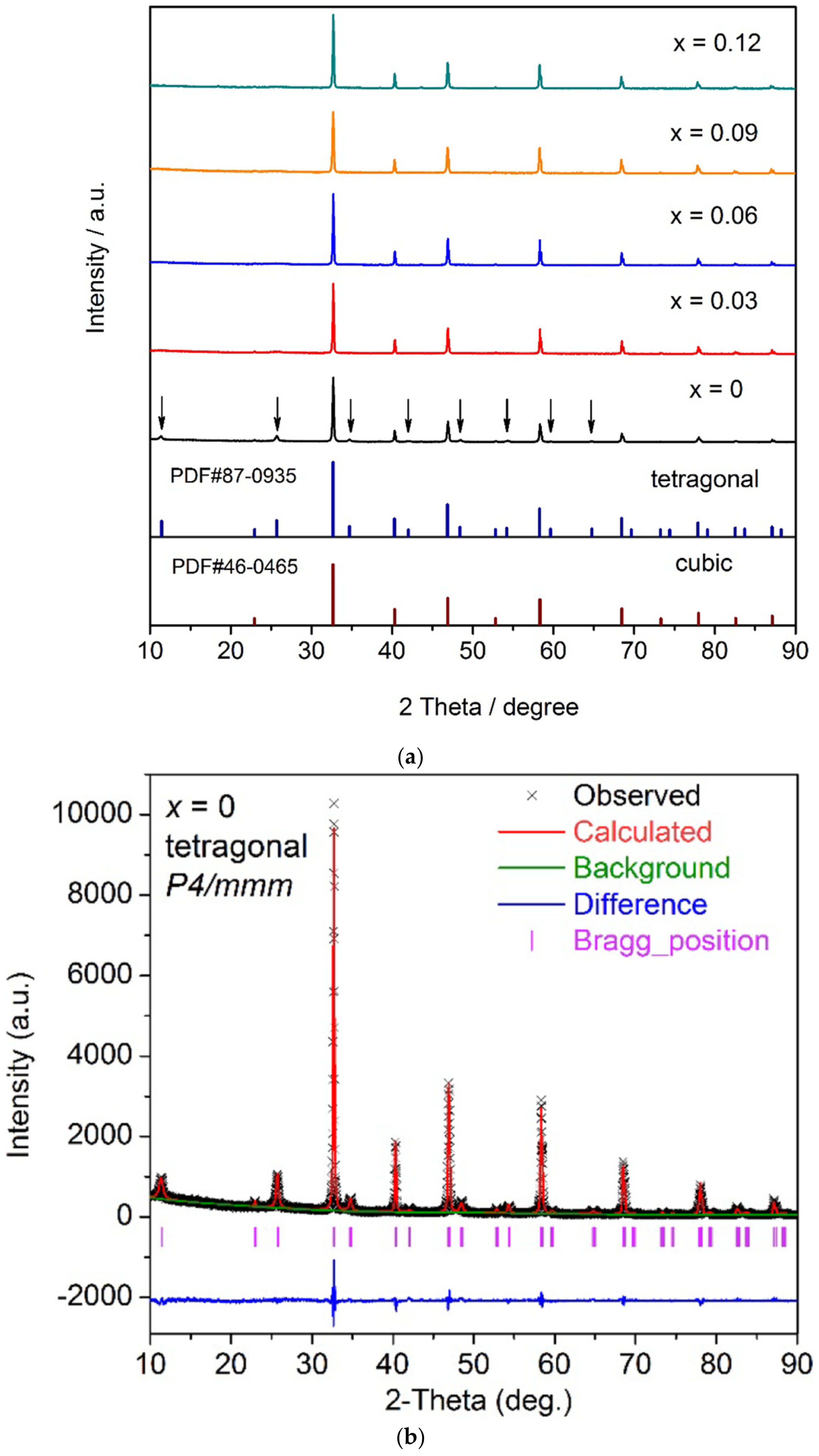
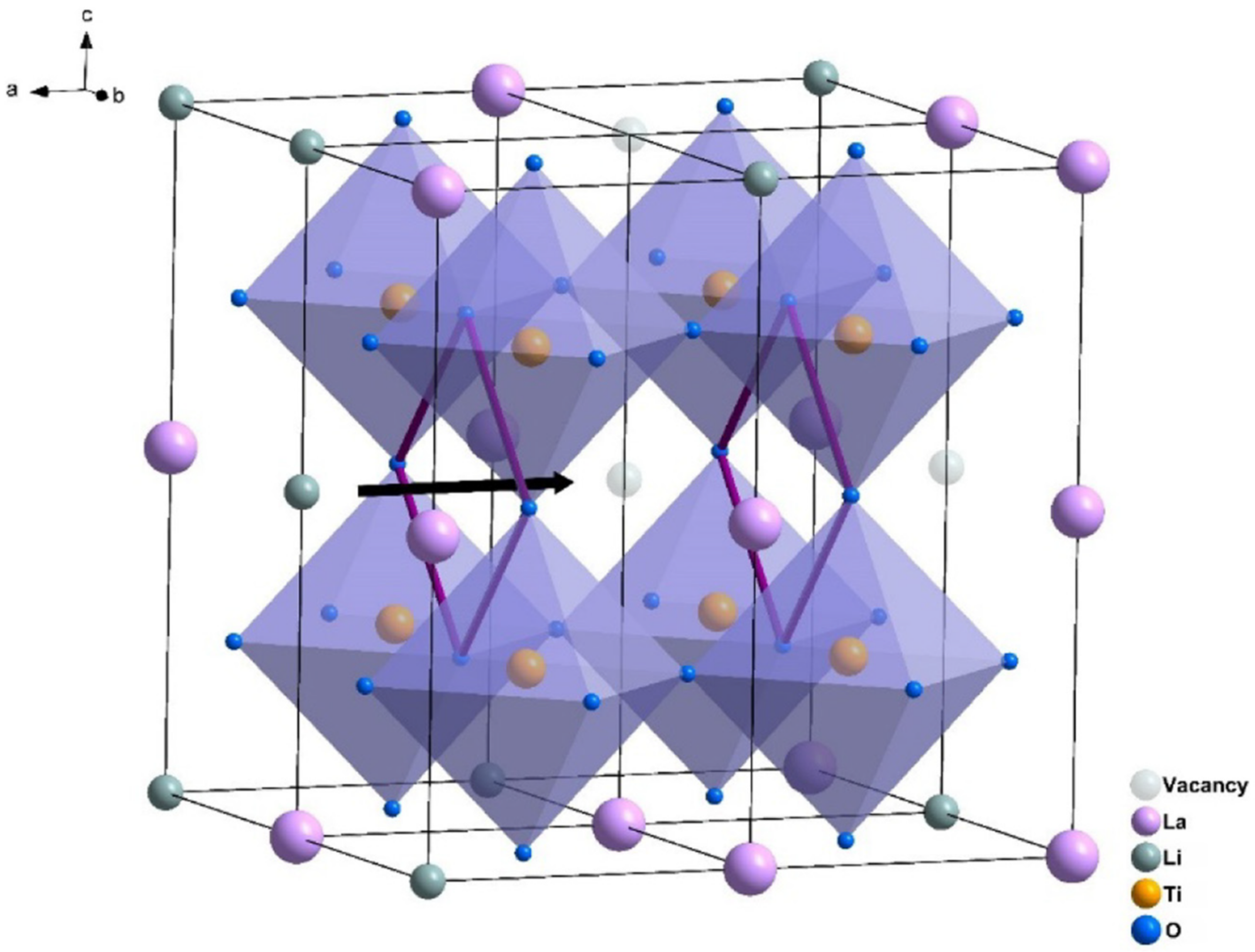
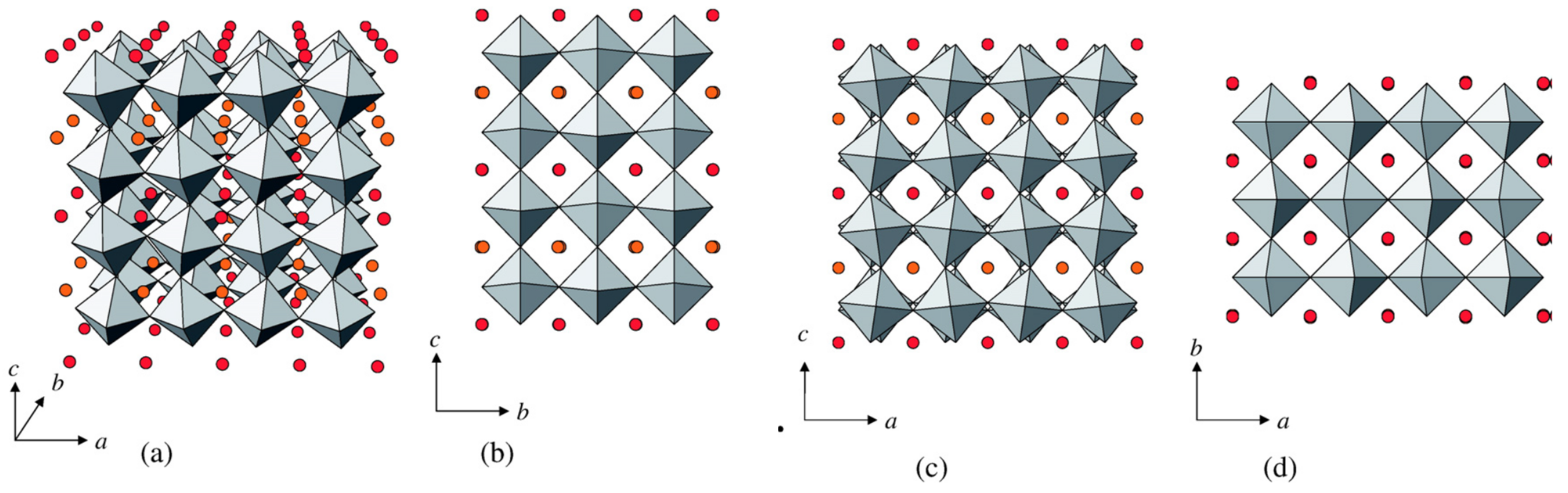

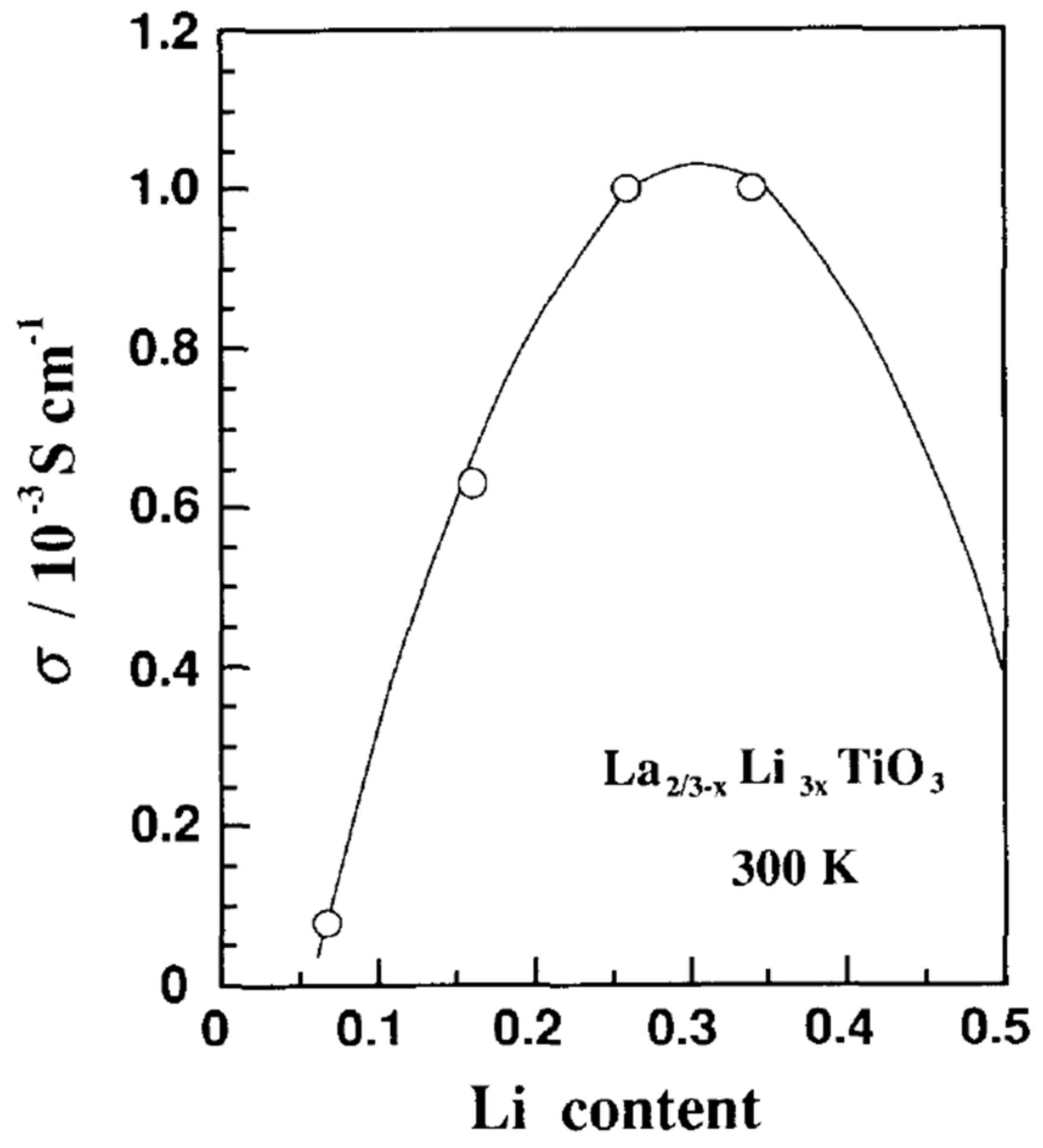



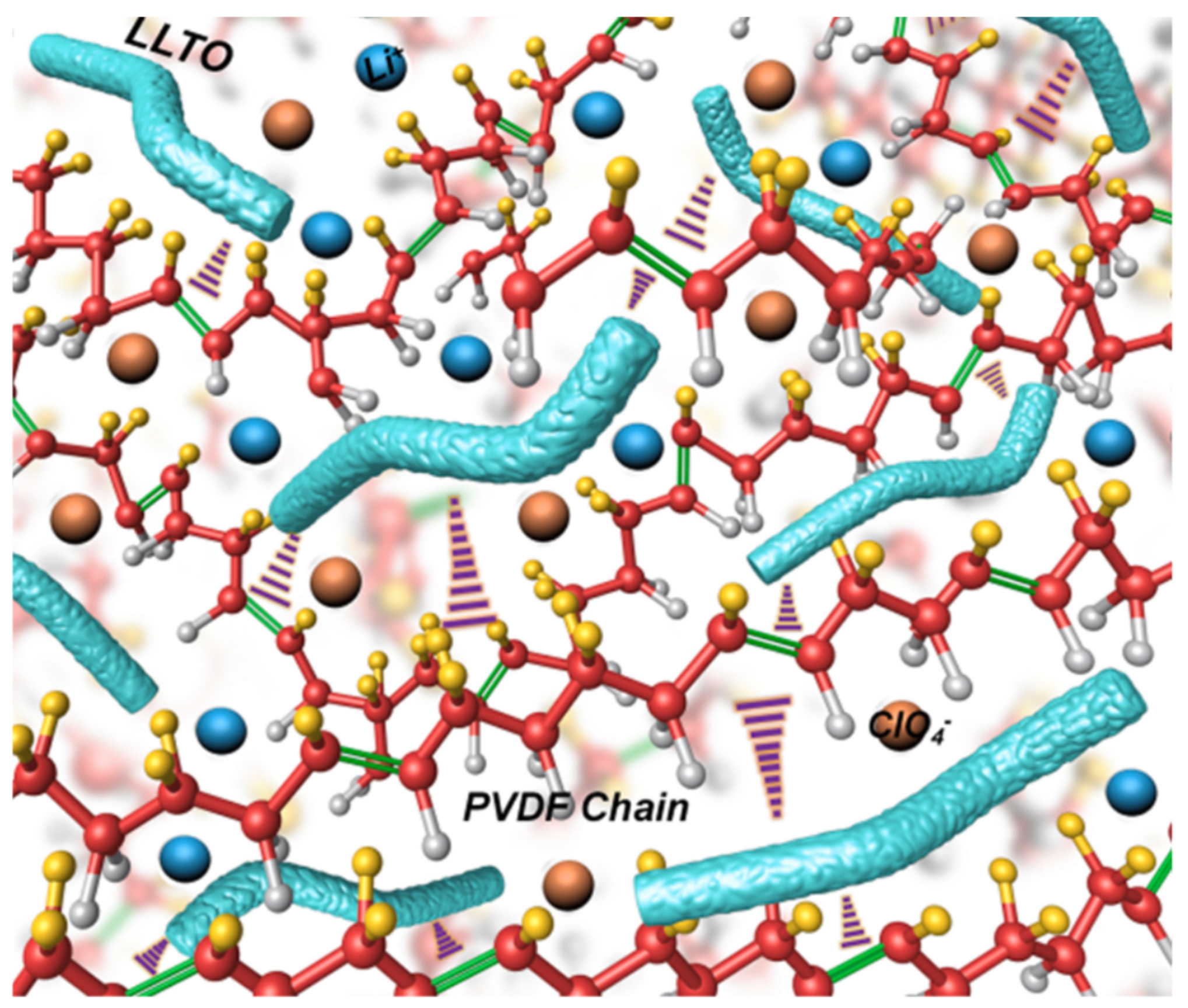
| Property | Polymer | Inorganic (Ceramic) | Hybrid/Composite |
|---|---|---|---|
| ionic conductivity | good at high temperature (60 °C) | high even at RT | high |
| mechanical strength | poor | excellent | medium |
| interface | good (low impedance) | poor (high impedance) | good |
| Final Product | Reagents | Gel Formation | Calcination | Sintering | Ref. |
|---|---|---|---|---|---|
| pristine LLTO and Sr doped LLTO Li0.35La0.55TiO3 Li0.35La0.35Sr0.03TiO3 | Sr(NO3)2, La2O3, LiNO3, C6H8O7×H2O | 80 °C for 2 h combustion at 250 °C | 650 °C for 6 h | 1250 °C for 4 h | [6] |
| Li0.35La0.55TiO3 | Ti, HCl, C4H6O6 (tartaric acid), LiNO3, La2O3 | 90 to 120 °C | 800, 900, 1000 and 1100 °C | 1250 °C | [51] |
| Eu doped Li0.5La0.5TiO3 | Ti[OCH(CH3)2]4, LiNO3, La(NO3)3×6H2O, Eu2O3, C6H8O7 (citric acid), (CH2OH)2 (ethylene glycol) | 120 °C for one hour | 350 °C for 2 h | 800 °C for 3 h | [52] |
| Al doped LLTO (Li0.33La0.56)1.005Ti0.99Al0.01O3 | LiNO3, La2(NO3)3×6H2O, Ti[OCH(CH3)2]4, Al(NO3)3×9H2O, (CH2)2(OH)2, C6H8O7, Li2O | 70 °C for 12 h to form a gel then heating to 100 °C to form a resin | 350 °C for 6 h + 750 °C for 3 h | 1350 °C for 6 h | [53] |
| Li0.35La0.55TiO3 | LiNO3, La(NO3)3×6H2O, Ti(OC4H9)4 | 80 °C for gel formation and drying at 150 °C | combustion at 350 °C for 4 h + calcination at 900 °C for 2 h | no sintering | [54] |
| Li0.33La0.56TiO3 | La(NO3)3×4H2O, LiNO3, Ti(OC3H7)4 | 95 °C for 2 h and 100 °C for 12 h | combustion at 450 °C for 30 min + calcination at 800–1200 °C for 12 h | 1150 °C for 10 h | [32] |
| Final Product | Reagents | Calcination | Sintering | Ref. |
|---|---|---|---|---|
| Li0.34La0.51TiO2.94 | La2O3, Li2CO3, TiO2 | 800 °C for 4 h + two heat treatments with intermediary grinding at 1150 °C for 12 h | 1350 °C for 6 h | [61] |
| Li0.33La0.56TiO3 | Li2CO3, La2O3, TiO2 | 800 °C for 8 h | 1250 °C + 1350 °C for 12 h followed by quenching | [57] |
| Li0.33La0.56−yTiO3−3yF3y (y = 0.017, 0.05) Li0.33+3yLa0.56−yTiO3 (y = 0, 0.01, 0.02, 0.04) | Li2CO3, La2O3, TiO2, LiF | 650 °C for 12 h | 1350 °C for 1.5 h followed by quenching | [5] |
| Li0.5−xLa0.5NaxTiO3 | Li2CO3, Na2CO3, La2O3, TiO2 | 1100 °C for 4 h | 1300–1330 °C for 6 h | [69] |
| LLTO doped with rare earths | La2O3, Li2CO3, Na2CO3, TiO2, SrCO3, BaCO3, MgO | 800 °C for 4 h, 1150–1200 °C for 6 to 12 h with intermediary grinding | 1350–1400 °C for 3 to 10 h | [73] |
| Li0.33La0.56TiO3−yFy 0 ≤ y ≤ 0.183 | La2O3, TiO2, Li2CO3, LiF | 800 °C for 2 h | 1200 °C for 10 h | [74] |
| Method | Parameters | Observations | Ref. |
|---|---|---|---|
| e-beam evaporation | LLTO target chamber pressure 7 × 10−2 Pa O2/Ar ratio 1:2 target to substrate distance 30 cm beam power 300–600 W | [67] | |
| PLD KrF laser 248 nm wavelength, aimed at 45° on rotating target | LixLa2/3−xTiO3 (x = 0.1, 0.2, 0.3, 0.4, 0.5) target chamber pressure: 1 × 10−6 to 5 × 10−6 torr target substrate distance 59 mm substrate temperature: RT pulse frequency: 10 Hz laser power: 180 mJ/pulse | amorphous film | [64] |
| RF magnetron sputtering | chamber pressure: 1 Pa O2/Ar ratio 30% O2, 70% Ar magnetron power 80 W | [78] |
| Composition | Synthesis Conditions | Crystallization | Lattice Constants | Ref. |
|---|---|---|---|---|
| Li0.35La0.567TiO3 | 800 °C | Tetragonal P4/mmm | a = 5.48869 c = 7.71678 | [51] |
| Li0.35La0.567TiO3 | 900 °C | a = 5.47065 c = 7.77080 | ||
| Li0.35La0.567TiO3 | 1000 °C | a = 5.47239 c = 7.77586 | ||
| Li0.35La0.567TiO3 | 1100 °C | a = 5.49422 c = 7.75065 | ||
| Li0.33La0.556TiO3 | 1250 °C | [62] | ||
| Li0.4La0.533TiO3 | 1250, 1300 °C | |||
| Li0.26La0.61TiO3 | 1000 °C | a = 3.875 c = 7.739 | [56] | |
| Li0.33La0.556TiO3 | Slow cooling | [57] | ||
| Li0.438La0.52TiO3 | a = 3.87 c ≈ 2a | |||
| Li0.501La0.499TiO3 | a = 3.87 c ≈ 2a | |||
| Li0.5La0.5TiO3 | 1350 °C | a = 3.6 c = 7.2 | [87] | |
| Li0.5La0.5TiO3 | 900 °C | a = 3.93 c = 7.86 | [88] | |
| Li0.35La0.55TiO3 | 1100 °C | Orthorhombic Pmmm Cmmm | a = 7.74351 b = 7.74209 c = 7.73723 | [51] |
| Li0.35La0.55TiO3 | 1250 °C | a = 7.74610 b = 7.73459 c = 7.73991 | ||
| Li0.16La0.613TiO3 | 1250, 1300, 1350 °C | Orthorhombic Pmmm | [62] | |
| Li0.33La0.556TiO3 | 1300, 1350 °C | |||
| Li0.4La0.533TiO3 | 1350 °C | |||
| Li0.09La0.636TiO3 | a = 3.864 b = 3.875 c = 7.786 | [57] | ||
| Li0.189La0.603TiO3 | ||||
| Li0.29La0.57TiO3 | 1400, 1460 °C | Orthorhombic Cmmm | a = 7.737(1) b = 7.742(1) c = 7.785(1) | [63] |
| Li0.33La0.556TiO3 | quenching | Cubic Pm3m | [57] |
| Main Material Characteristic | Effect on Conductivity | Variables Determining the Main Material Parameter | Other Parameters Affected by Optimization of Main Material Characteristic | Requirement for Improving Conductivity |
|---|---|---|---|---|
| grain boundary | decrease | sintering temperature, cooling speed | Li+ concentration, crystal symmetry | high temperature, slow cooling |
| domain boundary | decrease | sintering temperature, Li content | Li+ concentration, grain boundary | low sintering temperature, high Li content |
| crystal symmetry | increase if symmetry is high | sintering temperature, cooling speed, Li content | Li+ concentration, grain boundary | high temperature, quenching |
| crystal structure | increase if more space is available for Li transport | chemical composition, synthesis parameters | unit cell volume, bottleneck size, TiO6 octahedron size and tilt, Li+ concentration, vacancy concentration | increase La3+ substitution degree (with larger radius cation) |
| Li+/vacancy concentration | linear | chemical composition, synthesis parameters, reaction dynamics | crystal structure, grain boundary | decrease La3+ substitution degree (with larger radius cation) |
| Sample | Highest Conductivity at RT (S/cm) | Ref. |
|---|---|---|
| liquid organic electrolyte | ~10−2 | [6] |
| large grain size | 4.86 × 10−4 | [57] |
| pristine Li0.35La0.55TiO3 | 9.15 × 10−4 | [6] |
| Sr2+/La3+ substitution | 1.5 × 10−3 up to 1.95 × 10−3 | [6,73] |
| Ti4+/Al3+ substitution | 3.17 × 10−4 | [1] |
| O2−/F− substitution | 2.3 × 10−3 (30 °C) | [5] |
| PVDF/LLTO (15% LLTO wt.) | 5.8 × 10−4 | [107] |
| PEO/LLTO (5% LLTO wt.) | 5.53 × 10−5 (25 °C) and 4.65 × 10−4 (60 °C) | [108] |
| Vr-LLTO/PEO | 1.04 × 10−4 (25 °C) | [119] |
| PE-LLTO | 0.38 × 10−3 | [123] |
| LLTO-PEO heterostructure | 1.49 × 10−4 (30 °C) | [120] |
| Battery/Electrolyte | Initial Specific Capacity | Capacity Retention | Ref. |
|---|---|---|---|
| PVDF-LLTO | ≈150–170 mAh/g | 140 mAh after 200 cycles | [107] |
| LLTO coating for cathode material | 135 mAh/g | 80% after 200 cycles | [118] |
| PEO/LiTFSI/LLTO | ≈135 mAh/g | 123 mAh/g after 100 cycles at 60 °C | [108] |
| LLTO-PE | ≈150.3 mAh/g | 88.7% after 1000 cycles | [123] |
| Li|LLTO-PVDF|LiFePO4 | >150 mAh/g | 91.7% after 1000 cycles | [2] |
| Metallic Li battery|fluorinated LLTO-PEO electrolyte | >125 mAh/g | >80% after 100 cycles at 90 °C | [122] |
| Al-LLTO aqueous battery Li metal anode, LiCoO2 cathode | 150–200 mAh/g | 59.3% after 100 cycles | [1] |
| Organic type Li-oxygen battery, Al-LLTO electrolyte | 300 mAh/g | 100% after 100 cycles | [53] |
| LiTFSI-BL-SN | 170–180 mAh/g | 90% after 500 cycles | [24] |
| SiOx/C anode | 800–830 mAh/g | 600–700 mAh/g after 40–50 cycles at a rate of 2 C 800 mAh after 100 cycles at a rate of 0.1 C | [25] |
| Metallic Li battery, Li4Ti5O12 electrode/PVDF electrolyte | 150 mAh/g | 119 mAh/g after 400 cycles at a rate of 5 C | [27] |
| Typical phone battery | 372 mAh/g | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okos, A.; Ciobota, C.F.; Motoc, A.M.; Piticescu, R.-R. Review on Synthesis and Properties of Lithium Lanthanum Titanate. Materials 2023, 16, 7088. https://doi.org/10.3390/ma16227088
Okos A, Ciobota CF, Motoc AM, Piticescu R-R. Review on Synthesis and Properties of Lithium Lanthanum Titanate. Materials. 2023; 16(22):7088. https://doi.org/10.3390/ma16227088
Chicago/Turabian StyleOkos, Alexandru, Cristina Florentina Ciobota, Adrian Mihail Motoc, and Radu-Robert Piticescu. 2023. "Review on Synthesis and Properties of Lithium Lanthanum Titanate" Materials 16, no. 22: 7088. https://doi.org/10.3390/ma16227088
APA StyleOkos, A., Ciobota, C. F., Motoc, A. M., & Piticescu, R.-R. (2023). Review on Synthesis and Properties of Lithium Lanthanum Titanate. Materials, 16(22), 7088. https://doi.org/10.3390/ma16227088







