Abstract
Nanocomposite films of BiFeO3-Bi2Fe4O9 were fabricated on a sapphire substrate Al2O3 using the method of gas discharge high-frequency cathodic sputtering of a ceramic target with a stoichiometric composition in an oxygen atmosphere. The results of the film analysis using X-ray structural analysis, Raman scattering, XPS, and atomic force microscopy are presented. The lattice parameters, surface topography, chemical composition of the films, concentration, and average sizes of the crystallites for each phase were determined. It was shown that the ratio of the BiFeO3 to Bi2Fe4O9 phases in the obtained film is approximately 1:2. The sizes of the crystallites range from 15 to 17 nm. The optical and magnetic properties of the nanocomposite layers were studied, and the band gap width and magnetization hysteresis characteristic of ferromagnetic behavior were observed. The band gap width was found to be 1.9 eV for the indirect and 2.6 eV for the direct interband transitions. The magnetic properties are characterized by a hysteresis loop resembling a “wasp-waist” shape, indicating the presence of magnetic anisotropy.
1. Introduction
The increased interest of researchers in multiferroics, in which ferromagnetic and ferroelectric ordering coexist, is associated with the potential applications [1,2,3]. The connection between the ferroelectric and ferromagnetic or antiferromagnetic properties in magnetoelectric materials is widely used in multifunctional memory devices and spintronics [3,4]. One of the promising multiferroics is bismuth ferrite BiFeO3 (and its derivatives), which exhibits high temperatures of ferroelectric (TC ≈ 1100 K) and magnetic ordering (TN ≈ 645 K) [5]. At room temperature, the crystal structure has the R3c space group. The spontaneous polarization is oriented along the [111] direction of the pseudocubic perovskite unit cell, and the antiferromagnetic ordering of the G-type occurs such that the magnetic moments of the iron ions rotate in a spiral [101]. According to neutron diffraction data, the period of the cycloid is 62 nm [6]. Below the Neel temperature TN, bismuth ferrite has a complex, spatially modulated, magnetic structure of the cycloidal type, which does not allow for ferromagnetic properties. The destruction of its spatially modulated spin structure is a necessary condition for the occurrence of the magnetoelectric effect, which can be achieved by doping bismuth ferrite with rare-earth elements, creating nanostructured and thin film systems, or under the influence of high magnetic fields and pressures. Another way to suppress modulation is to create multiphase compounds. Recently, a new direction has emerged in condensed matter physics, studying multiphase systems consisting of compounds with different crystallographic structures and types of magnetic ordering, such as the composite BiFeO3-Bi2Fe4O9. The questions about the peculiarities of the formation of BiFeO3-Bi2Fe4O9 composites, the nature of the interaction, and the details of the mechanism linking their magnetic subsystems are currently widely discussed in the scientific literature. In [7,8,9], it is reported that nanocomposites based on the perovskite-like bismuth orthoferrite BiFeO3 and the mullite-like ferrite Bi2Fe4O9 demonstrate effects related to the exchange interaction at the interface of these phases.
Mullite Bi2Fe4O9, which is often observed as an impurity phase of BiFeO3 in the solid state, also belongs to the family of multiferroics [10,11]. Unlike BiFeO3, it has an orthorhombic crystal structure (space group Pbam) [12]. The magnetic properties of polycrystalline Bi2Fe4O9 depend on the synthesis method and the size of the crystallites. A sample with a crystallite size of 200–450 nm, synthesized using ethylenediaminetetraacetic acid [13], exhibits weak magnetization at room temperature. Polycrystalline Bi2Fe4O9 with a micron-sized grain, obtained by melting, undergoes an antiferromagnetic phase transition at 250–260 K [14,15]. Polycrystalline Bi2Fe4O9 ceramics with a grain size less than 200 nm are characterized by magnetic hysteresis at room temperature, which disappears upon heating [16].
In recent years, interest in bismuth ferrite has increased due to the discovery of a large magnetoelectric effect (ME) in BiFeO3-based films [17]. The observed ME is associated with the destruction of the spatially modulated structure in thin films, which is caused by high internal strain fields (due to the lattice mismatch between the film and substrate) [18]. Therefore, the development of technology for obtaining thin structures of multiferroic materials based on BiFeO3 on different substrates becomes a relevant direction.
It should also be noted that BiFeO3/Bi2Fe4O9 nanocomposites exhibit higher photocatalytic activity (compared to pure BiFeO3 and Bi2Fe4O9 individually) in the decomposition of rhodamine B and in the production of H2 from water under visible light. Such a heterostructure of BiFeO3/Bi2Fe4O9 and its synthesis strategy can provide new insights into the development of highly efficient photocatalysts [19].
This work presents the results of the synthesis and investigations of the structure, morphology, and optical and magnetic properties of a nanocomposite film BiFeO3-Bi2Fe4O9, synthesized on an Al2O3 sapphire substrate. To the best of our knowledge, there are few studies on thin films of Bi2Fe4O9 and BiFeO3-Bi2Fe4O9.
2. Materials and Methods
Gas discharge RF sputtering of BiFeO3 films was carried out on a “Plasma 50SE” installation (Manufacturer: ELITEH LLC, Moscow, Russia), designed for producing thin single-crystal films of complex oxides by reactive RF sputtering. The diagram of experimental setup for producing heteroepitaxial films is shown in Figure 1. Single-crystal Al2O3 (c-plane) 0.5 mm thick was used as a substrate. The initial temperature of the substrate is ~673 K, the oxygen pressure in the chamber is 80 Pa, the input RF power is 140 W. A ceramic disk of stoichiometric composition BiFeO3 with a diameter of 50 mm and a thickness of 3 mm was used as a sputtering target.
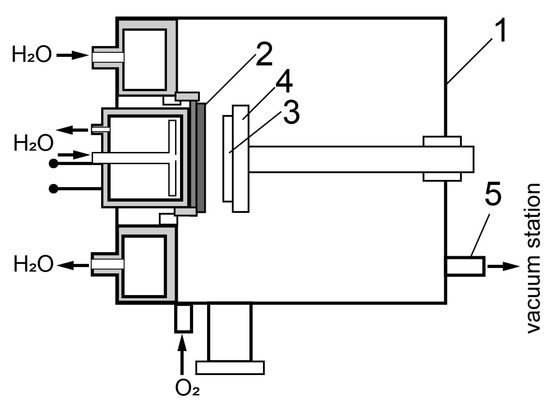
Figure 1.
Scheme of the experimental setup for producing heteroepitaxial films. 1—Vacuum chamber, 2—ceramic target, 3—substrate, 4—ceramic heater, 5—output to vacuum station.
An X-ray diffraction pattern of a thin film sample on a substrate was taken on an XRD-7000 diffractometer from Shimadzu (Kyoto, Japan). The sample was placed on an attachment for studying thin films and shooting was carried out. The shooting parameters were as follows: tube voltage 30 kV; current 30 mA; X-ray wavelength λCuKα = 0.15406 nm; ray focusing scheme—grazing reflection geometry (grazing incidence X-Ray diffraction—GIXD); incident beam slit DS = 0.15°; graphite monochromator on a reflected beam; the angle of the incident beam Θ is fixed at value = 2°; scanning range −15–80°; scanning step −0.01°; scanning speed −0.2°/min; sample rotation speed −30 rpm. The measurements were carried out at room temperature T = 25 °C (298 K). The diffraction patterns were refined using Rietveld refinement via FULLPROF software (Version—July 2017).
Ntegra Spectra atomic force microscope was used to measure the surface topology of the as-grown samples and record Raman spectra (laser λ = 532 nm). Magnetic measurements were carried out at room temperature using a vibrating sample magnetometer Lakeshore VSM 7404 (Lake Shore Cryotronics, Inc., Westerville, OH, USA). The chemical composition of the film was determined using energy dispersive X-ray spectroscopy (EDX-spectra, ASPEX Express, Delmont, PA, USA).
For measuring the optical properties were using an integrating sphere Avasphere-50 (Avantes, Apeldoorn, The Netherlands) in the wavelength range of λ~250–800 nm. The combined deuterium/halogen lamp AvaLight-DH-S-BAL (Avantes, Apeldoorn, The Netherlands) was used as the light source, and its emission was delivered to the samples via a 600 µm fiber optic light guide. Photonic signals were collected using a bifurcated 600 µm light guide and recorded with an MS3504i spectrometer (SOL-Instruments, Minsk, Belarus) combined with an HS-101(HR)-2048 × 122 CCD camera (Hamamatsu, Hamamatsu City, Japan) and a personal computer. The final data of the spectrophotometric coefficients Tt and Rd were defined as
where and are the transmission and reflection spectra of the samples; and are the reference signal spectra, measured with quartz plates; is the integrating sphere signal with covered input and open output ports; is the signal for a sphere with open optical ports. The calculation of the spectral dependence of the coefficients of optical absorption and light scattering was carried out using the inverse method of the Monte Carlo numerical simulation, developed and described in detail in the works [20,21,22].
Overview and region C1s and O1s X-ray photoelectron (XPS) spectra of the samples were collected on SPECS instrument (Specs GmbH, Berlin, Germany) using MgK-α excitation (Eex = 1254 eV). The recorded spectra were calibrated to pure graphite C1s energy (284.6 eV). XPS data were treated with CasaXPS software package (version 2.3.24, Casa Software Ltd., Teignmouth, UK). Shirley type background and mixed Gauss (70%)–Lorentz (30%) functions were used for the deconvolution of spectra under fixed FWHM for all spectral components.
3. Results
The results of the X-ray diffraction studies of a film on a leucosapphire Al2O3 (C-plane) substrate annealed at 550 °C for 2 h are presented in Figure 2. The analysis of the diffraction pattern showed the presence of peaks in good agreement with the standard declared values for BiFeO3 (ICSD # 98-015-7424, R3c), which can be attributed to rhombohedral planes with space group R3c. High intensity peaks related to Bi2Fe4O9 (ICSD #98-002-6808, Pbam) were also observed.

Figure 2.
X-ray diffraction pattern of the BiFeO3-Bi2Fe4O9 film.
The volatile nature of bismuth may be responsible for the observed impurity phases. Previously, it was reported that during the synthesis process, the formation of stoichiometric compounds, such as Bi2Fe4O9 and Bi25FeO40, is inevitable [23,24,25]. According to previous research, this phenomenon is due to the product transformation during the crystal growth process [26].
The average crystallite size (D) was calculated using the Scherer formula:
where K is the shape factor (0.9), λ is the X-ray wavelength, βhkl is the full width at half maximum (FWHM) and θ is the Bragg angle. The calculation was carried out using the peak (012) (θ = 22.42°) for the BiFeO3 phase and (121) (θ = 27.793°) for the Bi2Fe4O9 phase, the average crystallite sizes were 14.9 nm and 17.3 nm, respectively.
The analysis of diffraction patterns using the Rietveld method shows that for the sample under study, the diffraction profile consists of the space group R3c, Pbam, and I23 with the contribution of BiFeO3 (33.4%), Bi2Fe4O9 (63.4%) and a small amount of Bi25FeO40 (3.2%) (Figure 3, Table 1). The content of Bi25FeO40 (ICSD #98-004-1937, I23) is quite small and within acceptable limits, and the contribution of its influence on the properties of the BiFeO3-Bi2Fe4O9 composite can be excluded. The refined lattice parameters and crystallite size are presented in Table 1.
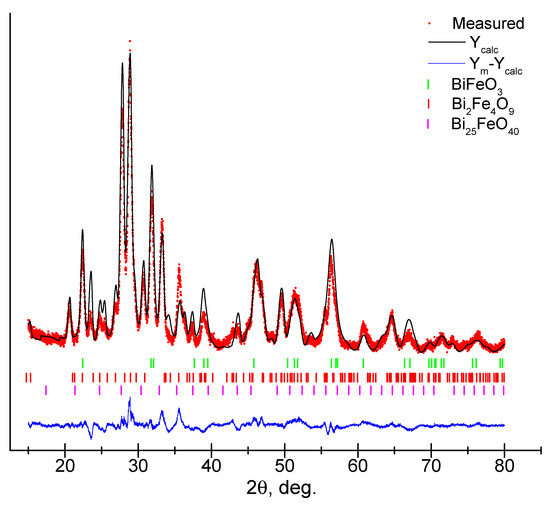
Figure 3.
Refined X-ray diffraction pattern of a thin film using the Rietveld method.

Table 1.
Structural parameters and average crystallite size.
Raman scattering is one of the most powerful tools used to study structural features. Figure 4 shows the Raman spectrum in the range 100–1000 cm−1, showing the characteristic peaks expected for the BiFeO3 and Bi2Fe4O9 phases.

Figure 4.
Raman spectra of BiFeO3-Bi2Fe4O9 film.
Note that for the compounds BiFeO3 and Bi2Fe4O9, despite being well-studied by Raman spectroscopy methods, there are discrepancies in the results in the literature. According to group theory [27,28], a rhombohedral BFO with space group R3c is characterized by thirteen (4A1 + 9E) active modes. Also, in [29], they showed that the mullite compound Bi2Fe4O9 is characterized by the presence of forty-two combination modes in the phonon spectrum out of a total number of ninety zone-center (Ӷ-point) modes in the Raman spectrum. However, as in the case of BiFeO3, for Bi2Fe4O9, in practice, a smaller number of phonon modes is observed compared to the above group-theoretic representation [28,30]. Table 2 shows the experimentally observed values of the Raman modes. The spectrum shows two distinct peaks at 143 and 175 cm−1 and eight weaker peaks in the range 218–609 cm−1. In the case of Bi2Fe4O9, clearly defined modes can be distinguished at 280, 328, and 423 cm−1. The observed Raman modes are in good agreement with the Raman spectra of BiFeO3 and Bi2Fe4O9 single crystals [29,31,32].

Table 2.
Raman mode values (in cm−1).
Thus, the results of the Raman analysis are in good agreement with the results of the X-ray diffraction analysis, confirming the coexistence of both rhombohedral BiFeO3 and orthorhombic Bi2Fe4O9.
The surface topography of a BiFeO3-Bi2Fe4O9 composite thin film with a scale area of 5 µm × 5 µm obtained by AFM is shown in Figure 5. The BiFeO3-Bi2Fe4O9 film has a dense surface without voids and cracks. The dense surface morphology and the absence of cracks and voids confirm the uniform deposition of thin films, suitable for the subsequent design of devices based on them. The analysis of the AFM image of the film shows that the grains on the surface are large compared to the size of their crystallites calculated using the Scherrer formula. In general, the grain size was in the range of 20–80 nm, and the average size was about 48 nm. The widely varying values of the crystallite size and grain size indicate that the latter are not single crystalline, but rather consist of many crystallites. The root mean square roughness (Rms) of the BiFeO3-Bi2Fe4O9 thin film was 11.14 nm.
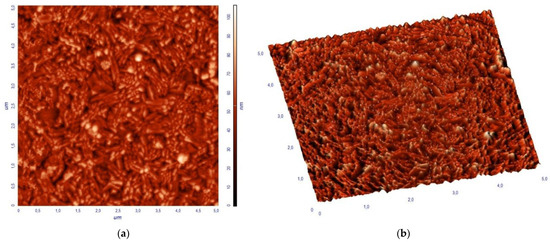
Figure 5.
AFM images of the BiFeO3-Bi2Fe4O9 thin films: (a) 2D, (b) 3D.
The results of studying the full energy spectrum of the BiFeO3-Bi2Fe4O9 composite film using energy dispersive X-ray spectroscopy (EDX) are presented in Figure 6. The elemental composition of the sample corresponds to the chemical formulas of the components and corresponds to the results of X-ray diffraction analysis. The EDX spectra were taken in several selected areas of the sample, and all showed the expected presence of Bi, Fe, and O. An Al peak associated with the Al2O3 substrate is also observed. The presence of a carbon peak is related to the research methodology.

Figure 6.
EDX spectrum of the BiFeO3-Bi2Fe4O9 film.
The optical properties of the BiFeO3-Bi2Fe4O9 nanocomposite were studied using UV-visible diffuse reflectance spectroscopy and converted into absorption spectra using the Monte Carlo method [20,22]. The absorption spectrum in the wavelength range 250–800 nm is shown in Figure 7.

Figure 7.
UV-visible spectra of a thin film (a) and Tauc plot of (αhν)2 and (αhν)0.5 versus hν for the optical band gap Eg (b).
As can be seen, the sample exhibits absorption in the ultraviolet and visible regions of light. The optical band gap (Eg) can be estimated using the Tauc relation [33,34]:
where α is the absorption coefficient, hν is the photon energy, K is a constant, n is the coefficient characterizing the direct or indirect optical transition. The value of the band gap is determined by interpolating the linear part of the graph to the X axis. The linear regions on the Tauc plots (Figure 7b) indicate either an indirect optical band gap of 1.9 eV or a direct optical band gap of 2.6 eV. The existing literature indicates a wide band gap range (2.1–2.8 eV) for BiFeO3 and indicates the existence of both direct and indirect electron photoexcitation at room temperature in BiFeO3. However, the indirect charge transfer mechanism is thought to predominate at low room temperatures [35]. In the case of Bi2Fe4O9 films, the optical band gap is in the range of 2.05–2.2 eV [36,37,38]. From the theoretical studies of density functional theory [37], the band gap of Bi2Fe4O9 is considered to be indirect. For the Bi2Fe4O9-BiFeO3 sample, a band gap of 2.1 eV was observed [39]. Based on an analysis of the literature and the fact that the Bi2Fe4O9 phase predominates in our sample, we believe that in our case, an indirect optical transition occurs.
(αhν) = K(hν − Eg)n
Figure 8a shows the results of a panoramic scan of a thin film. Only C 1s, O 1s, Bi 4f and Fe 2p peaks were detected, indicating the absence of impurities, which is also confirmed by the EDX spectrum. The C 1s peak is due to carbon impurities [40,41]. Three regions associated with the Bi 4f, Fe 2p, and O 1s in the panoramic XPS spectrum of the sample were studied.

Figure 8.
XPS spectra of BiFeO3-Bi2Fe4O9 composite film: panoramic spectra (a); Bi 4f (b); Fe 2p (c); O 1s (d).
The high resolution Bi 4f spectra for the BiFeO3-Bi2Fe4O9 composite are shown in Figure 8b. The Bi 4f doublet consists of two peaks at 163.81 and 158.5 eV, which confirm the 3+ oxidation state of Bi, mainly representing the Bi-O bond. The spin-orbit splitting energy was Δ = 5.31 eV, which is comparable to the literature data [41,42,43] and is in good agreement with the theoretical value of 5.31 eV [44]. Two selected sub-peaks located at 163.74 and 158.31 eV are attributed to Bi (4f 5/2)-O and Bi (4f 7/2)-O bonds, while the other sub-peaks, located at 164.75 and 159, 11 eV, can be attributed to the Bi-O-Fe bond in the FeO6 octahedron, caused by oxygen vacancies and cation defects [45,46].
The spectra of the main level of Fe 2p are shown in Figure 8c. The asymmetric nature of the peaks and the presence of satellite peaks indicate the presence of iron in the oxidation states of Fe2+ and Fe3+. As shown in Figure 8c, the curve is divided into five components. The peaks of the Fe 2p doublets are concentrated at 724.31 eV (Fe 3+ 2p1/2) and 710.63 eV (Fe3+ 2p3/2), which represent Fe-O bonds [45]. The spin-orbit splitting energy of pure Fe 2p is 13.68 eV, which is close to the theoretical value (Δ Fe 2p) of 13.6 eV for Fe2O3 [45]. The peaks of the Fe 2p doublets located at 725.63 eV and 712.29 eV can be attributed to the (Fe 2p1/2)2–O3 and (Fe 2p3/2)–O3 bonds, and the subpeaks located at 723.83 and 710.23 to the bonds Fe-O, Fe-O-Bi bonds in the FeO6 octahedron, oxygen vacancies, and cation defects [47].
According to the ratio of the fitted peak areas for Fe3+ and Fe2+, the concentration ratio of Fe3+ and Fe2+ is approximately 1.43, indicating a relatively high Fe2+ content, which in turn may affect the magnetic properties of the composite.
To confirm the presence of oxygen vacancies, the spectrum of the O 1s level was studied. Figure 8d shows the O 1s spectra after deconvolution with the approximation of the fitted peaks. The spectrum is well-described by the superposition of three components centered at 527.5, 529.3, and 531.4 eV, respectively. A strong peak at 529.3 eV is assigned to the characteristic signal from lattice O. The shoulder at about 531.4 eV belongs to defective O components, such as oxygen vacancies, and the peak at 527.5 to physisorbed oxygen [42].
Figure 9a shows the magnetic hysteresis loops M–H of the BiFeO3-Bi2Fe4O9 composite structure, measured at room temperature in magnetic fields up to 20 kOe. Figure 9b shows the M–H loop considering the subtraction of the diamagnetic contribution of the substrate. The loop exhibits a ferromagnetic character without reaching saturation. The presence of magnetic hysteresis indicates the ferromagnetic properties of the resulting films, while the substrate exhibits diamagnetic properties.
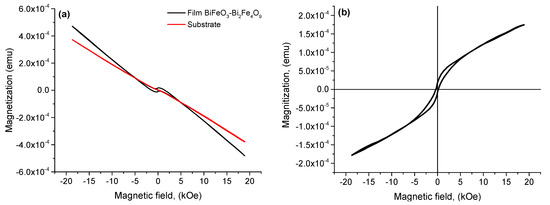
Figure 9.
M−H magnetic hysteresis loops before (a) and after (b) subtraction of the diamagnetic contribution of the substrate.
Since in the maximum magnetic fields achieved by a laboratory vibration magnetometer the magnetization did not reach saturation, the law of approach to saturation (LAS) was applied to estimate the value of saturation magnetization MS. The LAS determines the dependence of magnetization (M) on the applied field (H), where the applied field (H) is much greater than the coercive field (HC). LAS is usually used to describe magnetization in a region of strong magnetic field, where the rotation of the magnetic domain plays a significant role. According to [48] this value is determined by the expression:
A typical LAS fitting curve for the BiFeO3-Bi2Fe4O9 is shown in Figure 10. For this sample, the saturation magnetization, remanence, and coercive force were Ms = 1.59 × 10−4 emu, Mr = 1.33 × 10−5 emu and HC = 270 Oe, respectively and the Mr/Ms = 0.0833.

Figure 10.
Fitting to the law of approach to saturation (LAS) for Bi2Fe4O9−BiFeO3.
Note that a wasp-waist hysteresis loop was observed in the sample. Work by Lawrence H. Bennett and Edward Della Torre [49] examined how wasp-waist hysteresis loops arise in composite materials containing two ferromagnetic materials with different coercivities. Roberts and his colleagues, as well as Tauxe and his colleagues, used numerical simulations to determine the causes of this phenomenon. They found that the coexistence of materials with different properties, such as magnetic hardness and softness, anisotropy and coercive fields, or a mixture of single-domain and superparamagnetic particles can explain the appearance of the wasp-waist hysteresis loop [50,51,52].
4. Conclusions
By using the radio frequency cathode sputtering method of a ceramic target with a stoichiometric composition of BiFeO3 in an oxygen atmosphere, the nanocomposite films BiFeO3-Bi2Fe4O9 were obtained on a sapphire substrate Al2O3. Based on the results of the film analysis using X-ray structural analysis, Raman scattering, XPS, and atomic force microscopy, the structural parameters, topography, chemical composition of the films, concentration, and average sizes of the crystallites for each phase were determined. The results of the structural analysis demonstrated the formation of a two-phase structure, consisting of BiFeO3 (33.4%), Bi2Fe4O9 (63.4%), and a small amount of Bi25FeO40 (3.2%). The grain sizes, determined by AFM, were approximately 48 nm, and the root mean square roughness (Rms) of the film was 11 nm. The bandgap width was 1.9 eV for an indirect and 2.6 eV for a direct interband transition. The magnetic properties were characterized by a hysteresis loop resembling a wasp-waist, indicating the presence of magnetic anisotropy. The saturation magnetization, remanence, and coercive force were Ms = 1.59 × 10–4 emu, Mr = 1.33 × 10–5 emu, and HC = 270 Oe, respectively, with a Mr/Ms ratio of 0.0833. The results of the research can be useful in creating new materials for magnetoelectronics.
Author Contributions
Conceptualization, F.O. and N.A.; methodology, S.K. and N.A.; validation, S.S., J.M. and K.G.; formal analysis, S.S., M.A. and K.G.; investigation, A.P., M.A., J.M. and N.A.; resources, A.P. and F.O.; data curation, S.K.; writing—original draft preparation, S.K. and N.A.; writing—review and editing, S.S., A.P. and F.O.; visualization, M.A.; supervision, S.K., F.O. and K.G.; project administration, F.O.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out with the financial support of the Russian Science Foundation grant, project number 23-22-00130.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be provided upon request to the corresponding author.
Acknowledgments
The CzechNanoLab project LM2023051 funded by MEYS CR is gratefully acknowledged for the financial support of the measurements at CEITEC Nano Research Infrastructure. The authors also acknowledge the shared services center “Surface and novel materials” of UdmFRC of UB RAS for the XPS data acquisition using the SPECS spectrometer. The X-ray diffraction studies were performed on the equipment of the Analytical Center for Collective Use of the Dagestan Federal Research Center of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Ramazanov, S.; Sobola, D.; Gajiev, G.; Orudzhev, F.; Kaspar, P.; Gummetov, A. Multiferroic/Polymer Flexible Structures Obtained by Atomic Layer Deposition. Nanomaterials 2022, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Sando, D.; Agbelele, A.; Rahmedov, D.; Liu, J.; Rovillain, P.; Toulouse, C.; Infante, I.C.; Pyatakov, A.P.; Fusil, S.; Jacquet, E.; et al. Crafting the Magnonic and Spintronic Response of BiFeO3 Films by Epitaxial Strain. Nat. Mater. 2013, 12, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Fina, I.; Marti, X.; Kim, Y.H.; Hesse, D.; Alexe, M.; Lee, J.H.; Fina, I.; Kim, Y.H.; Hesse, D.; et al. Spintronic Functionality of BiFeO3 Domain Walls. Adv. Mater. 2014, 26, 7078–7082. [Google Scholar] [CrossRef]
- Smolenskii, G.A.; Yudin, V.M.; Sher, E. Weak ferromagnetism of some BiFeO3-Pb(Fe0.5Nb0.5)O3 perovskites. Sov. Phys. Solid State 1965, 6, 2936. [Google Scholar]
- Sosnowska, I.; Peterlin-Neumaier, T.; Steichele, E. Spiral Magnetic Ordering in Bismuth Ferrite. J. Phys. C Solid State Phys. 1982, 15, 4835–4846. [Google Scholar] [CrossRef]
- Maity, T.; Goswami, S.; Bhattacharya, D.; Roy, S. Superspin Glass Mediated Giant Spontaneous Exchange Bias in a Nanocomposite of BiFeO3-Bi2Fe4O9. Phys. Rev. Lett. 2013, 110, 107201. [Google Scholar] [CrossRef]
- Maity, T.; Roy, S. Asymmetric Ascending and Descending Loop Shift Exchange Bias in Bi2Fe4O9-BiFeO3 Nanocomposites. J. Magn. Magn. Mater. 2020, 494, 165783. [Google Scholar] [CrossRef]
- Pleshakov, I.V.; Volkov, M.P.; Lomanova, N.A.; Kuz’min, Y.I.; Gusarov, V.V. Magnetic Characteristics of a Nanocomposite Based on Bismuth Ferrites. Tech. Phys. Lett. 2020, 46, 1072–1075. [Google Scholar] [CrossRef]
- Park, Y.A.; Song, K.M.; Lee, K.D.; Won, C.J.; Hur, N. Effect of Antiferromagnetic Order on the Dielectric Properties of Bi2Fe4O9. Appl. Phys. Lett. 2010, 96, 338992. [Google Scholar] [CrossRef]
- Dutta, D.P.; Sudakar, C.; Mocherla, P.S.V.; Mandal, B.P.; Jayakumar, O.D.; Tyagi, A.K. Enhanced Magnetic and Ferroelectric Properties in Scandium Doped Nano Bi2Fe4O9. Mater. Chem. Phys. 2012, 135, 998–1004. [Google Scholar] [CrossRef]
- Kirsch, A.; Murshed, M.M.; Litterst, F.J.; Gesing, T.M. Structural, Spectroscopic, and Thermoanalytic Studies on Bi2Fe4O9: Tunable Properties Driven by Nano- and Poly-Crystalline States. J. Phys. Chem. C 2019, 123, 3161–3171. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Xu, Y.; He, Y.; Chen, W. Synthesis and Characterization of Bi2Fe4O9 Powders. Mater. Chem. Phys. 2011, 128, 388–391. [Google Scholar] [CrossRef]
- Alvarez, G.; Contreras, J.; Conde-Gallardo, A.; Montiel, H.; Zamorano, R. Detection of Para–Antiferromagnetic Transition in Bi2Fe4O9 Powders by Means of Microwave Absorption Measurements. J. Magn. Magn. Mater. 2013, 348, 17–21. [Google Scholar] [CrossRef]
- Papaefthymiou, G.C.; Viescas, A.J.; Le Breton, J.M.; Chiron, H.; Juraszek, J.; Park, T.J.; Wong, S.S. Magnetic and Mössbauer Characterization of the Magnetic Properties of Single-Crystalline Sub-Micron Sized Bi2Fe4O9 Cubes. Curr. Appl. Phys. 2015, 15, 417–422. [Google Scholar] [CrossRef]
- Tian, Z.M.; Yuan, S.L.; Wang, X.L.; Zheng, X.F.; Yin, S.Y.; Wang, C.H.; Liu, L. Size Effect on Magnetic and Ferroelectric Properties in Bi2Fe4O9 Multiferroic Ceramics. J. Appl. Phys. 2009, 106, 343219. [Google Scholar] [CrossRef]
- Zvezdin, A.K.; Pyatakov, A.P. Phase transitions and the giant magnetoelectric effect in multiferroics. Phys.-Uspekhi 2004, 47, 416. [Google Scholar] [CrossRef]
- Yuan, G.L.; Or, S.W.; Chan, H.L.W.; Liu, Z.G. Reduced Ferroelectric Coercivity in Multiferroic Bi0.825Nd0.175FeO3 Thin Film. J. Appl. Phys. 2007, 101, 818138. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Y.; Qiu, Y.; Liu, Y.; Xiong, R.; Shi, J.; Wei, J. Facial Synthesis and Photoreaction Mechanism of BiFeO3/Bi2Fe4O9 Heterojunction Nanofibers. ACS Sustain. Chem. Eng. 2017, 5, 4630–4636. [Google Scholar] [CrossRef]
- Wang, L.; Steven, L.; Jacques, P.D. Monte Carlo Modeling of Light Transport in Multi-Layered Tissues in Standard C; University of Texas M. D. Anderson Cancer Center: Houston, TX, USA, 1992. [Google Scholar]
- Giraev, K.M.; Ashurbekov, N.A.; Magomedov, M.A.; Murtazaeva, A.A.; Medzhidov, R.T. The Effect of Pathological Processes on Absorption and Scattering Spectra of Samples of Bile and Pancreatic Juice. Opt. Spectrosc. Engl. Transl. Opt. I Spektrosk. 2015, 119, 162–170. [Google Scholar] [CrossRef]
- Orudzhev, F.; Alikhanov, N.; Amirov, A.; Rabadanova, A.; Selimov, D.; Shuaibov, A.; Gulakhmedov, R.; Abdurakhmanov, M.; Magomedova, A.; Ramazanov, S.; et al. Porous Hybrid PVDF/BiFeO3 Smart Composite with Magnetic, Piezophotocatalytic, and Light-Emission Properties. Catalysts 2023, 13, 874. [Google Scholar] [CrossRef]
- Huo, Y.; Jin, Y.; Zhang, Y. Citric Acid Assisted Solvothermal Synthesis of BiFeO3 Microspheres with High Visible-Light Photocatalytic Activity. J. Mol. Catal. A Chem. 2010, 331, 15–20. [Google Scholar] [CrossRef]
- Selbach, S.M.; Tybell, T.; Einarsrud, M.A.; Grande, T. Size-Dependent Properties of Multiferroic BiFeO3 Nanoparticles. Chem. Mater. 2007, 19, 6478–6484. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.H.; Zhang, B.P.; Wang, Y.; Nan, C.W. Controlled Fabrication of BiFeO3 Uniform Microcrystals and Their Magnetic and Photocatalytic Behaviors. J. Phys. Chem. C 2010, 114, 2903–2908. [Google Scholar] [CrossRef]
- Dai, Z.; Akishige, Y. Electrical Properties of Multiferroic BiFeO3 Ceramics Synthesized by Spark Plasma Sintering. J. Phys. D Appl. Phys. 2010, 43, 445403. [Google Scholar] [CrossRef]
- Fukumura, H.; Harima, H.; Kisoda, K.; Tamada, M.; Noguchi, Y.; Miyayama, M. Raman Scattering Study of Multiferroic BiFeO3 Single Crystal. J. Magn. Magn. Mater. 2007, 310, e367–e369. [Google Scholar] [CrossRef]
- Bielecki, J.; Svedlindh, P.; Tibebu, D.T.; Cai, S.; Eriksson, S.G.; Börjesson, L.; Knee, C.S. Structural and Magnetic Properties of Isovalently Substituted Multiferroic BiFeO3: Insights from Raman Spectroscopy. Phys. Rev. B Condens. Matter. Mater. Phys. 2012, 86, 184422. [Google Scholar] [CrossRef]
- Iliev, M.N.; Litvinchuk, A.P.; Hadjiev, V.G.; Gospodinov, M.M.; Skumryev, V.; Ressouche, E. Phonon and Magnon Scattering of Antiferromagnetic Bi2Fe4O9. Phys. Rev. B Condens. Matter. Mater. Phys. 2010, 81, 024302. [Google Scholar] [CrossRef]
- Mohapatra, S.R.; Swain, A.; Yadav, C.S.; Kaushik, S.D.; Singh, A.K. Unequivocal Evidence of Enhanced Magnetodielectric Coupling in Gd3+ Substituted Multiferroic Bi2Fe4O9. RSC Adv. 2016, 6, 112282–112291. [Google Scholar] [CrossRef]
- Alikhanov, N.M.R.; Rabadanov, M.K.; Orudzhev, F.F.; Gadzhimagomedov, S.K.; Emirov, R.M.; Sadykov, S.A.; Kallaev, S.N.; Ramazanov, S.M.; Abdulvakhidov, K.G.; Sobola, D. Size-Dependent Structural Parameters, Optical, and Magnetic Properties of Facile Synthesized Pure-Phase BiFeO3. J. Mater. Sci. Mater. Electron. 2021, 32, 13323–13335. [Google Scholar] [CrossRef]
- Friedrich, A.; Biehler, J.; Morgenroth, W.; Wiehl, L.; Winkler, B.; Hanfland, M.; Tolkiehn, M.; Burianek, M.; Mühlberg, M. High-Pressure Phase Transition of Bi2Fe4O9. J. Phys. Condens. Matter 2012, 24, 145401. [Google Scholar] [CrossRef]
- Zeljković, S.; Ivas, T.; Maruyama, H.; Nino, J.C. Structural, Magnetic and Optical Properties of BiFeO3 Synthesized by the Solvent-Deficient Method. Ceram. Int. 2019, 45, 19793–19798. [Google Scholar] [CrossRef]
- Tu, C.S.; Chen, P.Y.; Jou, Y.S.; Chen, C.S.; Chien, R.R.; Schmidt, V.H.; Haw, S.C. Polarization-Modulated Photovoltaic Conversion in Polycrystalline Bismuth Ferrite. Acta Mater. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Daboczi, M.; Zhang, M.; Briscoe, J.; Kim, J.S.; Yan, H.; Dunn, S. Origin of the switchable photocurrent direction in BiFeO3 thin films. Mater. Horiz. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Liu, Z.; Huang, G.; Hou, J.; Zhang, G. Visible-light photovoltaic effect in multiferroic Bi2Fe4O9 thin film. Mater. Lett. 2022, 309, 131411. [Google Scholar] [CrossRef]
- Ameer, S.; Jindal, K.; Tomar, M.; Jha, P.K.; Gupta, V. Growth of highly oriented orthorhombic phase of Bi2Fe4O9 thin films by pulsed laser deposition. Mater. Today Proc. 2021, 47, 1646–1650. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, R. Morphology and optical absorption of Bi2Fe4O9 crystals via mineralizer-assisted hydrothermal synthesis. Particuology 2013, 11, 581–587. [Google Scholar] [CrossRef]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and their evaluation as non-noble metal catalysts for efficient reduction of 4-nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Y.; Guo, Q.; Liu, J.; Wang, Y.; Yang, M.; Xia, H. Controllable synthesis of TiO2@Fe2O3 core-shell nanotube arrays with double-wall coating as superb lithium-ion battery anodes. Sci. Rep. 2017, 7, 40927. [Google Scholar] [CrossRef]
- Gomez-Iriarte, G.A.; Pentón-Madrigal, A.; de Oliveira, L.A.S.; Sinnecker, J.P. XPS study in BiFeO3 surface modified by argon etching. Materials 2022, 15, 4285. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.Y.; Liu, W.F.; Xu, X.L.; Li, X.; Zhang, H.; Gao, J.; Li, D.J. Room temperature exchange bias in multiferroic BiFeO3 nano-and microcrystals with antiferromagnetic core and two-dimensional diluted antiferromagnetic shell. J. Nanopart. Res. 2017, 19, 182. [Google Scholar] [CrossRef]
- Bharathkumar, S.; Sakar, M.; Balakumar, S. Versatility of electrospinning in the fabrication of fibrous mat and mesh nanostructures of bismuth ferrite (BiFeO3) and their magnetic and photocatalytic activities. Phys. Chem. Chem. Phys. 2015, 17, 17745–17754. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Tan, G.; Liu, W.; Ren, H. Structural, electrical and magnetic properties of (Bi0.9RE0.1)(Fe0.97Co0.03)O3 (RE = Nd and Gd) thin films. Mater. Res. Bull. 2014, 52, 143–150. [Google Scholar] [CrossRef]
- Quan, Z.; Hu, H.; Xu, S.; Liu, W.; Fang, G.; Li, M.; Zhao, X. Surface chemical bonding states and ferroelectricity of Ce-doped BiFeO3 thin films prepared by sol–gel process. J. Sol-Gel Sci. Technol. 2008, 48, 261–266. [Google Scholar] [CrossRef]
- Wang, J.B.N.J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef]
- Tamilselvan, A.; Balakumar, S.; Sakar, M.; Nayek, C.; Murugavel, P.; Kumar, K.S. Role of oxygen vacancy and Fe–O–Fe bond angle in compositional, magnetic, and dielectric relaxation on Eu-substituted BiFeO3 nanoparticles. Dalton Trans. 2014, 43, 5731–5738. [Google Scholar] [CrossRef] [PubMed]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of Structure-Property Relationships in Nickel Ferrite Nanoparticles Annealed at Different Temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Bennett, L.H.; Della Torre, E. Analysis of Wasp-Waist Hysteresis Loops. J. Appl. Phys. 2005, 97, 984370. [Google Scholar] [CrossRef]
- Gupta, S.; Tomar, M.; Gupta, V. Magnetic Hysteresis of Cerium Doped Bismuth Ferrite Thin Films. J. Magn. Magn. Mater. 2015, 378, 333–339. [Google Scholar] [CrossRef]
- Roberts, A.P.; Cui, Y.; Verosub, K.L. Wasp-Waisted Hysteresis Loops: Mineral Magnetic Characteristics and Discrimination of Components in Mixed Magnetic Systems. J. Geophys. Res. Solid Earth 1995, 100, 17909–17924. [Google Scholar] [CrossRef]
- Tauxe, L.; Mullender, T.A.T.; Pick, T. Potbellies, Wasp-Waists, and Superparamagnetism in Magnetic Hysteresis. J. Geophys. Res. Solid Earth 1996, 101, 571–583. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).