Influence Analysis of Polyvinyl Alcohol on the Degradation of Artificial Leather with Cellulose Nitrate Coating Originating from a Suitcase Stored in the Collection of the Auschwitz-Birkenau State Museum in Oświęcim, Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objects
2.2. Aging Conditions Study
2.3. Chemical Surface Structure Analysis of Cellulose Nitrate after Artificial Aging of Samples
2.3.1. Fourier-Transform Infrared Attenuated Total Reflection Spectroscopy (FTIR-ATR)
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
3. Results
3.1. Chemical Structure of Cellulose-Nitrate-Based Artificial Leather after Aging, Determined via the FTIR-ATR Method
3.2. Elemental Composition of Cellulose-Nitrate-Based Artificial Leather after Aging, Determined via X-ray Photoelectron Spectroscopy (XPS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cywiński, P.A.; Lachendro, J.; Setkiewicz, P. Auschwitz from A to Z; Auschwitz-Birkenau State Museum: Oświęcim, Poland, 2013; p. 125. [Google Scholar]
- Kanigel, R. Faux Real Genuine Leather and 200 Year of Inspired Fakes; University of Pensylvania Press: Philadelphia, PA, USA, 2010; p. 31. [Google Scholar]
- Worden, E.C. Nitrocellulose Industry; Constable and Company Ltd.: London, UK, 1911; pp. 360–395. [Google Scholar]
- United States Patent Office. Manufacture of Artificial Leather or Leather Cloth. U.S. Patent 352,726, 16 November 1886. Available online: https://patentimages.storage.googleapis.com/pdfs/US352726.pdf (accessed on 19 May 2023).
- Tuttle, F. The Story of Coated Fabrics, II—Rubber and Pyroxylin Coatings. Text. Res. 1944, 14, 228–232. [Google Scholar] [CrossRef]
- Rath, H. Lehrbuch der Textilchemie: Einschl. Der Textil-Chemischen Technologie; Springer: Berlin/Heidelberg, Germany, 1952; p. 360. [Google Scholar]
- Kunstleder, D.G. Enzyklopädie der Textilchemischen Technologie; Springer: Berlin/Heidelberg, Germany, 1930; pp. 691–693. [Google Scholar]
- Shaschoua, Y. Mesocycles in conserving plastics. Stud. Conserv. 2016, 61, 208–213. [Google Scholar] [CrossRef]
- Springate, M.E. Cellulose Nitrate Plastic (Celluloid) in Archaeological Assemblages: Identification and Care. Northeast. Hist. Archaeol. 1997, 26, 63–72. [Google Scholar] [CrossRef]
- Shashoua, Y. A safe plase: Storage strategies for plastics. GCI Newsl. 2014, 29, 13–15. Available online: https://www.getty.edu/conservation/publications_resources/newsletters/pdf/v29n1.pdf (accessed on 14 October 2023).
- Shashoua, Y.; Schilling, M.; Mazurek, J. The effectiveness of conservation adsorbents at inhibiting degradation of cellulose acetate. In Proceedings of the 17th Triennial Conference ICOM-CC—Modern Materials and Contemporary Art, International Council of Museums, Melbourne, Australia, 15–19 September 2014. [Google Scholar]
- Paulocik, C.; Williams, R.C. The chemical composition and conservation of late 19th and early 20th century sequins. J. Can. Assoc. Conserv. 2010, 35, 46–61. [Google Scholar]
- Meincke, A.; Hausdorf, D.; Gadsden, N.; Baumeister, M. Early cellulose nitrate coatings on furniture of the Company of Master Craftsmen. J. Am. Inst. Conserv. 2013, 48, 1–24. [Google Scholar] [CrossRef]
- Sirkis, L.S. The History, Deterioration and Conservation of Cellulose Nitrate and Other Early Plastic. Bachelor’s Thesis, University of London Institute of Archaeology, London, UK, 1982. Available online: https://www.academia.edu/34391817/the_history_deterioration_and_conservation_of_cellulose_nitrate_and_other_early_plastic_objects (accessed on 13 October 2023).
- Quye, A.; Littlejohn, D.; Pethric, R.A.; Steward, R.A. Accelerated ageing to study the degradation of cellulose nitrate museum artefacts. Polym. Degrad. Stab. 2011, 96, 1934–1939. [Google Scholar] [CrossRef]
- Louvet, A.; Lavedrine, B.; Flieder, F. Size Exclusion Chromatography and Mass Spectrometry of Photographic Bases in Cellulose Nitrate Degradation. J. Photogr. Sci. 2016, 43, 30–35. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rybitwa, D.; Rahnama, M.; Wilczyński, S. Microorganisms colonising historical cardboard objects from the Auschwitz-Birkenau State Museum in Oświęcim, Poland and their disinfection with vaporised hydrogen peroxide (VHP). Int. Biodeterior. Biodegrad. 2020, 152, 104997. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Gutarowska, B.; Rybitwa, D.; Pietrzak, K.; Machnowski, W.; Wrzosek, H.; Papis, A.; Walawska, A.; Otlewska, A.; Szulc, J.; et al. Vapourised hydrogen peroxide (VHP) and ethylene oxide (EtO) methods for disinfecting historical cotton textiles from the Auschwitz-Birkenau State Museum in Oswięcim, Poland. Int. Biodeterior. Biodegrad. 2018, 133, 42–51. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institut: Los Angeles, CA, USA, 2000; pp. 100–120. [Google Scholar]
- Margariti, C. The application of FTIR microspectroscopy in a non-invasive and non-destructive way to the study and conservation of mineralised excavated textiles. Herit. Sci. 2019, 7, 63. [Google Scholar] [CrossRef]
- Yan, Y.; Wen, C.; Jin, M.; Duan, L.; Zhang, R.; Luo, C.; Xiao, J.; Ye, Z.; Gao, B.; Liu, P.; et al. FTIR spectroscopy in cultural heritage studies: Non-destructive analysis of Chinese Handmade Papers. Chem. Res. Chin. Univ. 2019, 35, 586–591. [Google Scholar] [CrossRef]
- Carter, E.A.; Swarbrick, B.; Harrison, T.H.; Ronai, L. Rapid identification of cellulose nitrate and cellulose acetate film in historic photograph collections. Herit. Sci. 2020, 8, 51. [Google Scholar] [CrossRef]
- Nunes, S.; Ramacciotti, F.; Neves, A.; Angelin, E.M.; Ramos, A.M.; Roldão, É.; Wallaszkovits, N.; Armijo, A.A.; Melo, M.J. A diagnostic tool for assessing the conservation condition of cellulose nitrate and acetate in heritage collections: Quantifying the degree of substitution by infrared spectroscopy. Herit. Sci. 2020, 8, 33. [Google Scholar] [CrossRef]
- De Sá, S.F.; da Cruz, S.M.; Callapez, M.E.; Carvalho, V. Plastics that made history—The contribution of conservation science for the history of the Portuguese Plastics Industry. Conserv. Património 2020, 35, 85–100. [Google Scholar] [CrossRef]
- Neves, A.; Friedel, R.; Callapez, M.E.; Swank, S.D. Safeguarding our dentistry heritage: A study of the history and conservation of nineteenth–twentieth century dentures. Herit. Sci. 2023, 11, 142. [Google Scholar] [CrossRef]
- Quye, A.; Littlejohn, D.; Pethric, R.A.; Steward, R.A. Investigation of inherent degradation in cellulose nitrate museum artefacts. Polym. Degrad. Stab. 2011, 96, 1369–1376. [Google Scholar] [CrossRef]
- Shashoua, Y.; Bradley, S.M.; Daniels, V.D. Degradation of cellulose nitrate adhesive. Stud. Conserv. 1992, 37, 113–119. [Google Scholar] [CrossRef]
- Lozano MV, C.; Elsasser, C.; Angelin, E.M.; Pamplona, M. Shedding Light on Degradation Gradients in Celluloid: An ATR-FTIR Study of Artificially and Naturally Aged Specimens. Polymers 2023, 15, 522. [Google Scholar] [CrossRef]
- Berthumeyrie, S.; Collin, S.; Bussiere, P.; Therias, S. Photooxidation of cellulose nitrate: New insights into degradation mechanisms. J. Hazard. Mater. 2014, 272, 137–147. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Rahnama, M. Application of a medical diode laser (810 nm) for disinfecting small microbiologically contaminated spots on degraded collagenous materials for improved biosafety in objects of exceptional historical value from the Auschwitz-Birkenau State Museum and Protection of Human Health. Front. Microbiol. 2020, 11, 596852. [Google Scholar] [CrossRef]
- Short, R.D.; Munro, H.S.; Ward, R.J.; McBriar, I.; Chan, H.S.O.; Tan, K.L. An XPS investigation of the radiation induced degradation reaction of cellulose nitrate: Part 1—Argon Plasma Induced Surface Reactions. Polym. Degrad. Stab. 1991, 31, 211–218. [Google Scholar] [CrossRef]
- Short, R.D.; Munro, H.S. Conclusions drawn from a study of cellulose nitration in technical mixed acids by X-ray photoelectron spectroscopy and 13C nuclear magnetic resonance. Polymer 1993, 34, 2714–2719. [Google Scholar] [CrossRef]
- Heckman, H.; Kepley, V.L.; Mahanthappa, M.K.; McQueen, A.; Mullen, K. Investigation of Cellulose Nitrate Motion Picture Film Chemical Decomposition & Associated Fire Risk; University of Wisconsin: Madison, WI, USA, 2015; pp. 25–38. Available online: https://hcommons.org/deposits/item/hc:12627/ (accessed on 28 August 2023).
- Elasser, C.; Mayr, V.; Montag, P.; EM Angelin Hilbig, H.; Grosse, C.U.; Pamplona, M. Mock-Ups in Plastic Conservation Research: Processing and Aging of 3D Celluloid Specimens Simulating Historical Objects. Polymers 2023, 15, 852. [Google Scholar] [CrossRef] [PubMed]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitrocellulose synthesis from miscanthus cellulose. Propellants Explos. Pyrotech. 2018, 43, 96–100. [Google Scholar] [CrossRef]
- Li, L.; Frey, M. Preparation and characterization of cellulose nitrate-acetate mixed ester fibers. Polymer 2010, 51, 3774–3783. [Google Scholar] [CrossRef]
- Kawanobe, W. Synthetic resin in restoration of cultural property. Kobunshi 2007, 56, 588–592. [Google Scholar] [CrossRef]
- Horie, V. Materials for Conservation. Organic Consolidants, Adhesives and Coatings; Routledge: New York, NY, USA, 2010; pp. 142–145. [Google Scholar]
- Leiggi, P.; May, P. Vertebrate Paleontological Techniques; Cambridge University Press: Cambridge, UK, 1994; Volume 1, pp. 3–35. [Google Scholar]
- Peng, H.; Wang, S. Properties and Reinforcing Mechanism of Cellulose Reinforced Polyvinyl Alcohol Hydrogel Membranes. In Proceedings of the 2nd International Symposium on Mechanical Engineering and Material Science (ISMEMS 2017), Suzhou, China, 17–19 November 2017; pp. 25–28. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawady, W.K.; Nassa, M.A. Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohydr. Polym. 2010, 79, 694–699. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Długa, A.; Kowalonek, J.; Kaczmarek, H. Bionanocellulose/Poly(Vinyl Alcohol) Composites Produced by In-Situ Method and Ex-Situ/Impregnation or Sterilization Methods. Materials 2021, 14, 6340. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Houda, T.; Lu, R.; Hayakawa, N. Ultraviolet degradation of poly(vinyl alcohol) used in restoration of historical and cultural properties. Polym. Degrad. Stab. 2015, 114, 30–36. [Google Scholar] [CrossRef]
- Okada, Y.; Kawanobe, W.; Hayakawa, N.; Tsubokura, S.; Chujo, R.; Fujimatsu, H.; Takizawa, T.; Hirai, T. Whitening of polyvinyl alcohol used as restoration material for Shohekiga. Polym. J. 2011, 43, 74–77. [Google Scholar] [CrossRef]
- Neves, A.; Ramos, A.M.; Callapez, M.E.; Friedel, R.; Réfrégiers, M. Novel markers to early detect degradation on cellulose nitrate-based heritage at the submicrometer level using synchrotron UV-Vis multispectral luminescence. Sci. Rep. 2021, 11, 20208. [Google Scholar] [CrossRef] [PubMed]
- Edge, M.; Allen, N.S.; Hayes, M.; Riley PN, K. Mechanisms Of Deterioration In Cellulose Nitrate Base Archival Cinematograph Film. Eur. Polym. J. 1990, 26, 623–630. [Google Scholar] [CrossRef]
- Tsang, J. Safe Handling of Plastics in a Museum Environment. WAAC Newsl. 2010, 32, 16–22. [Google Scholar]
- King, R.; Grau-Bové, J.; Curran, K. Plasticiser loss in heritage collections: Its prevalence, cause, effect, and methods for analysis. Herit. Sci. 2020, 8, 123. [Google Scholar] [CrossRef]
- Derrick, M.; Daniel, V.; Parker, A. Evaluation Of Storage And Display Conditions For Cellulose Nitrate Objects. Stud. Conserv. 2013, 39 (Suppl. 2), 207–211. [Google Scholar] [CrossRef]
- Selwitz, C. Cellulose Nitrate in Conservation; J. Paul Getty Trust: Los Angeles, CA, USA, 1988; pp. 41–55. [Google Scholar]


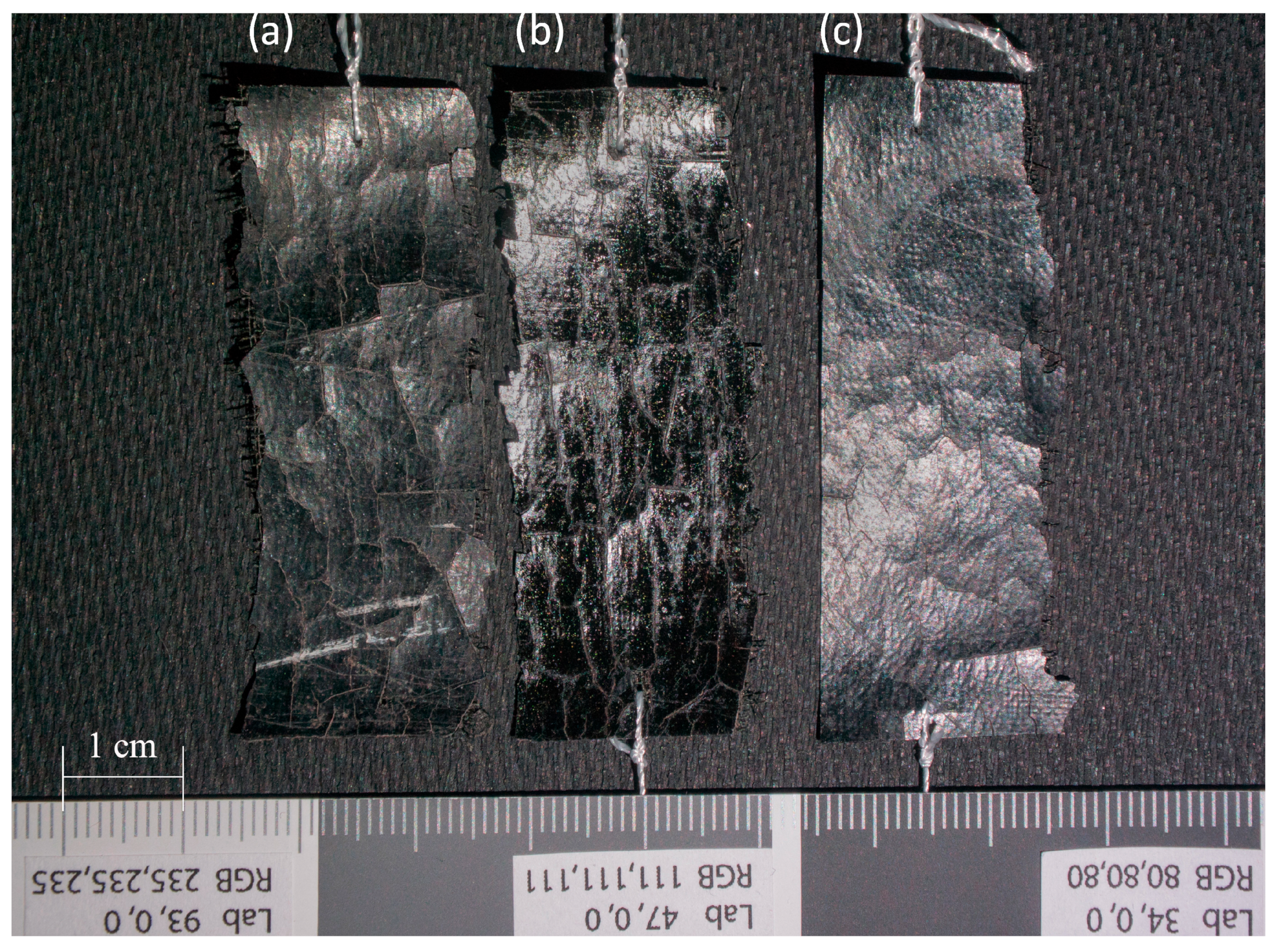

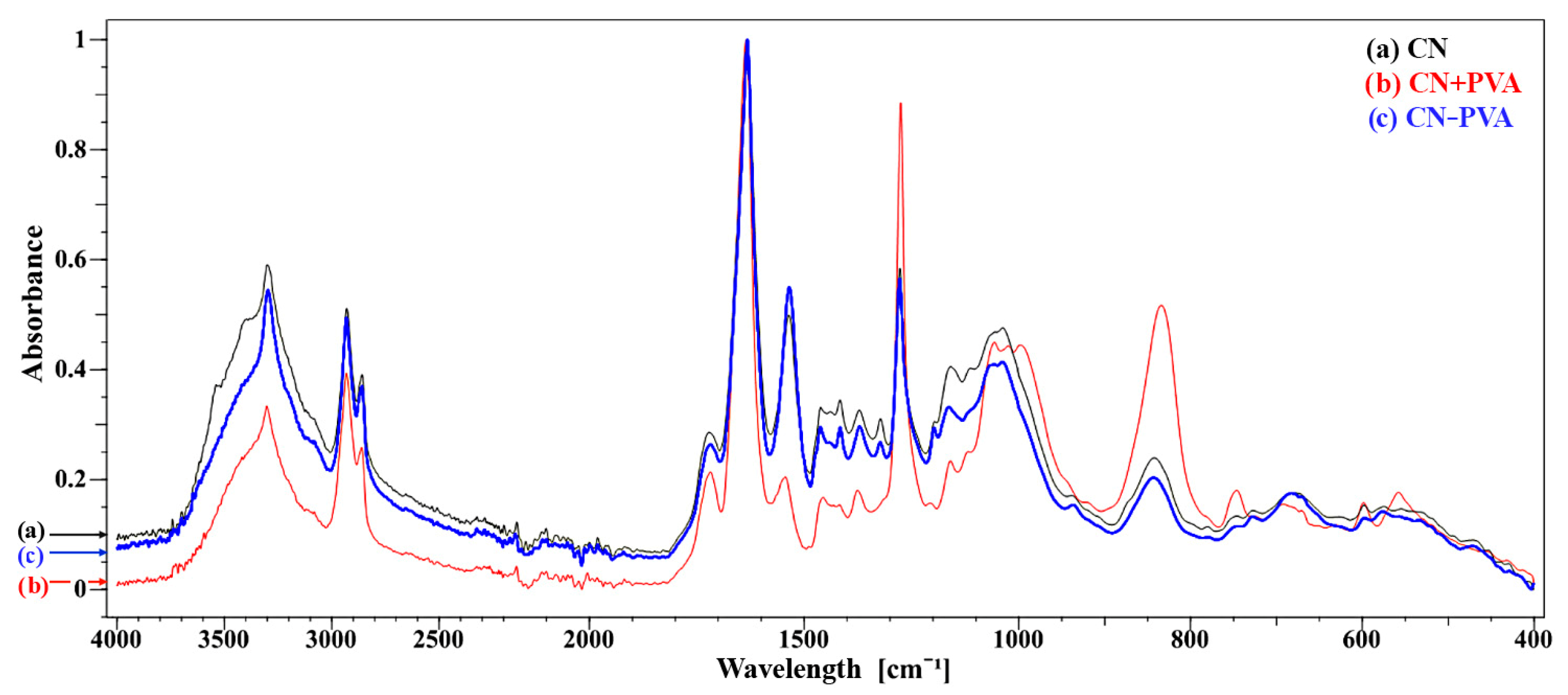
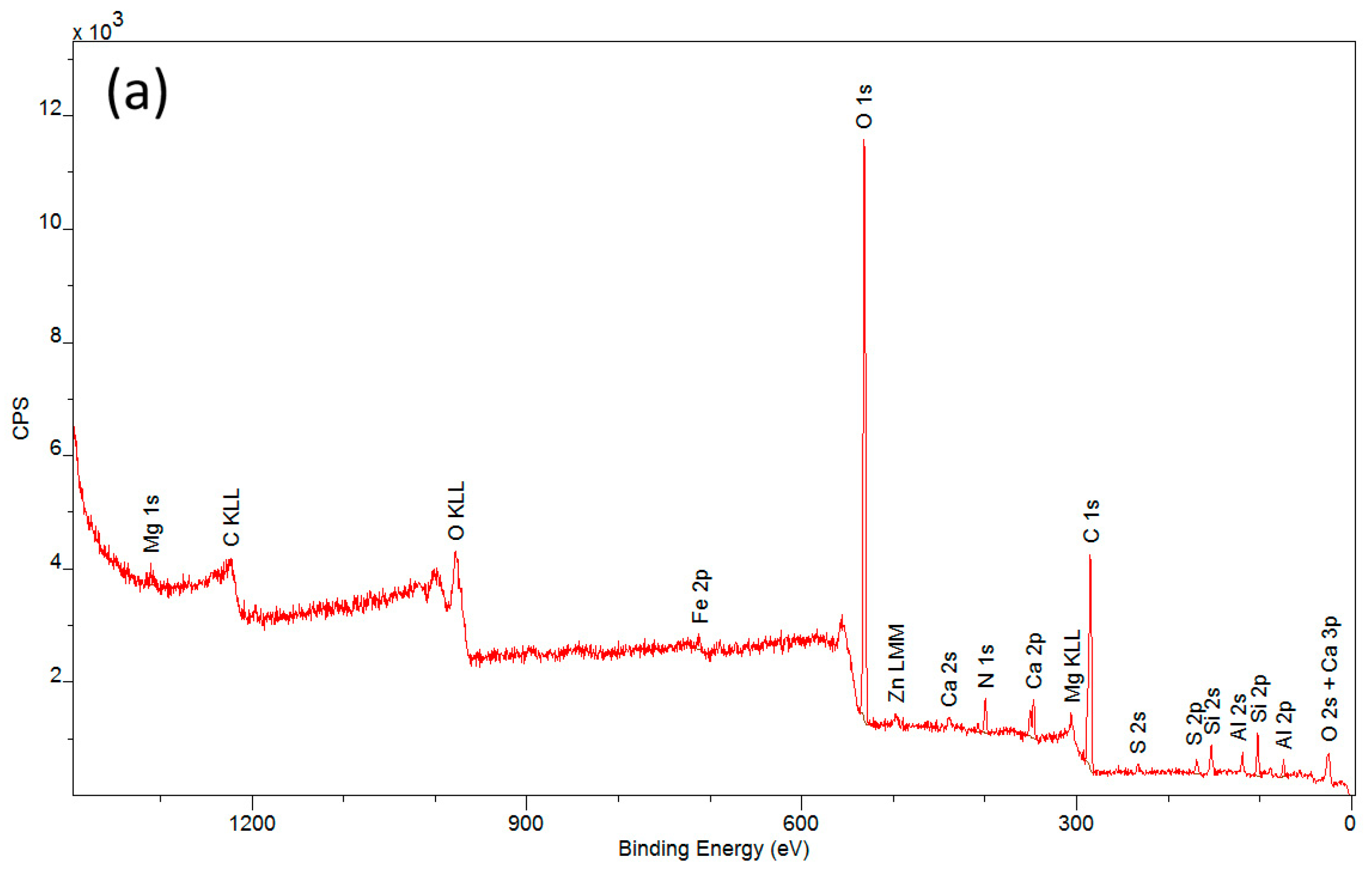
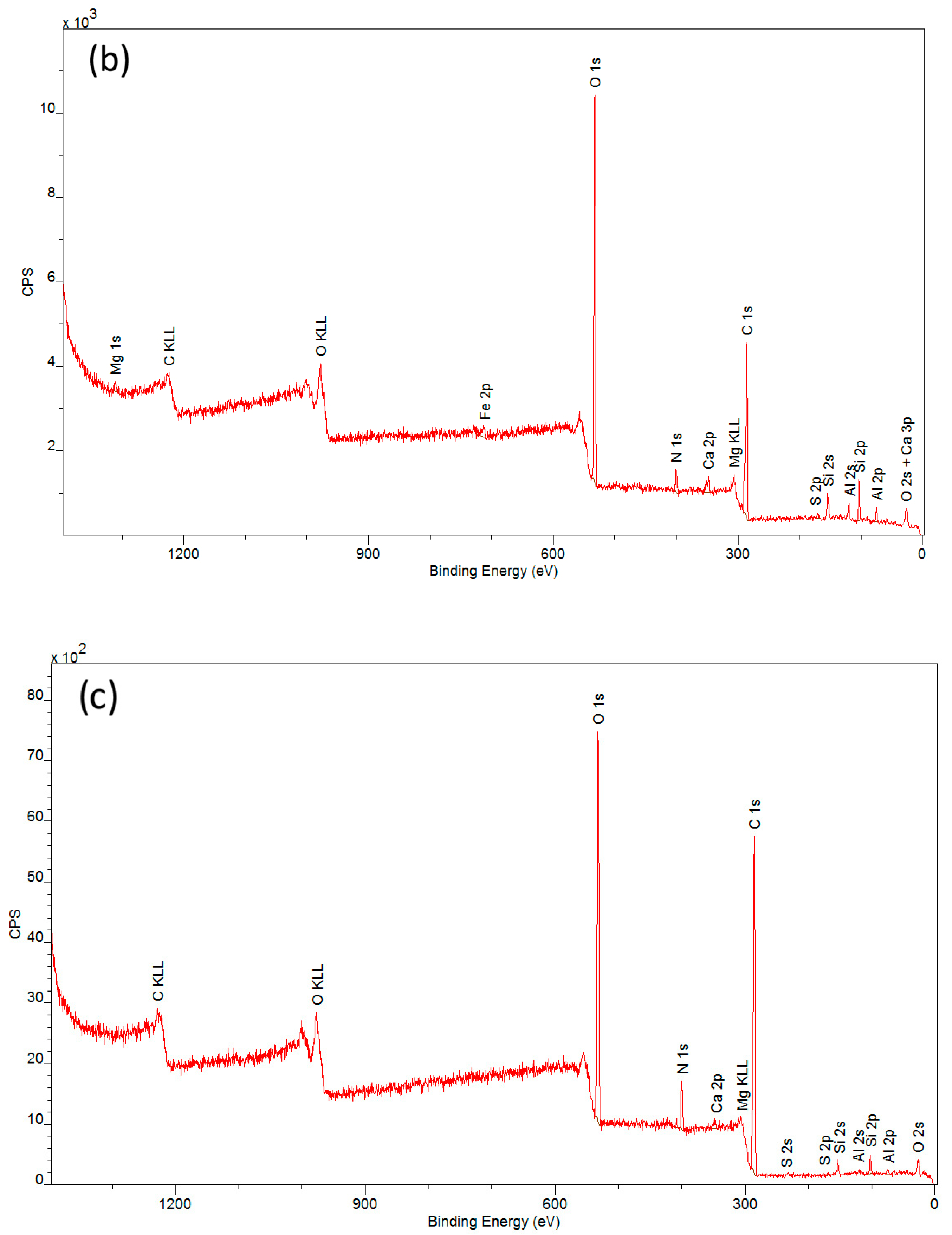
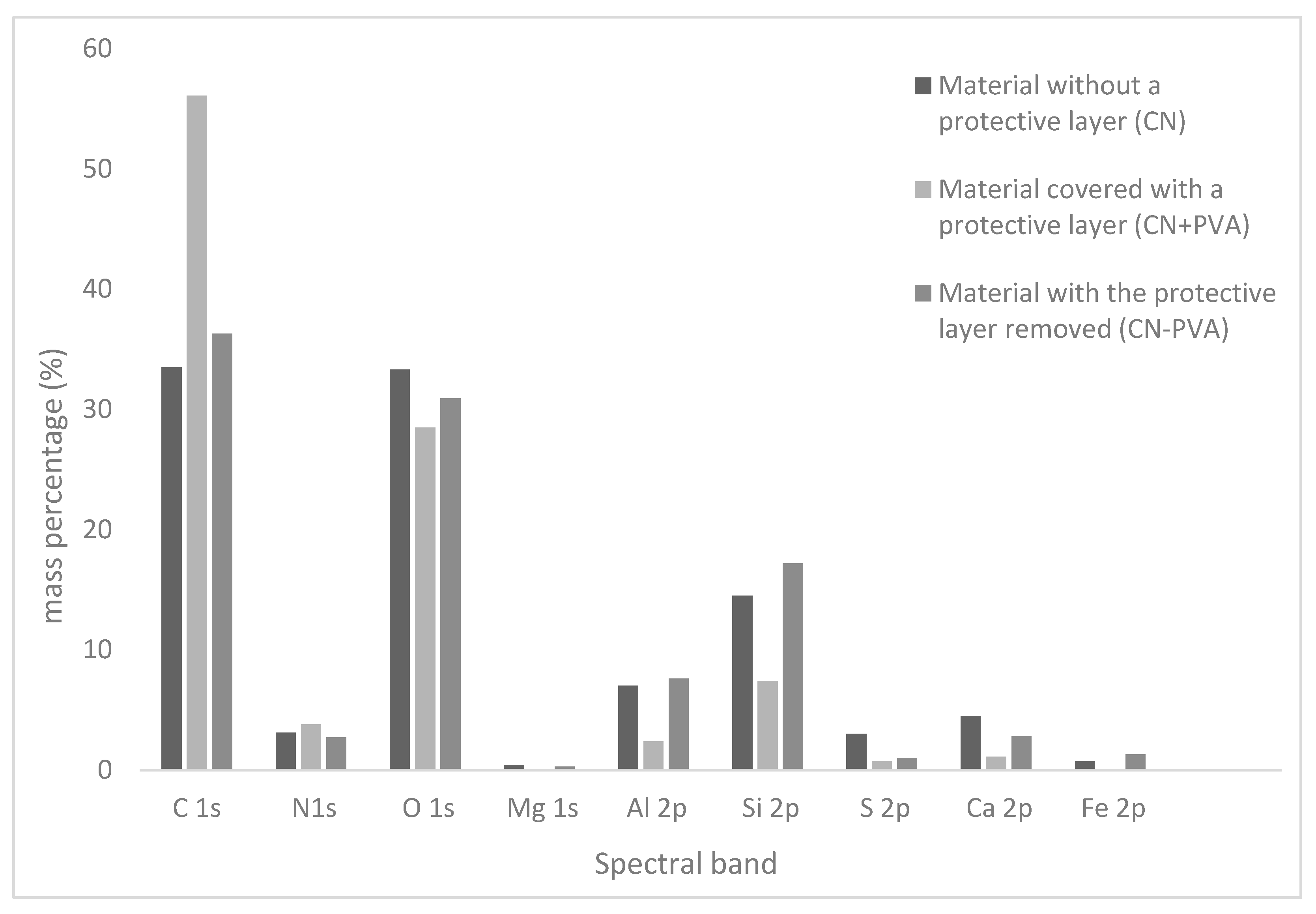
| Spectral Band | CN | CN + PVA | CN − PVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EB/eV | % At | % Mass | EB/eV | % At | % Mass | EB/eV | % At | % Mass | |
| C 1s | 284.99 | 45.7 | 33.5 | 285.00 | 65.5 | 56.1 | 284.98 | 48.9 | 36.3 |
| N 1s | 399.49 | 3.7 | 3.1 | 399.50 | 3.8 | 3.8 | 399.98 | 3.1 | 2.7 |
| O 1s | 531.99 | 34.1 | 33.3 | 532.50 | 25.0 | 28.5 | 531.98 | 31.3 | 30.9 |
| Mg 1s | 1310.49 | 0.3 | 0.4 | - | - | - | 1311.48 | 0.2 | 0.3 |
| Al 2p | 73.99 | 4.3 | 7.0 | 75.00 | 1.3 | 2.4 | 74.48 | 4.5 | 7.6 |
| Si 2p | 102.49 | 8.5 | 14.5 | 102.00 | 3.7 | 7.4 | 102.98 | 10.0 | 17.2 |
| S 2p | 168.49 | 1.5 | 3.0 | 168.50 | 0.3 | 0.7 | 169.48 | 0.5 | 1.0 |
| Ca 2p | 346.99 | 1.9 | 4.5 | 348.00 | 0.4 | 1.1 | 347.48 | 1.1 | 2.8 |
| Fe 2p | 711.99 | 0.2 | 0.7 | - | - | - | 713.48 | 0.4 | 1.3 |
| Element/Group | CN | CN + PVA | CN − PVA |
|---|---|---|---|
| N [%] | 3.10 | 3.80 | 2.70 |
| C [%] | 33.50 | 56.10 | 36.30 |
| O [%] | 33.30 | 28.50 | 30.90 |
| N:C | 1:11 | 1:15 | 1:13 |
| C:O | 1:1 | 4.6:1 | 1.2:1 |
| O-NO2 [%At] | 10.7 | 13.4 | 8.0 |
| DS | 0.40 | 0.50 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrzębiowska, N.; Wawrzyk, A.; Uroda, N. Influence Analysis of Polyvinyl Alcohol on the Degradation of Artificial Leather with Cellulose Nitrate Coating Originating from a Suitcase Stored in the Collection of the Auschwitz-Birkenau State Museum in Oświęcim, Poland. Materials 2023, 16, 7033. https://doi.org/10.3390/ma16217033
Jastrzębiowska N, Wawrzyk A, Uroda N. Influence Analysis of Polyvinyl Alcohol on the Degradation of Artificial Leather with Cellulose Nitrate Coating Originating from a Suitcase Stored in the Collection of the Auschwitz-Birkenau State Museum in Oświęcim, Poland. Materials. 2023; 16(21):7033. https://doi.org/10.3390/ma16217033
Chicago/Turabian StyleJastrzębiowska, Nel, Anna Wawrzyk, and Natalia Uroda. 2023. "Influence Analysis of Polyvinyl Alcohol on the Degradation of Artificial Leather with Cellulose Nitrate Coating Originating from a Suitcase Stored in the Collection of the Auschwitz-Birkenau State Museum in Oświęcim, Poland" Materials 16, no. 21: 7033. https://doi.org/10.3390/ma16217033
APA StyleJastrzębiowska, N., Wawrzyk, A., & Uroda, N. (2023). Influence Analysis of Polyvinyl Alcohol on the Degradation of Artificial Leather with Cellulose Nitrate Coating Originating from a Suitcase Stored in the Collection of the Auschwitz-Birkenau State Museum in Oświęcim, Poland. Materials, 16(21), 7033. https://doi.org/10.3390/ma16217033








