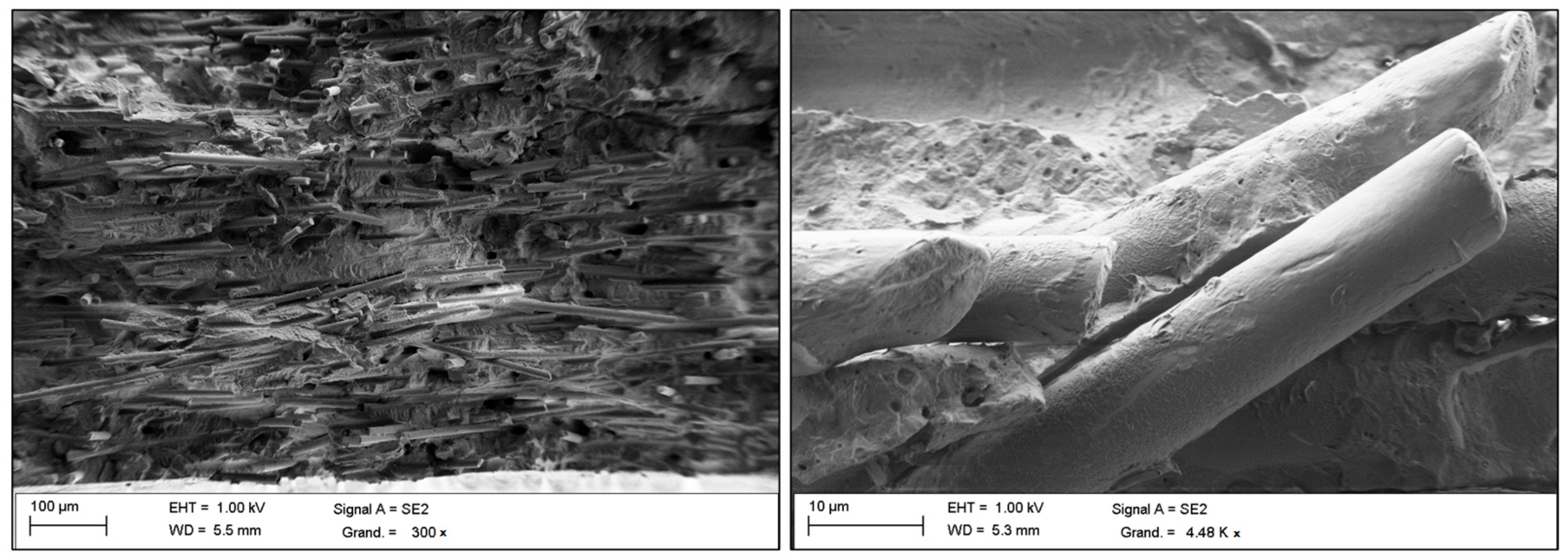
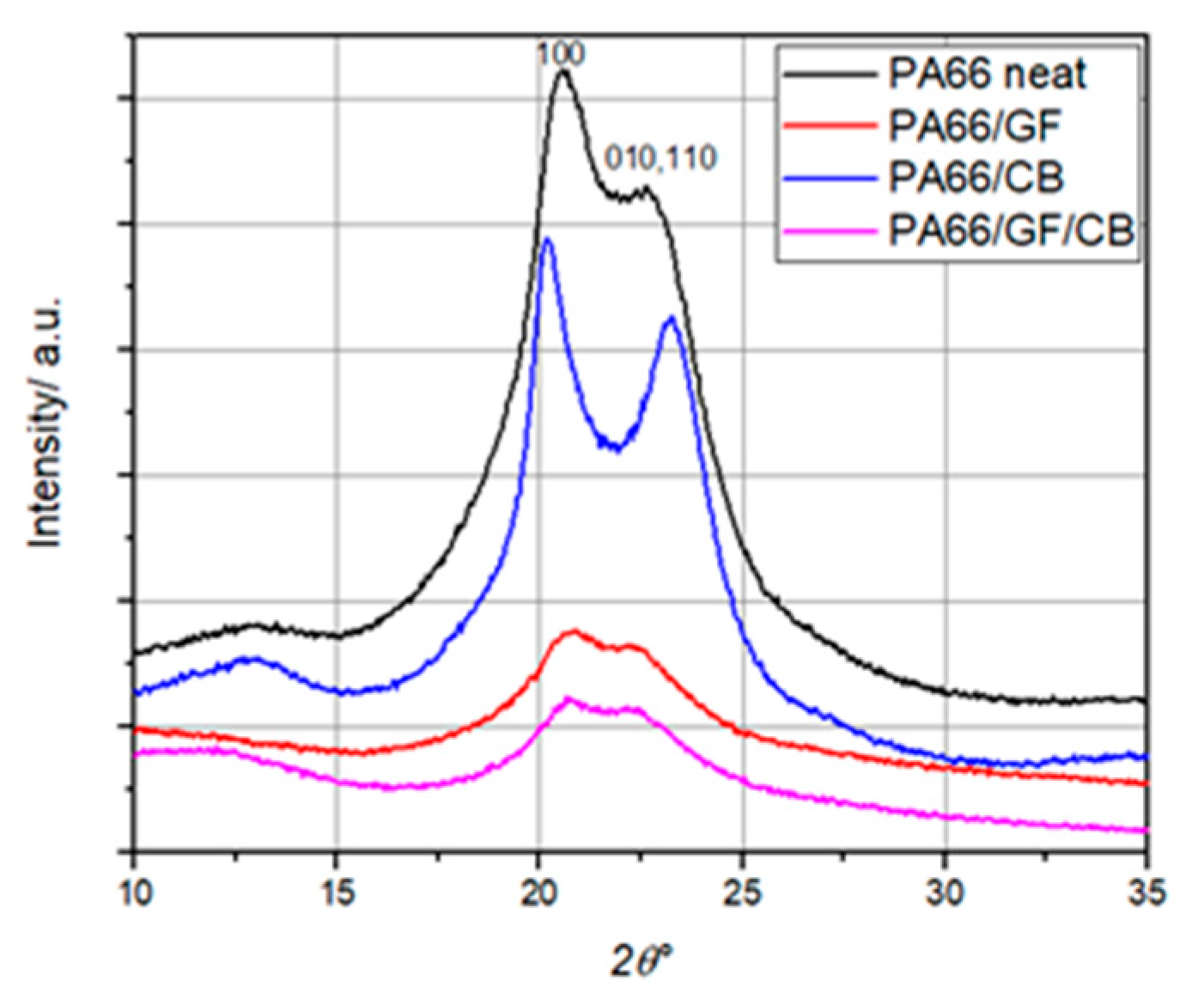
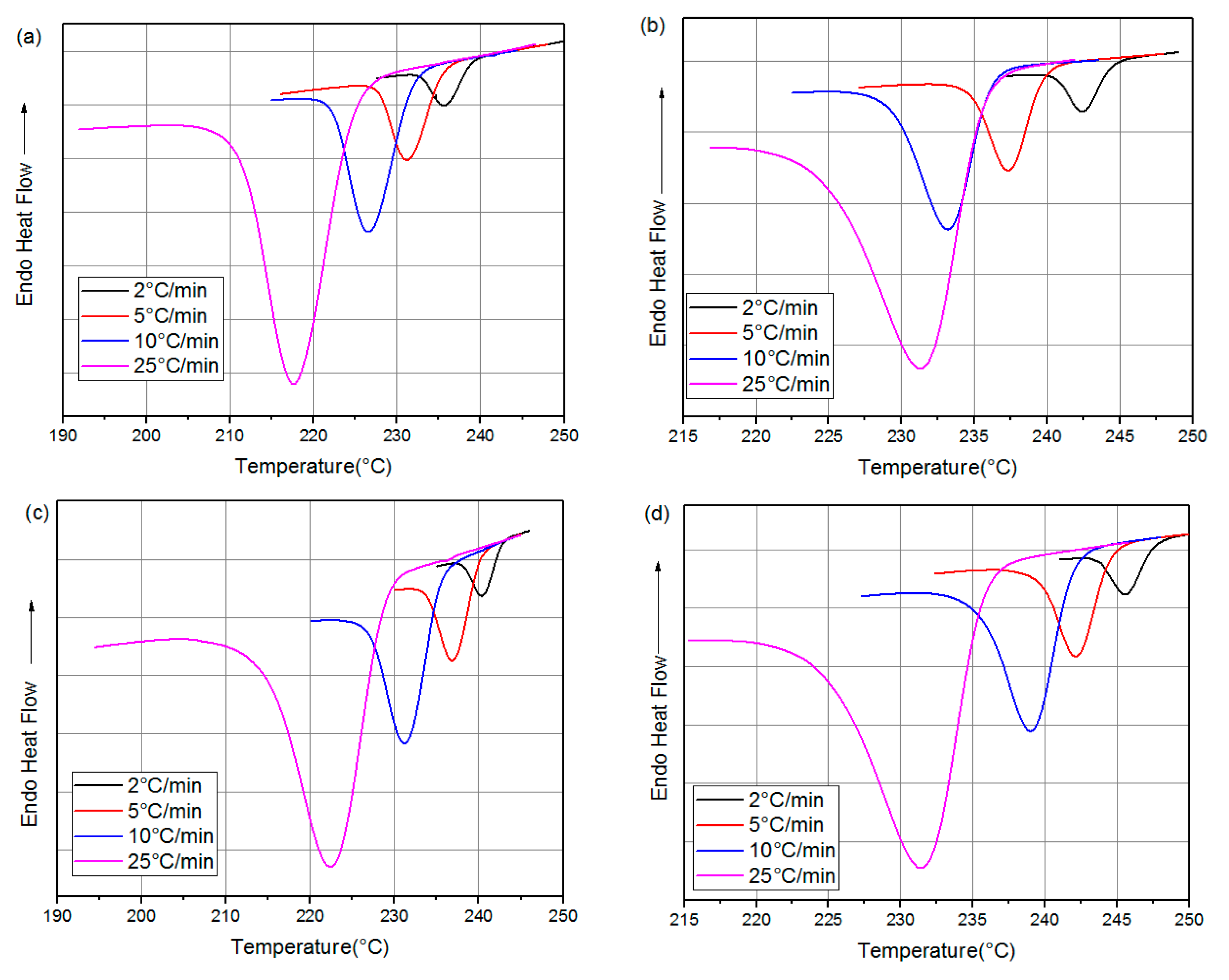
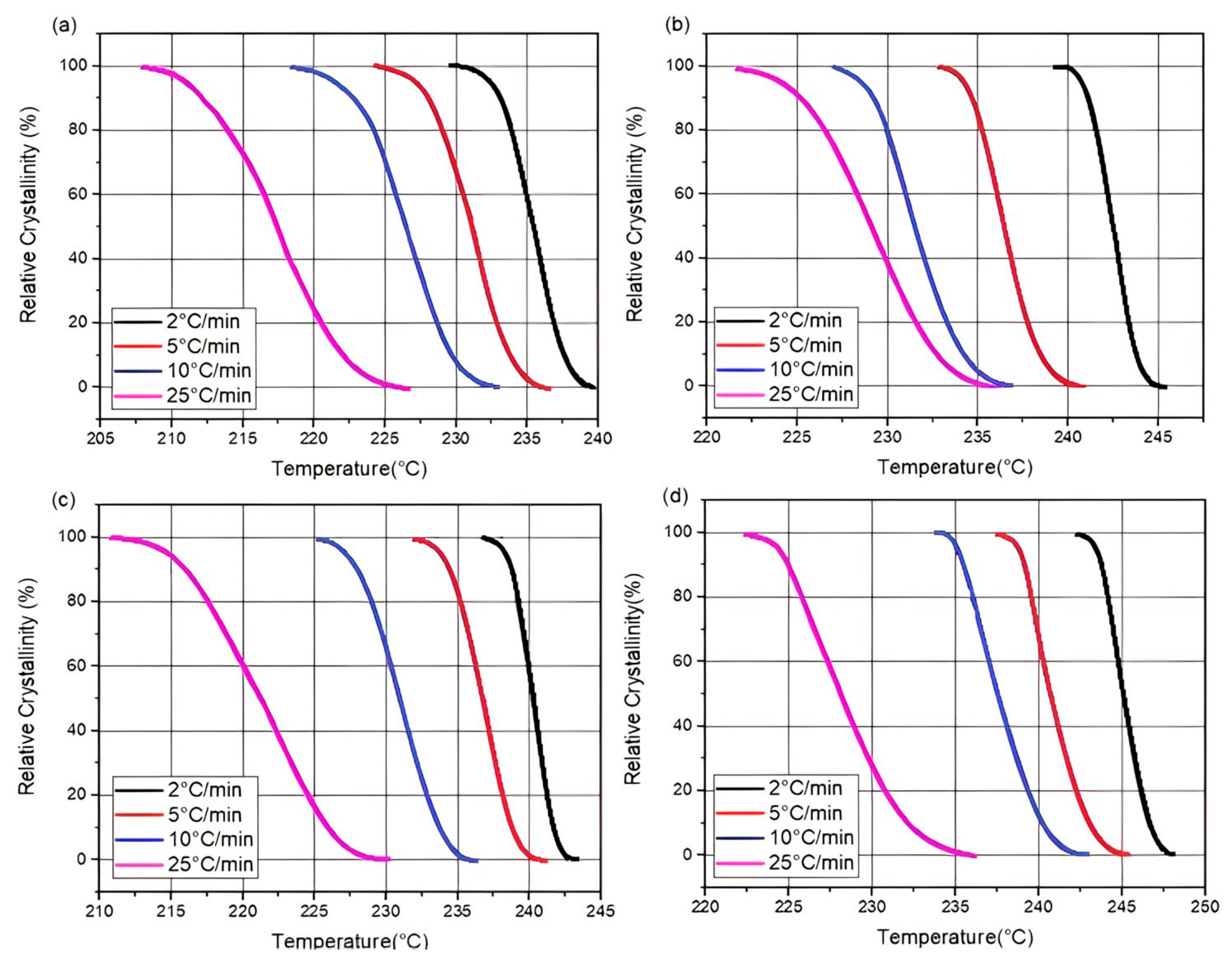
1. Introduction
Polyamides are commonly used in various applications due to their high strength and durability. Polyamide 66 (PA66) is a semicrystalline thermoplastic with excellent thermal and physical properties [
1] and high mechanical properties (rigidity, wear, and creep resistance) and boasts exceptional short-term thermal endurance due to its high melting point of 260 °C [
2,
3].
Industrial polymer-based composites use mineral fillers and fibers as reinforcing agents [
4,
5,
6]. These agents enhance the polymer’s mechanical properties while also decreasing production expenses [
3]. When polyamide 66 is reinforced with glass fiber (PA66/GF) and carbon black (PA66/CB), the resulting composite material has excellent mechanical properties. Concurrently, it is commonly understood that fibers can significantly enhance the crystallization process of various thermoplastic polymers with semi-crystalline properties [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. Glass fibers (GFs) are a popular reinforcement material for composites because of their high strength-to-weight ratio and resistance to corrosion. When added to PA66, glass fibers can significantly improve the composite’s strength and stiffness and increase the elastic modulus above the glass transition temperature of semi-crystalline polyamide [
2,
8,
20]. Carbon black (CB) is also frequently used to enhance the thermal and mechanical properties of the mechanical parts of PA66, such as resistance to wear caused by sliding (abrasion), electrical conductivity, and thermal stability [
21]. Like GF, carbon black modifies the crystallization behavior of semi-crystalline polymers [
22,
23].
The combination of glass fibers and carbon black with PA66 creates a composite material that exhibits superior properties. These properties include high impact, abrasion, and fatigue resistance. This makes it ideal for use in various applications, such as automotive parts, aircraft components, and sporting equipment [
1,
24].
The crystallization kinetics of semi-crystalline polymers, which have significant influences on the crystal structures, morphologies, and polymer properties, is a critical parameter for polymer processing [
25]. The study of non-isothermal crystallization kinetics is very relevant to understanding the properties of composite materials [
26]. PA66 crystallization typically occurs during cooling from the melt state. During the cooling process, the polymer chains arrange themselves in a highly ordered manner, forming crystals. Crystals grow and interlock, leading to the formation of a solid material with a highly ordered structure [
27].
Polyamide crystallization is influenced by several factors such as temperature, cooling rate, and additives or fillers. In addition, it is influenced by the polymerization degree. It typically occurs during the solidification process of the polymer, either during molding or cooling of the melted polymer [
19]. The effectiveness of composite materials utilizing a semi-crystalline polymer matrix relies not only on the proportion of the two base components but also on the ratio of crystallinity and the crystal structure of the matrix [
1,
2]. Globally, the crystallization process plays a crucial role that affects the final properties of polyamide. Its control is of high importance in optimizing the performance of this technical polymer in various applications.
In recent years, most researchers have focused on studying the non-isothermal crystallization kinetics of polymers and the way in which they can be affected by reinforcing fillers [
1,
2,
3,
4,
5,
6,
28,
29]. For example, Layachi et al. [
1] studied the non-isothermal crystallization kinetics of 15 wt.% glass fiber or 0.4 wt.% carbon black reinforced PA66 at different cooling rates. The results show that the PA66 crystallization rate is increased in the presence of these additives. What is more, Mo’s method can provide a good explanation of the non-isothermal crystallization of neat PA66 and PA66 composites [
1,
2]. Arroyo et al. [
28] studied the effect of short GFs on the isothermal crystallization of a polypropylene matrix. They determined that the presence of a GF reinforcing agent has a notable impact on the nucleation of the polymer. In addition, the incorporation of GFs can expedite the crystallization process, resulting in a shorter crystallization time.
When reinforcing fillers and additives are added to semi-crystalline polymeric matrices, the crystallization capabilities of the polymer matrix are modified [
1,
2]. This includes changes in crystallization kinetics, crystal perfection, and crystallinity index [
29]. Studies have shown that solid particles in such materials often act as nucleating agents during crystallization. As a result, this is likely to affect the mechanical properties of composites. Frihi et al. [
29] studied the influences of glass fiber contents on PA66 composites’ crystallization properties. They determined that the PA66/GF crystallinity ratio is significantly influenced by GF composition and processing conditions.
In the process of thermoplastic composite development, a semi-crystalline structure is created alongside interfacial bonds [
2,
29]. Understanding and managing the role of the reinforcing fibers in matrix crystallization is crucial. The polymer crystalline morphology, which is influenced by nucleation rates and crystal growth, is a crucial factor that affects the mechanical and physical properties of the final product. Therefore, gaining a better understanding and control of this phenomenon is of substantial interest [
30,
31]. Makhlouf et al. [
2] highlighted a reduction in the nucleation effect for composites containing 30 wt.% GF. This was attributed to the fact that the PA66 matrix approaches its saturation point at approximately 30 wt.% GF. The observation of a nucleation optimum at 30% can be attributed to the increase in the crowding effect of glass fibers that gradually impinge into each other and mutually cover each other with increasing contents so that only a part of their surface remains fully active for crystal nucleation. In parallel, the observation from a recent structural study [
29] that the PA66 matrix crystallinity gradually saturates at a glass fiber content of 30% is most probably because the increasing number of glass fibers is able to compensate—to some extent—the reduced nucleation activity of the glass fibers. Then, the overall PA66 crystallinity is influenced by the reduction of glass fiber nucleation activity at a GF content higher than 30 wt.%. This is in conjunction with the intrinsic crystallization capability of PA66 upon quiescent conditions. Additionally, the thermal stability of PA66/GF composites surpasses that of neat PA66. This is because of the bonding that occurs between the PA66 matrix and the GF.
The non-isothermal crystallization kinetics and reaction mechanisms of PA66 and its composites need to be understood to better control thermal crystallization and maintain mechanical properties [
32]. The activation energy is required to overcome the energy barrier between the disordered liquid phase and the ordered solid phase. Once this energy is supplied, the process of nucleation can occur [
33]. In the context of crystallization, activation energy refers to the energy required to initiate the formation of crystalline neat. The activation energy can also affect the crystal growth rate, as a higher activation energy means the reaction will occur slower. An evaluation of the activation energy of a non-isothermal crystallization is critical in materials science and engineering as it can provide valuable information on the nucleation and growth processes of the crystalline phase during cooling. This information can be used to optimize processing conditions and tailor the properties of the composite material. It can also be used for developing customized composite materials with tailored properties for specific applications [
34,
35].
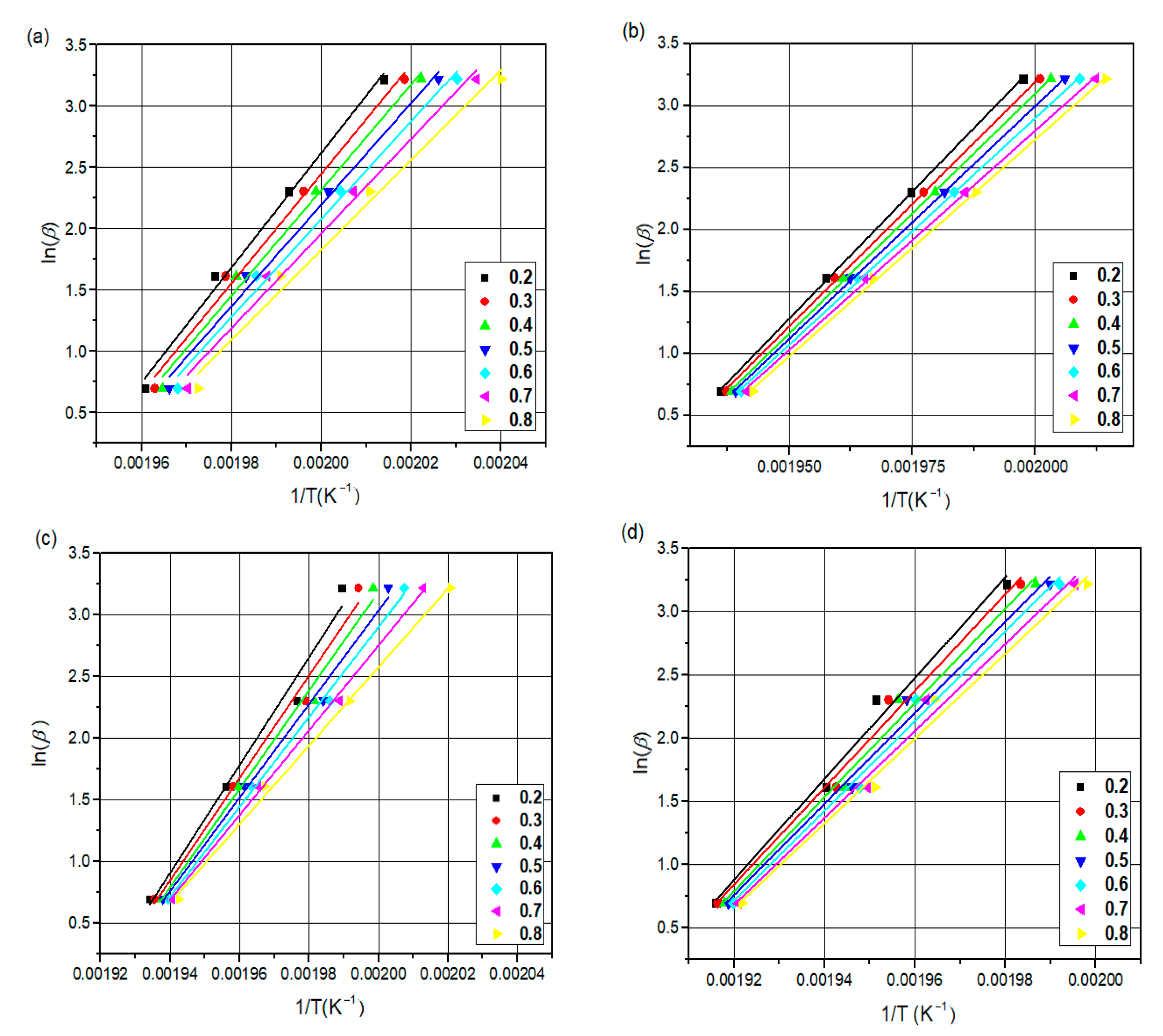
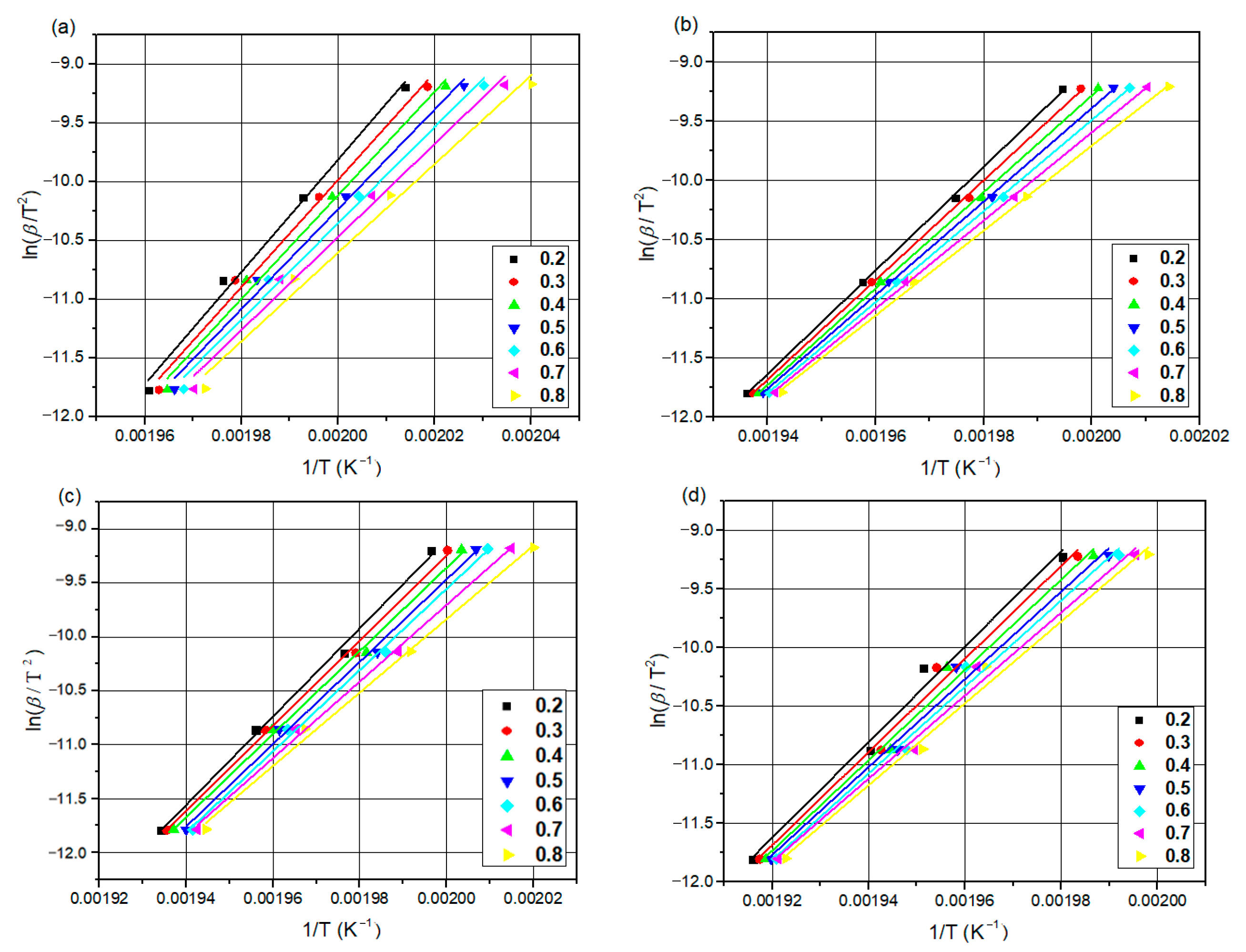
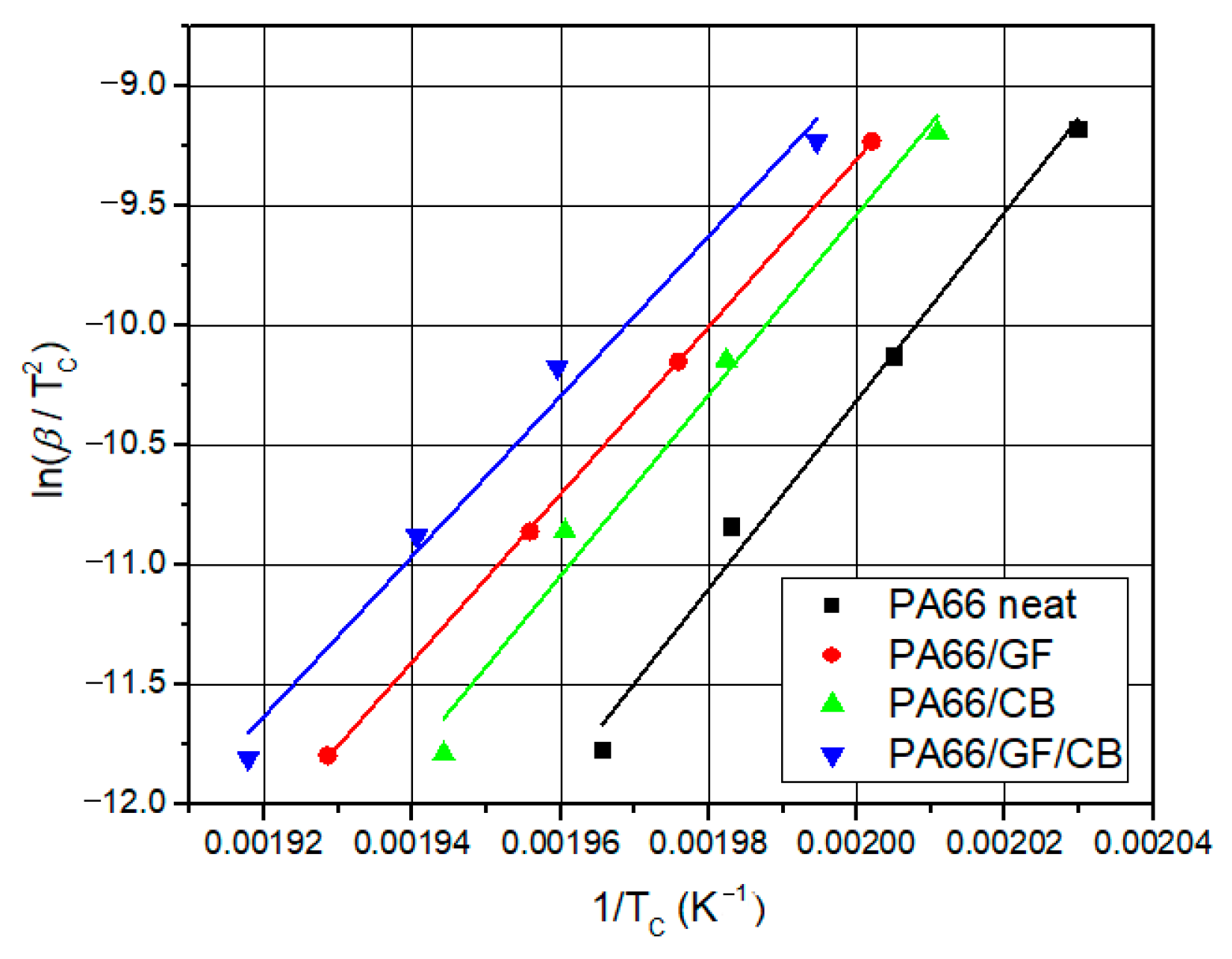
The differential scanning calorimetry (DSC) technique is commonly used for the kinetic analysis of polymers and for evaluating how material properties change over time and temperature [
19]. Applying kinetic methods can yield kinetic parameters (relative crystallinity and activation energy) from DSC measurements [
36,
37]. There are many methods for analyzing the kinetic data of non-isothermal reactions, and they can be generally divided into model-fitting methods and iso-conversive methods [
38,
39,
40,
41]. Model-fitting methods are, for example, the Kissinger and Coats–Redfern (C–R) methods [
40,
41]; iso-conversive methods can be classified into integral and differential iso-conversive methods [
42,
43,
44]. The most popular iso-conversive integral methods are the Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) methods. Both methods calculate accurate activation energies [
45,
46].
The kinetic analysis of PA66 can guide the crystallization process of PA66 in an industrial context. It can also establish a theoretical basis for the development of an action plan and the industrialization of the product. This study complements the previous one [
1,
2]: Makhlouf and Layachi et al. [
1,
2] examined the development of crystalline spherulites and crystallization kinetics of PA66/GF composites. Additionally, the optimal loading rate of GF for producing composites tailored for specific applications was revealed.
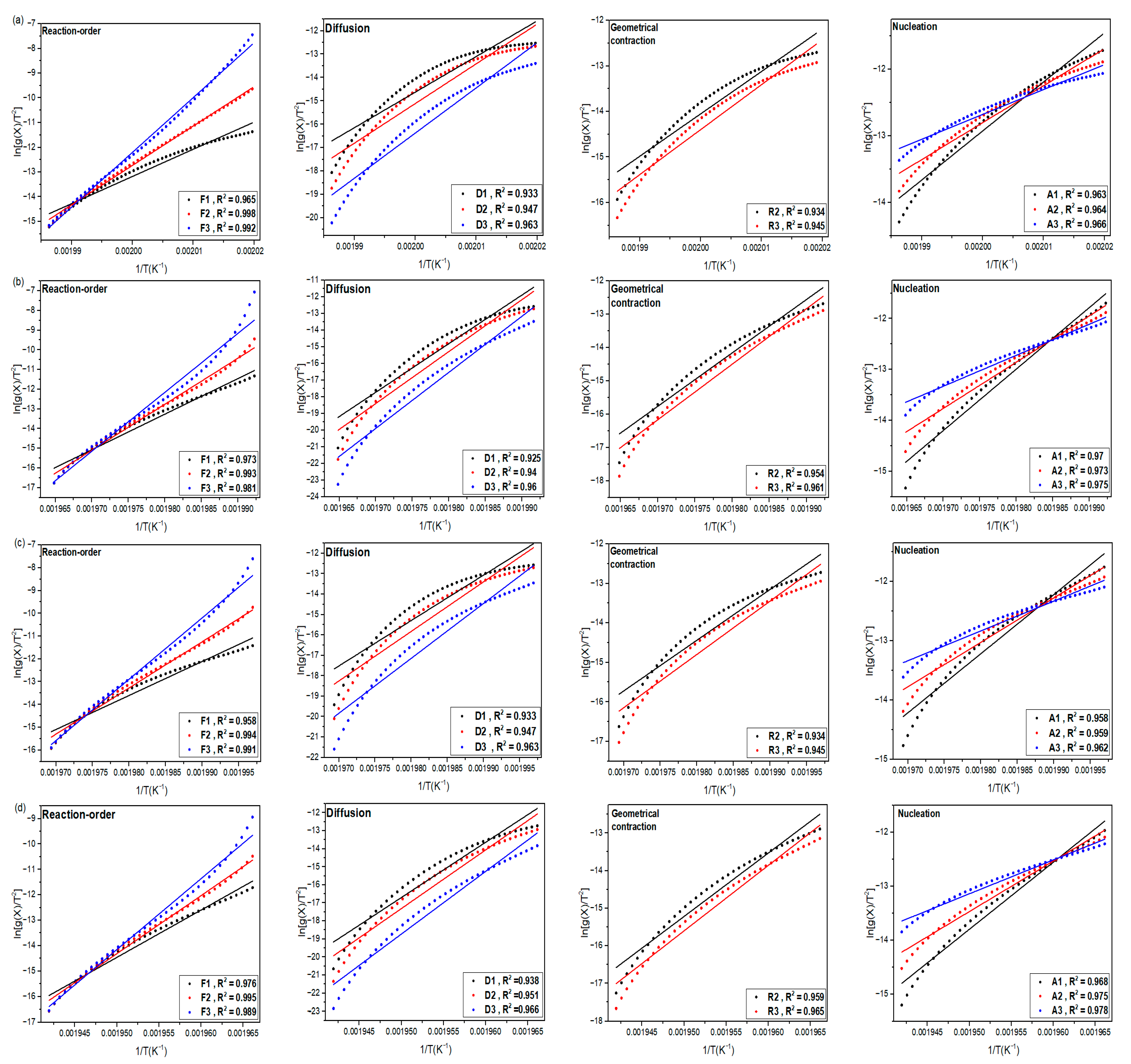
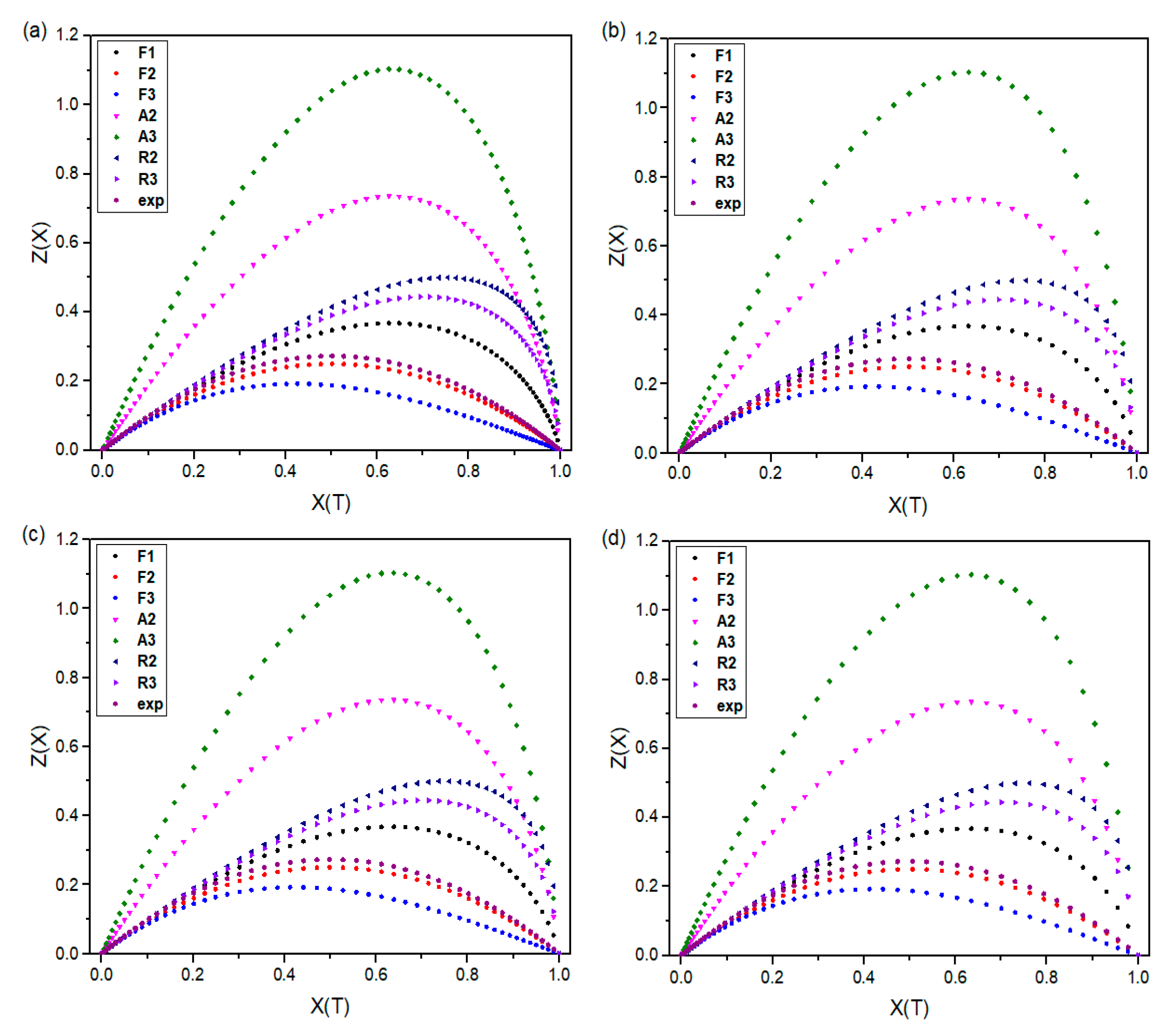
This study introduces the kinetics and mechanisms of non-isothermal crystallization of a PA66 matrix reinforced with 30 wt.% glass fiber and 0.4 wt.% carbon black. The novelty of the present work is related to (1) the exploration of the kinetic non-isothermal behavior of PA66 composites that are reinforced with both glass fiber and carbon black, through differential scanning calorimetry (DSC), and (2) the determination of the kinetic parameters and activation energy (Ea) and the evaluation of the suitability of the methods proposed. The activation energy of neat PA66, PA66/GF, PA66/CB, and PA66/GF/CB was evaluated using iso-conversive integral methods (Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS)) and model-fitting methods (Kissinger and Coats–Redfern (C–R)). The Criado and Coats–Redfern methods [
40,
41] were used to identify possible reaction models of neat PA66 and related composites, as well as the nucleating effect of GF and CB on the PA66 matrix, to gain a better understanding of the thermal crystallization mechanism of PA66 and its composites.
4. Conclusions
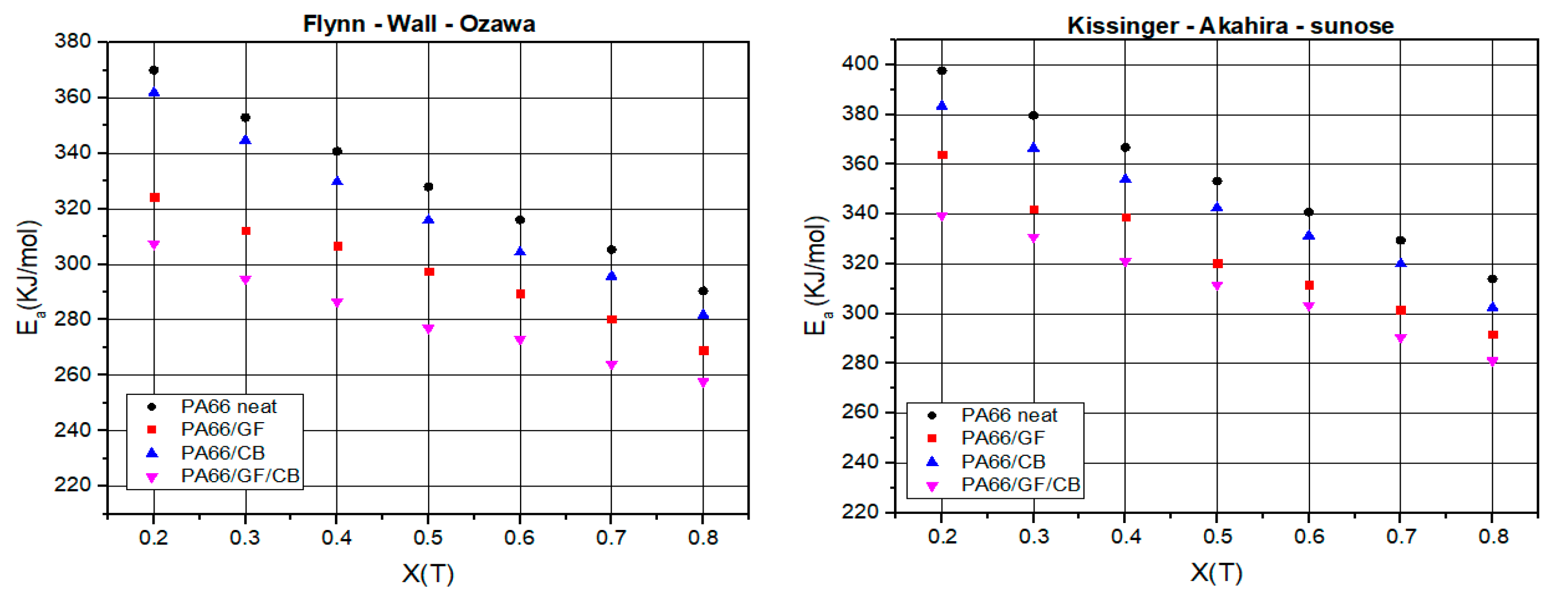
This paper investigates the effect of GF on PA66 crystallization behavior. The addition of a small amount of carbon black was investigated, owing to its widespread occurrence in industrial parts. Thermal crystallization kinetics of neat PA66 and its composites were analyzed using model-free and model-fitting approaches. The thermal crystallization results of the composites compared to neat PA66 show that GF promotes a nucleation effect. This significantly increases the crystallization kinetics and crystallinity ratio of the PA66 matrix in the composites. The crystallization rate also increased with the addition of CB despite the rather low content. The experimental results indicate that the presence of GF and CB improves the thermal stability of PA66 composites. Moreover, the average energy of neat PA66, as determined by the FWO method, stands at 329.1 KJ/mol, surpassing the energies of PA66/GF, PA66/CB, and PA66/GF/CB, which measure 297.1, 319.7, and 280.1 KJ/mol, respectively. It is clear from this that GF and CB contribute to the crystallization of PA66 in a synergistic manner. Crystallization of neat PA66, PA66/GF, PA66/CB, and PA66/GF/CB follows the second-order (F2) with the integral form g(X(T)) = and the differential form f(X(T) = , i.e., the reactions are decelerated, showing that PA66 matrix presents good affinity with GF and CB fillers. The results provide useful insights into the crystallization mechanism of pure PA66 and its composites.