Progress in the Synthesis Process and Electrocatalytic Application of MXene Materials
Abstract
:1. Introduction
2. The Principal Techniques for Producing MXene Materials
2.1. Etching of Synthetic MXene Materials and Methods for Their Derivation
2.1.1. Etching with Hydrofluoric Acid (HF)
2.1.2. Modified Etching Method for Synthesizing MXene Materials
2.1.3. Etching Using Modified Fluoride-Based Acid
2.1.4. Molten Salt Etching Synthesis of MXene
2.1.5. Electrochemical Etching Method
2.2. Hydrothermal Synthesis
2.3. Physically Assisted Synthesis of MXene
3. The Applications of MXene-Based Materials
3.1. Electrocatalysts for the HER Based on MXene
3.1.1. Acidic Solution HER Electrocatalysts
3.1.2. Electrocatalysts for HER in Neutral or Near-Neutral Solutions
3.1.3. Electrocatalysts for HER in Alkaline Solutions
3.1.4. MXene Materials for Hydrogen Storage Applications
3.2. Electrocatalysts Based on MXene for the OER
3.3. MXene-Based Electrocatalysts for the ORR
3.4. MXene-Based Electrocatalysts for the NRR
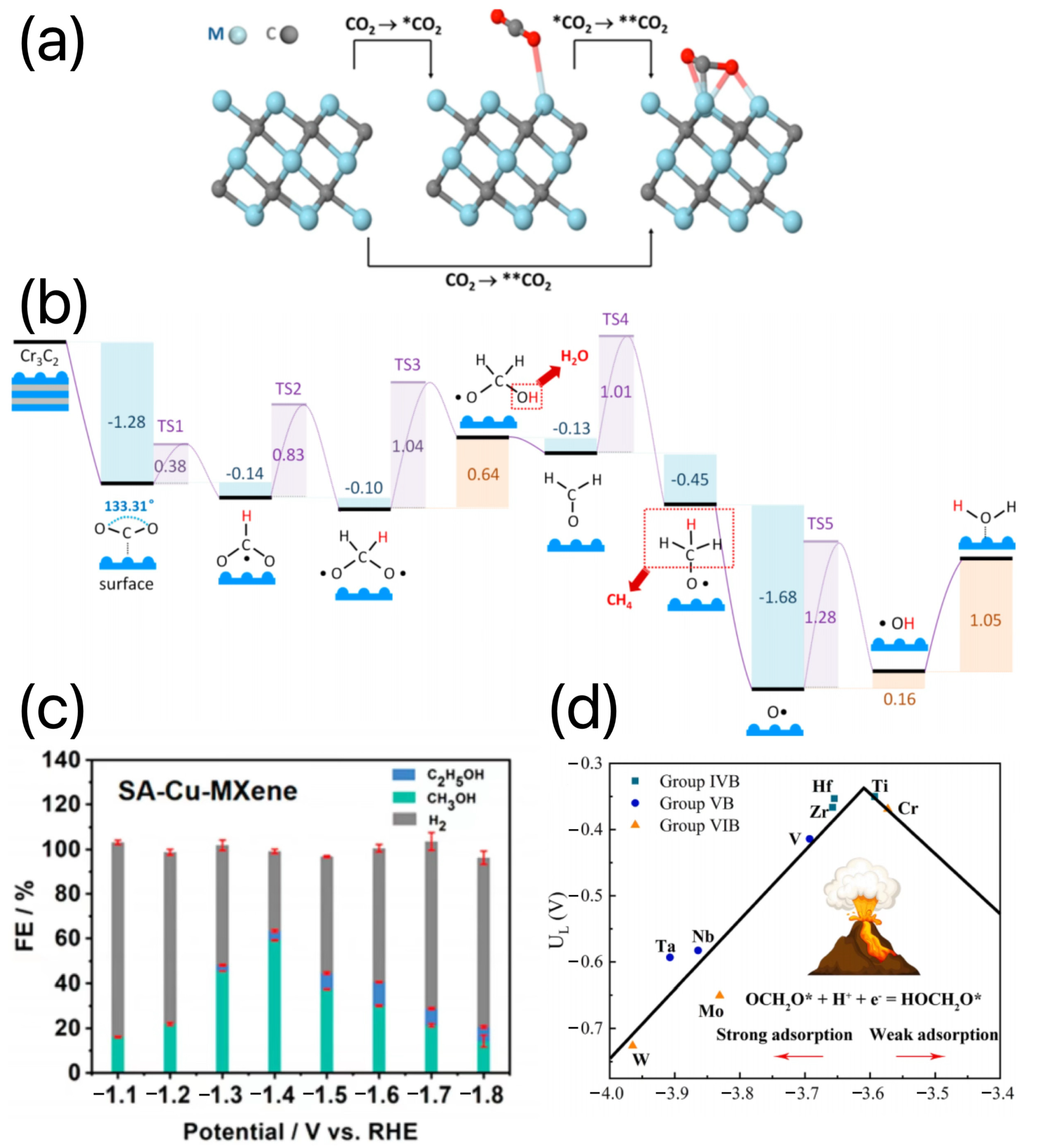
3.5. MXene-Based Electrocatalysts for the CO2RR
- Control of surface properties: This occurs through crystal surface control, morphology and size control, defect control, and other strategies to design the catalyst to improve its surface properties. These methods can adjust the surface structure and chemical composition of the catalyst and improve the catalytic activity.
- Polymetallic MXene: Researchers can synthesize MXene materials composed of multiple metallic elements, using the synergistic and geometric effects of polymetallic MXene to directly produce directional electron distribution and more active sites, thereby improving electrocatalytic performance.
- Composite cocatalysis: MXenes can be used as conductive enhancers in composites with strong interfacial coupling and fast charge-transfer kinetics. Therefore, combining MXene with other functional materials to form composite materials can effectively improve the electrochemical properties of composite materials.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhuang, Z.; Lv, F.; Zhu, H.; Zhou, L.; Luo, M.; Zhu, J.; Lang, Z.; Feng, S.; Chen, W.; et al. The Marriage of the FeN4 Moiety and MXene Boosts Oxygen Reduction Catalysis: Fe 3d Electron Delocalization Matters. Adv. Mater. 2018, 30, e1803220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, e1804779. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the Synthesis of 2D MXenes. Adv. Mater. 2021, 33, e2103148. [Google Scholar] [CrossRef]
- Riazi, H.; Nemani, S.K.; Grady, M.C.; Anasori, B.; Soroush, M. Ti3C2MXene–polymer nanocomposites and their applications. J. Mater. Chem. A 2021, 9, 8051–8098. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Shuai, T.Y.; Zhan, Q.N.; Xu, H.M.; Huang, C.J.; Zhang, Z.J.; Li, G.R. Recent advances in the synthesis and electrocatalytic application of MXene materials. Chem. Commun. 2023, 59, 3968–3999. [Google Scholar] [CrossRef]
- Wu, Z.; Shang, T.; Deng, Y.; Tao, Y.; Yang, Q.H. The Assembly of MXenes from 2D to 3D. Adv. Sci. 2020, 7, 1903077. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, S.; Wang, Z.; Zhao, J.; Qiu, J. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Yu, X.; Yin, W.; Wang, T.; Zhang, Y. Decorating g-C3N4 Nanosheets with Ti3C2 MXene Nanoparticles for Efficient Oxygen Reduction Reaction. Langmuir 2019, 35, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, G.-F.; Ding, L.; Chen, X.; Ding, L.-X.; Wang, H. Efficient Electrocatalytic N2 Fixation with MXene under Ambient Conditions. Joule 2019, 3, 279–289. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, Z.; Yang, W.; Liu, S.; Zhang, X.; Yu, Y.; Cheong, W.C.; Zheng, L.; Ren, F.; Ying, G.; et al. MXene (Ti3C2) Vacancy-Confined Single-Atom Catalyst for Efficient Functionalization of CO2. J. Am. Chem. Soc. 2019, 141, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Le, H.T.; Nguyen, D.C.; Hoa, H.V.; Kim, N.H.; Lee, J.H. Recent advances in MXene-based nanocomposites for electrochemical energy storage applications. Prog. Mater. Sci. 2021, 117, 100733. [Google Scholar] [CrossRef]
- Kong, W.; Deng, J.; Li, L. Recent advances in noble metal MXene-based catalysts for electrocatalysis. J. Mater. Chem. A 2022, 10, 14674–14691. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.-M. Recent advances in structural engineering of MXene electrocatalysts. J. Mater. Chem. A 2020, 8, 10604–10624. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Chao, L.; Singh, L.; Murugadoss, G.; Rajeshkhanna, G. 2D MXene Nanomaterials as Electrocatalysts for Hydrogen Evolution Reaction (HER): A Review. Micromachines 2022, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Anne, B.R.; Kundu, J.; Kabiraz, M.K.; Kim, J.; Cho, D.; Choi, S.I. A Review on MXene as Promising Support Materials for Oxygen Evolution Reaction Catalysts. Adv. Funct. Mater. 2023, in press. [CrossRef]
- Peera, S.G.; Liu, C.; Sahu, A.K.; Selvaraj, M.; Rao, M.C.; Lee, T.G.; Koutavarapu, R.; Shim, J.; Singh, L. Recent Advances on MXene-Based Electrocatalysts toward Oxygen Reduction Reaction: A Focused Review. Adv. Mater. Interfaces 2021, 8, 2100975. [Google Scholar] [CrossRef]
- Sun, J.; Kong, W.; Jin, Z.; Han, Y.; Ma, L.; Ding, X.; Niu, Y.; Xu, Y. Recent advances of MXene as promising catalysts for electrochemical nitrogen reduction reaction. Chin. Chem. Lett. 2020, 31, 953–960. [Google Scholar] [CrossRef]
- Heo, J.; Her, N.; Jang, M.; Park, C.M.; Son, A.; Han, J.; Yoon, Y. Photocatalytic and electrocatalytic reduction of CO2 by MXene-based nanomaterials: A review. Crit. Rev. Environ. Sci. Technol. 2022, 53, 987–1008. [Google Scholar] [CrossRef]
- Ji, C.; Cui, H.; Mi, H.; Yang, S. Applications of 2D MXenes for Electrochemical Energy Conversion and Storage. Energies 2021, 14, 8183. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 3907–3911. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.-Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and Characterization of 2D Molybdenum Carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118–3127. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The Synthesis Process and Thermal Stability of V2C MXene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef]
- Wang, X.; Garnero, C.; Rochard, G.; Magne, D.; Morisset, S.; Hurand, S.; Chartier, P.; Rousseau, J.; Cabioc’h, T.; Coutanceau, C.; et al. A new etching environment (FeF3/HCl) for the synthesis of two-dimensional titanium carbide MXenes: A route towards selective reactivity vs. water. J. Mater. Chem. A 2017, 5, 22012–22023. [Google Scholar] [CrossRef]
- Ljubek, G.; Kralj, M.; Kraljić Roković, M. Fluorine-free mechanochemical synthesis of MXene. Mater. Sci. Technol. 2023, 39, 1645–1649. [Google Scholar] [CrossRef]
- Xiu, L.-Y.; Wang, Z.-Y.; Qiu, J.-S. General synthesis of MXene by green etching chemistry of fluoride-free Lewis acidic melts. Rare Met. 2020, 39, 1237–1238. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective Etching of Silicon from Ti3SiC2 (MAX) To Obtain 2D Titanium Carbide (MXene). Angew. Chem. Int. Ed. Engl. 2018, 57, 5444–5448. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, S.J.; Seo, D.; Chae, Y.; Anayee, M.; Lee, Y.; Gogotsi, Y.; Ahn, C.W.; Jung, H.-T. Etching Mechanism of Monoatomic Aluminum Layers during MXene Synthesis. Chem. Mater. 2021, 33, 6346–6355. [Google Scholar] [CrossRef]
- Rahman, U.U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.M.; Khan, A. MXenes as Emerging Materials: Synthesis, Properties, and Applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Naslund, L.A.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Jiang, F.; Wang, Y.; Mi, L.; Yu, Y.; Song, L. Fabrication and thermal stability of NH4HF2-etched Ti3C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Cockreham, C.B.; Zhang, X.; Li, H.; Hammond-Pereira, E.; Sun, J.; Saunders, S.R.; Wang, Y.; Xu, H.; Wu, D. Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment. ACS Appl. Energy Mater. 2019, 2, 8145–8152. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.A.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. Engl. 2018, 57, 15491–15495. [Google Scholar] [CrossRef]
- Liu, H.-J.; Dong, B. Recent advances and prospects of MXene-based materials for electrocatalysis and energy storage. Mater. Today Phys. 2021, 20, 100469. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Huang, Y.; Deng, Q.; Zhou, J.; et al. Photoluminescent Ti3C2 MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, J.; Ge, G.; Zhang, Y.; Jin, W.; Huang, W.; Shao, J.; Yang, J.; Dong, X. Stretchable Ti3C2Tx MXene/Carbon Nanotube Composite Based Strain Sensor with Ultrahigh Sensitivity and Tunable Sensing Range. ACS Nano 2018, 12, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xie, X.; Choi, S.; Zhao, Y.; Liu, H.; Wang, C.; Chang, S.; Wang, G. Sb2O3/MXene(Ti3C2Tx) hybrid anode materials with enhanced performance for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 12445–12452. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Li, Y.; Dai, J.; Song, Y. Hierarchical Ni2P/Cr2CTx(MXene) composites with oxidized surface groups as efficient bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2019, 7, 9324–9334. [Google Scholar] [CrossRef]
- Kumar, S.; Schwingenschlögl, U. Thermoelectric performance of functionalizedSc2CMXenes. Phys. Rev. B 2016, 94, 035405. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Shi, J.-j.; Cen, Y.-l.; Wu, M. Improvement of Visible-Light Photocatalytic Efficiency in a Novel InSe/Zr2CO2 Heterostructure for Overall Water Splitting. J. Phys. Chem. C 2019, 123, 12781–12790. [Google Scholar] [CrossRef]
- Xue, N.; Li, X.; Zhang, M.; Han, L.; Liu, Y.; Tao, X. Chemical-Combined Ball-Milling Synthesis of Fluorine-Free Porous MXene for High-Performance Lithium Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 10234–10241. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Pan, J.; Fan, W. Optical properties of two-dimensional semi-conductive MXene Sc2CO produced by sputtering. Optik 2020, 219, 165046. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.L.; Cheng, H.M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K.P. Direct Synthesis of Large-Area 2D Mo2C on In Situ Grown Graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ayoko, G.A.; Hu, C.; Sun, Z. Thermal reduction of sulfur-containing MAX phase for MXene production. Chem. Eng. J. 2020, 395, 125111. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Cheng, C. Ru-based electrocatalysts for hydrogen evolution reaction: Recent research advances and perspectives. Mater. Today Phys. 2020, 15, 100274. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Salah, B.; Lu, Q.; Abdullah, A.M.; Chitt, M.; Ghanem, A.; Al-Hajri, R.S.; Eid, K. Template-free synthesis of M/g-C3N4 (M = Cu, Mn, and Fe) porous one-dimensional nanostructures for green hydrogen production. J. Electroanal. Chem. 2023, 938, 117426. [Google Scholar] [CrossRef]
- Salah, B.; Abdelgawad, A.; Lu, Q.; Ipadeola, A.K.; Luque, R.; Eid, K. Synergistically interactive MnFeM (M = Cu, Ti, and Co) sites doped porous g-C3N4 fiber-like nanostructures for an enhanced green hydrogen production. Green. Chem. 2023, 25, 6032–6040. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-Dimensional Molybdenum Carbide (MXene) as an Efficient Electrocatalyst for Hydrogen Evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Handoko, A.D.; Steinmann, S.N.; Wei, F.; Seh, Z.W. Correction: Theory-guided materials design: Two-dimensional MXenes in electro- and photocatalysis. Nanoscale Horiz. 2019, 4, 1014. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; San Baek, D.; Joo, S.H.; Lee, K. MXene: An emerging two-dimensional material for future energy conversion and storage applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Pandey, M.; Thygesen, K.S. Two-Dimensional MXenes as Catalysts for Electrochemical Hydrogen Evolution: A Computational Screening Study. J. Phys. Chem. C 2017, 121, 13593–13598. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, T.; Xie, X.; Jiang, W.; Li, J.; Tian, B.; Su, C. Oxygen-Functionalized Ultrathin Ti3C2Tx MXene for Enhanced Electrocatalytic Hydrogen Evolution. ChemSusChem 2019, 12, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Tiwari, A.P.; Lee, M.; Choi, M.; Song, W.; Im, J.; Zyung, T.; Jung, H.-K.; Lee, S.S.; Jeon, S.; et al. Enhanced electrocatalytic activity by chemical nitridation of two-dimensional titanium carbide MXene for hydrogen evolution. J. Mater. Chem. A 2018, 6, 20869–20877. [Google Scholar] [CrossRef]
- Hui, X.; Ge, X.; Zhao, R.; Li, Z.; Yin, L. Interface Chemistry on MXene-Based Materials for Enhanced Energy Storage and Conversion Performance. Adv. Funct. Mater. 2020, 30, 2005190. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.W.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varadhan, P.; Fu, H.C.; Kim, H.; Zhang, D.; Chen, S.; Song, L.; Ma, D.; Wang, Y.; Alshareef, H.N.; et al. Heteroatom-Mediated Interactions between Ruthenium Single Atoms and an MXene Support for Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, e1903841. [Google Scholar] [CrossRef]
- Huang, J.-J.; Liu, X.-Q.; Meng, F.-F.; He, L.-Q.; Wang, J.-X.; Wu, J.-C.; Lu, X.-H.; Tong, Y.-X.; Fang, P.-P. A facile method to produce MoSe2/MXene hybrid nanoflowers with enhanced electrocatalytic activity for hydrogen evolution. J. Electroanal. Chem. 2020, 856, 113727. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.; Liu, S.; Li, J.; Bu, L.; Chen, J.; Xiao, X.; Choi, J.-H.; Gao, L.; Lee, J.-M. Confined growth of pyridinic N–Mo2C sites on MXenes for hydrogen evolution. J. Mater. Chem. A 2020, 8, 7109–7116. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, Z.; Wei, L.; Zhao, S.; Jian, X.; Chen, Y. Electrocatalytic hydrogen evolution under neutral pH conditions: Current understandings, recent advances, and future prospects. Energy Environ. Sci. 2020, 13, 3185–3206. [Google Scholar] [CrossRef]
- Murthy, A.P.; Govindarajan, D.; Theerthagiri, J.; Madhavan, J.; Parasuraman, K. Metal-doped molybdenum nitride films for enhanced hydrogen evolution in near-neutral strongly buffered aerobic media. Electrochim. Acta 2018, 283, 1525–1533. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Li, B.; Jiang, X.; Zhang, Y.; Li, M.; Song, S.; Chen, S.; Wang, M.; Shen, Y.; et al. Regulating the electronic configuration of ruthenium nanoparticles via coupling cobalt phosphide for hydrogen evolution in alkaline media. Mater. Today Phys. 2020, 12, 100182. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Liu, J.; Sun, F.; Yang, P.; Qiu, J. A hierarchically porous and hydrophilic 3D nickel–iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities. Nano Energy 2019, 63, 103880. [Google Scholar] [CrossRef]
- Mishra, I.K.; Zhou, H.; Sun, J.; Dahal, K.; Ren, Z.; He, R.; Chen, S.; Ren, Z. Highly efficient hydrogen evolution by self-standing nickel phosphide-based hybrid nanosheet arrays electrocatalyst. Mater. Today Phys. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Annabi, N.; Shin, S.R.; Tamayol, A.; Miscuglio, M.; Bakooshli, M.A.; Assmann, A.; Mostafalu, P.; Sun, J.Y.; Mithieux, S.; Cheung, L.; et al. Highly Elastic and Conductive Human-Based Protein Hybrid Hydrogels. Adv. Mater. 2016, 28, 40–49. [Google Scholar] [CrossRef]
- Liang, J.; Ding, C.; Liu, J.; Chen, T.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Heterostructure engineering of Co-doped MoS2 coupled with Mo2CTx MXene for enhanced hydrogen evolution in alkaline media. Nanoscale 2019, 11, 10992–11000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Lu, B. Plum pudding model inspired KVPO4F@3DC as high-voltage and hyperstable cathode for potassium ion batteries. Sci. Bull. 2020, 65, 1242–1251. [Google Scholar] [CrossRef]
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Yang, P.; Zhao, J.; Qiu, J. Multilevel Hollow MXene Tailored Low-Pt Catalyst for Efficient Hydrogen Evolution in Full-pH Range and Seawater. Adv. Funct. Mater. 2020, 30, 1910028. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Y.; Wang, Z.; Zhang, J.; Chen, X.; Dai, M.; Jia, D.; Zhou, Y.; Li, Z.; Hu, P. Efficiently Synergistic Hydrogen Evolution Realized by Trace Amount of Pt-Decorated Defect-Rich SnS2 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 37750–37759. [Google Scholar] [CrossRef]
- Tie, L.; Li, N.; Yu, C.; Liu, Y.; Yang, S.; Chen, H.; Dong, S.; Sun, J.; Dou, S.; Sun, J. Self-Supported Nonprecious MXene/Ni3S2 Electrocatalysts for Efficient Hydrogen Generation in Alkaline Media. ACS Appl. Energy Mater. 2019, 2, 6931–6938. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Feng, Y.; Qi, R.; Ren, J.; Zhu, Z. Stabilizing Ti3C2Tx-MXenes with TiOF2 nanospheres intercalation to improve hydrogen evolution reaction and humidity-sensing performance. Appl. Surf. Sci. 2019, 496, 143729. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Chen, Z.; Kumar, P.V.; Tsoukalou, A.; Kierzkowska, A.; Abdala, P.M.; Safonova, O.V.; Fedorov, A.; Müller, C.R. Single Site Cobalt Substitution in 2D Molybdenum Carbide (MXene) Enhances Catalytic Activity in the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 17809–17816. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Grauer, D.C.; Yano, J.; Long, J.R.; Yang, P.; Chang, C.J. Electrodeposited Cobalt-Sulfide Catalyst for Electrochemical and Photoelectrochemical Hydrogen Generation from Water. J. Am. Chem. Soc. 2013, 135, 17699–17702. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Du, Y.; Liao, W. Catalytic effect of Ti2C MXene on the dehydrogenation of MgH2. Int. J. Hydrogen Energy 2019, 44, 6787–6794. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Zang, L.; Sun, W.; Liu, S.; Huang, Y.; Yuan, H.; Tao, Z.; Wang, Y. Enhanced Hydrogen Storage Properties and Reversibility of LiBH4 Confined in Two-Dimensional Ti3C2. ACS Appl. Mater. Interfaces 2018, 10, 19598–19604. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, D.; Liu, X.; Fan, G.; Liu, B. Improving the hydrogen storage performance of lithium borohydride by Ti3C2 MXene. Int. J. Hydrogen Energy 2019, 44, 29297–29303. [Google Scholar] [CrossRef]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting Carbon Nitride and Titanium Carbide Nanosheets for High-Performance Oxygen Evolution. Angew. Chem. Int. Ed. Engl. 2016, 55, 1138–1142. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Yang, Y.; Yin, X.; Que, W.; Zhu, J. Three dimensional hierarchical network structure of S-NiFe2O4 modified few-layer titanium carbides (MXene) flakes on nickel foam as a high efficient electrocatalyst for oxygen evolution. Electrochim. Acta 2019, 296, 762–770. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. 2D MXene-Based Materials for Electrocatalysis. Trans. Tianjin Univ. 2020, 26, 149–171. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, B.; Li, S.; Zhou, L.; Lai, L.; Wang, Z.; Zhao, S.; Han, M.; Gao, K.; Lu, M.; et al. Interdiffusion Reaction-Assisted Hybridization of Two-Dimensional Metal-Organic Frameworks and Ti3C2Tx Nanosheets for Electrocatalytic Oxygen Evolution. ACS Nano 2017, 11, 5800–5807. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wei, Z.; Ma, C.; Xing, X.; Li, Z.; Luo, D. MXene Boosted CoNi-ZIF-67 as Highly Efficient Electrocatalysts for Oxygen Evolution. Nanomaterials 2019, 9, 775. [Google Scholar] [CrossRef]
- Zou, H.; He, B.; Kuang, P.; Yu, J.; Fan, K. Metal-Organic Framework-Derived Nickel-Cobalt Sulfide on Ultrathin Mxene Nanosheets for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 22311–22319. [Google Scholar] [CrossRef] [PubMed]
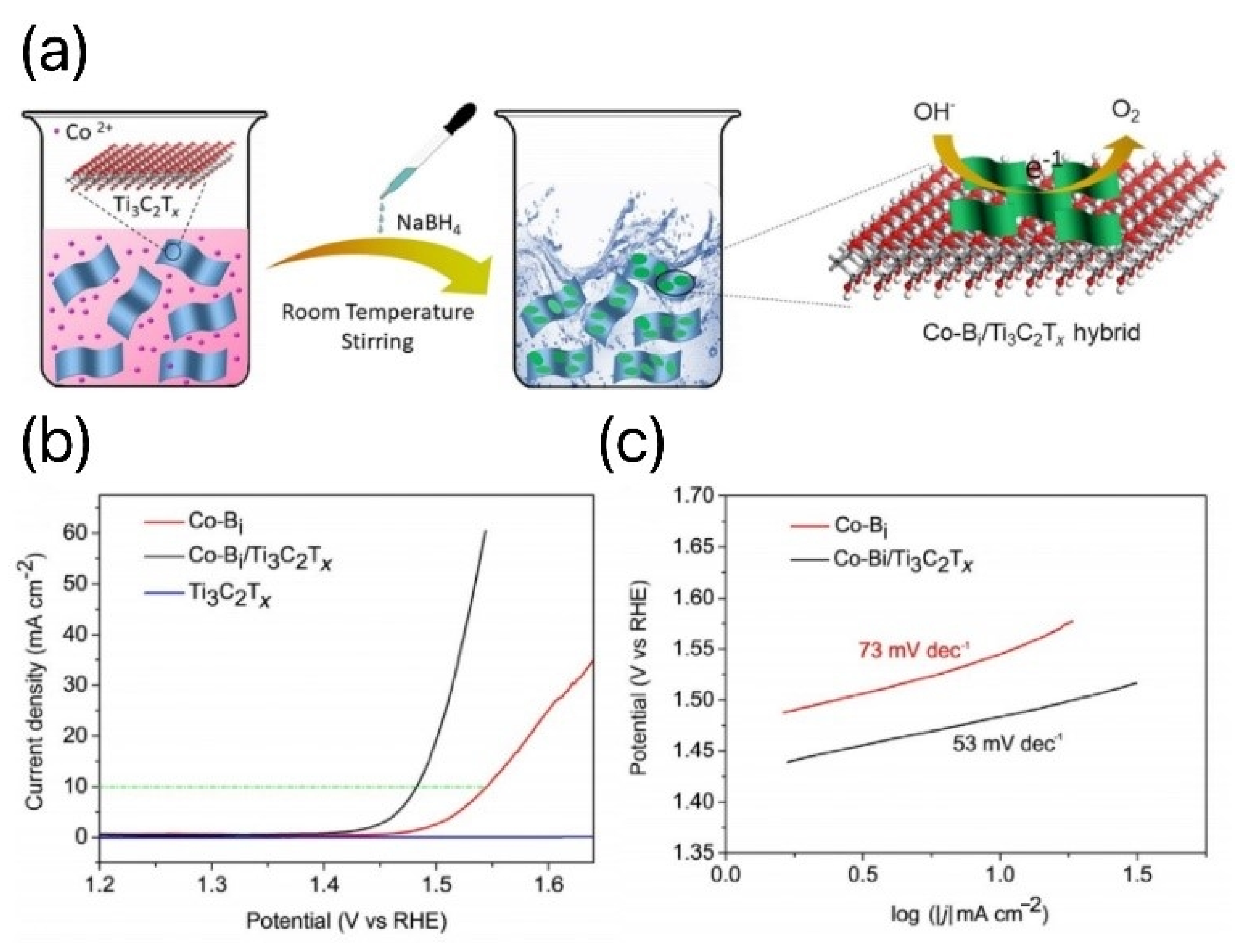
- Liu, J.; Chen, T.; Juan, P.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical Cobalt Borate/MXenes Hybrid with Extraordinary Electrocatalytic Performance in Oxygen Evolution Reaction. ChemSusChem 2018, 11, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Selvam, N.C.S.; Lee, J.; Choi, G.H.; Oh, M.J.; Xu, S.; Lim, B.; Yoo, P.J. MXene supported CoxAy (A = OH, P, Se) electrocatalysts for overall water splitting: Unveiling the role of anions in intrinsic activity and stability. J. Mater. Chem. A 2019, 7, 27383–27393. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal Strategy for HF-Free Facile and Rapid Synthesis of Two-dimensional MXenes as Multifunctional Energy Materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Benchakar, M.; Bilyk, T.; Garnero, C.; Loupias, L.; Morais, C.; Pacaud, J.; Canaff, C.; Chartier, P.; Morisset, S.; Guignard, N.; et al. MXene Supported Cobalt Layered Double Hydroxide Nanocrystals: Facile Synthesis Route for a Synergistic Oxygen Evolution Reaction Electrocatalyst. Adv. Mater. Interfaces 2019, 6, 1901328. [Google Scholar] [CrossRef]
- Tian, M.; Jiang, Y.; Tong, H.; Xu, Y.; Xia, L. MXene-Supported FeCo-LDHs as Highly Efficient Catalysts for Enhanced Electrocatalytic Oxygen Evolution Reaction. ChemNanoMat 2019, 6, 154–159. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Han, M.; Xi, Y.; You, H.; Hao, X.; Li, Z.; Zhou, J.; Song, D.; Wang, D.; et al. Environmentally-Friendly Exfoliate and Active Site Self-Assembly: Thin 2D/2D Heterostructure Amorphous Nickel–Iron Alloy on 2D Materials for Efficient Oxygen Evolution Reaction. Small 2019, 15, 1805435. [Google Scholar] [CrossRef]
- Umapathi, S.; Masud, J.; Swesi, A.T.; Nath, M. FeNi2Se4–Reduced Graphene Oxide Nanocomposite: Enhancing Bifunctional Electrocatalytic Activity for Oxygen Evolution and Reduction through Synergistic Effects. Adv. Sustain. Syst. 2017, 1, 1700086. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Ma, W.; Ma, R.; Wang, C.; Liang, J.; Liu, X.; Zhou, K.; Sasaki, T. A Superlattice of Alternately Stacked Ni-Fe Hydroxide Nanosheets and Graphene for Efficient Splitting of Water. ACS Nano 2015, 9, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
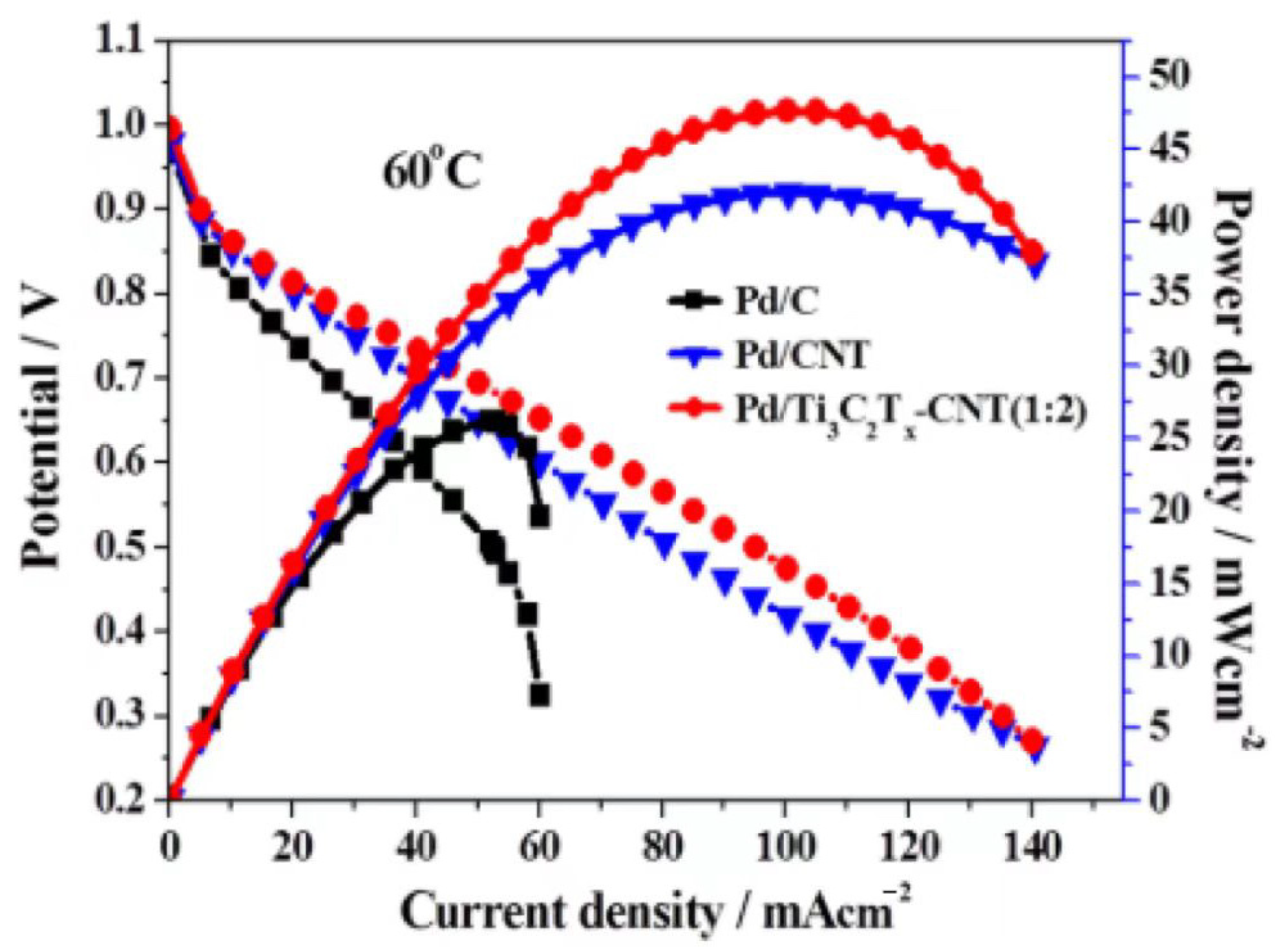
- Zhang, P.; Wang, R.; Xiao, T.; Chang, Z.; Fang, Z.; Zhu, Z.; Xu, C.; Wang, L.; Cheng, J. The High-Performance Bifunctional Catalyst Pd/Ti3C2Tx–Carbon Nanotube for Oxygen Reduction Reaction and Hydrogen Evolution Reaction in Alkaline Medium. Energy Technol. 2020, 8, 2000306. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, M.; Wang, X.; Huang, H. Theoretical Prediction of Catalytic Activity of Ti2C MXene as Cathode for Li–O2 Batteries. J. Phys. Chem. C 2019, 123, 17466–17471. [Google Scholar] [CrossRef]
- Wei, B.; Fu, Z.; Legut, D.; Germann, T.C.; Du, S.; Zhang, H.; Francisco, J.S.; Zhang, R. Rational Design of Highly Stable and Active MXene-Based Bifunctional ORR/OER Double-Atom Catalysts. Adv. Mater. 2021, 33, e2102595. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zheng, Y.; Wu, X.; Zhou, M.; Rong, X.; Wang, L.; Tang, Y.; Liu, X.; Qiu, L.; Cheng, C. Tunable Structured MXenes With Modulated Atomic Environments: A Powerful New Platform for Electrocatalytic Energy Conversion. Small 2022, 18, e2203281. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Fu, G.; Yang, H.B.; Yan, Y.; Chen, J.; Yu, Z.; Gao, J.; Gan, L.Y.; Liu, B.; Chen, P. Bifunctional N-CoSe2/3D-MXene as Highly Efficient and Durable Cathode for Rechargeable Zn–Air Battery. ACS Mater. Lett. 2019, 1, 432–439. [Google Scholar] [CrossRef]
- Wen, Y.; Ma, C.; Wei, Z.; Zhu, X.; Li, Z. FeNC/MXene hybrid nanosheet as an efficient electrocatalyst for oxygen reduction reaction. RSC Adv. 2019, 9, 13424–13430. [Google Scholar] [CrossRef]
- Jiang, L.; Duan, J.; Zhu, J.; Chen, S.; Antonietti, M. Iron-Cluster-Directed Synthesis of 2D/2D Fe–N–C/MXene Superlattice-like Heterostructure with Enhanced Oxygen Reduction Electrocatalysis. ACS Nano 2020, 14, 2436–2444. [Google Scholar] [CrossRef]
- Zhang, J.; He, D.; Su, H.; Chen, X.; Pan, M.; Mu, S. Porous polyaniline-derived FeNxC/C catalysts with high activity and stability towards oxygen reduction reaction using ferric chloride both as an oxidant and iron source. J. Mater. Chem. A 2014, 2, 1242–1246. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, H.; Wang, N.; Chen, B.; Mai, Y.; Feng, X. Patterning Graphene Surfaces with Iron-Oxide-Embedded Mesoporous Polypyrrole and Derived N-Doped Carbon of Tunable Pore Size. Small 2018, 14, 1702755. [Google Scholar] [CrossRef]
- Yang, X.; Jia, Q.; Duan, F.; Hu, B.; Wang, M.; He, L.; Song, Y.; Zhang, Z. Multiwall carbon nanotubes loaded with MoS2 quantum dots and MXene quantum dots: Non–Pt bifunctional catalyst for the methanol oxidation and oxygen reduction reactions in alkaline solution. Appl. Surf. Sci. 2019, 464, 78–87. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, X.; Lyu, F.; Zhong, Q.; Hu, H.; Pan, Q.; Zhang, Q. Integrating MXene nanosheets with cobalt-tipped carbon nanotubes for an efficient oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 1281–1286. [Google Scholar] [CrossRef]
- Cao, S.; Han, N.; Han, J.; Hu, Y.; Fan, L.; Zhou, C.; Guo, R. Mesoporous Hybrid Shells of Carbonized Polyaniline/Mn2O3 as Non-Precious Efficient Oxygen Reduction Reaction Catalyst. ACS Appl. Mater. Interfaces 2016, 8, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Y. 2D Early Transition Metal Carbides (MXenes) for Catalysis. Small 2019, 15, e1804736. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Han, J.; Jin, Z.; Han, Y.; Wang, F.; Niu, Y.; Wu, Y.; Xu, Y. High-Performance Electrocatalytic Conversion of N2 to NH3 Using Oxygen-Vacancy-Rich TiO2 In Situ Grown on Ti3C2Tx MXene. Adv. Energy Mater. 2019, 9, 1803406. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.; Huang, L.; Li, J.; Yao, L.; Zhu, Q.; Wang, W.; Abbas, W.; Naz, R.; Gu, J.; et al. Fluorine-free Ti3C2Tx (T = O, OH) nanosheets (∼50–100 nm) for nitrogen fixation under ambient conditions. J. Mater. Chem. A 2019, 7, 14462–14465. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Xie, X.-Y.; Li, X.; Ma, Y.; Liu, Q.; Fang, W.-H.; Shi, X.; Cui, G.; Sun, X. Ti3C2Tx(T = F, OH) MXene nanosheets: Conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3. J. Mater. Chem. A 2018, 6, 24031–24035. [Google Scholar] [CrossRef]
- Peng, W.; Luo, M.; Xu, X.; Jiang, K.; Peng, M.; Chen, D.; Chan, T.S.; Tan, Y. Spontaneous Atomic Ruthenium Doping in Mo2CTX MXene Defects Enhances Electrocatalytic Activity for the Nitrogen Reduction Reaction. Adv. Energy Mater. 2020, 10, 2001364. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Ong, W.J.; MacFarlane, D.R.; Zhao, X.; Cheetham, A.K.; Sun, C. Understanding of Electrochemical Mechanisms for CO2 Capture and Conversion into Hydrocarbon Fuels in Transition-Metal Carbides (MXenes). ACS Nano 2017, 11, 10825–10833. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, C.; Hu, R.; Du, Z.; Gu, J.; Cui, Y.; Chen, X.; Xu, W.; Cheng, Z.; Li, S.; et al. Selective Etching Quaternary MAX Phase toward Single Atom Copper Immobilized MXene (Ti3C2Clx) for Efficient CO2 Electroreduction to Methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Guo, Z.; Tang, C.; Sa, B.; Miao, N.; Zhou, J.; Sun, Z. Breaking the linear scaling relations in MXene catalysts for efficient CO2 reduction. Chem. Eng. J. 2022, 429, 132171. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, D.; Biswas, A.N.; Xie, Z.; Hwang, S.; Lee, J.H.; Meng, H.; Chen, J.G. Transition Metal Nitrides as Promising Catalyst Supports for Tuning CO/H2 Syngas Production from Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 11345–11348. [Google Scholar] [CrossRef] [PubMed]
- Handoko, A.D.; Chen, H.; Lum, Y.; Zhang, Q.; Anasori, B.; Seh, Z.W. Two-Dimensional Titanium and Molybdenum Carbide MXenes as Electrocatalysts for CO2 Reduction. iScience 2020, 23, 101181. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Peng, X.; Mi, Y.; Bao, H.; Zhao, S.; Liu, X.; Luo, J. Nitrogen doping and titanium vacancies synergistically promote CO2 fixation in seawater. Nanoscale 2020, 12, 17191–17195. [Google Scholar] [CrossRef]
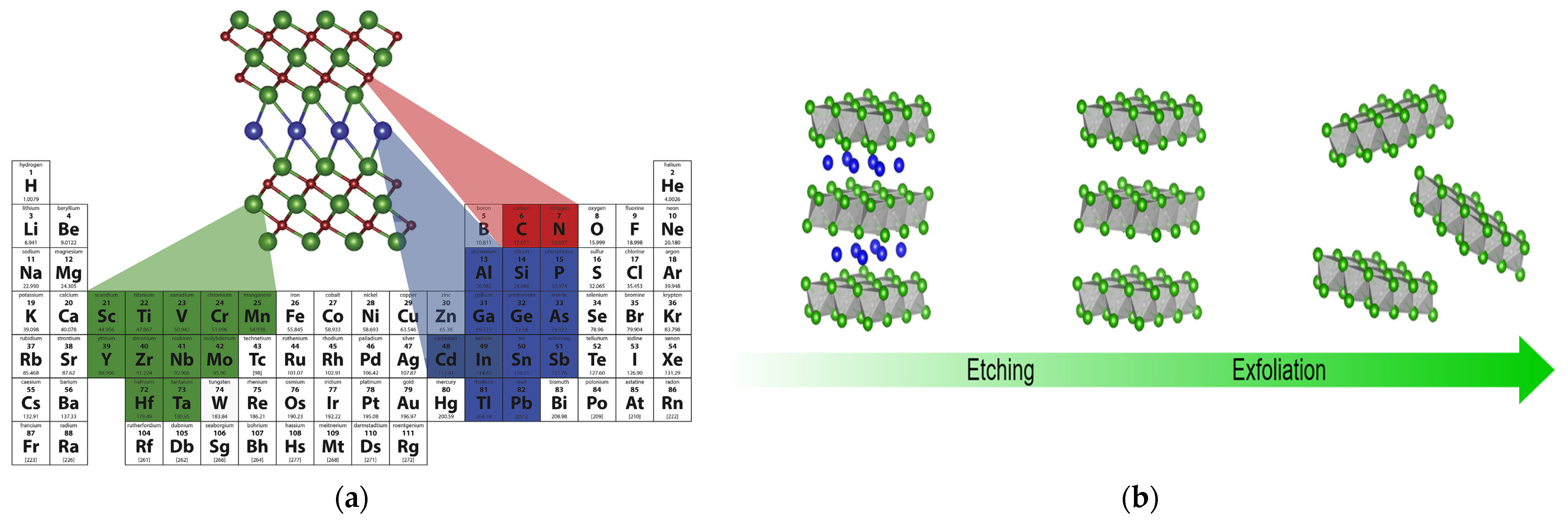
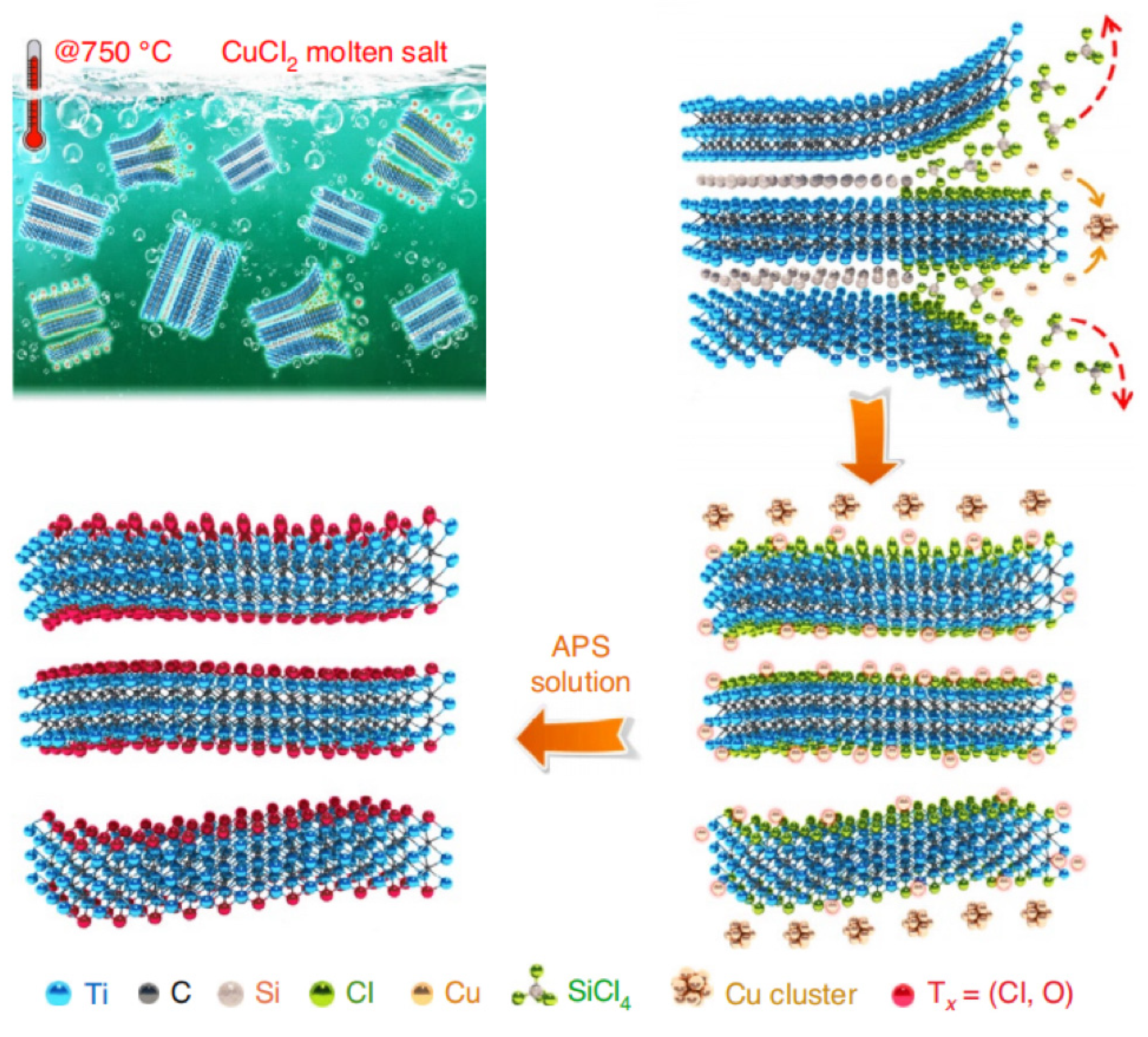
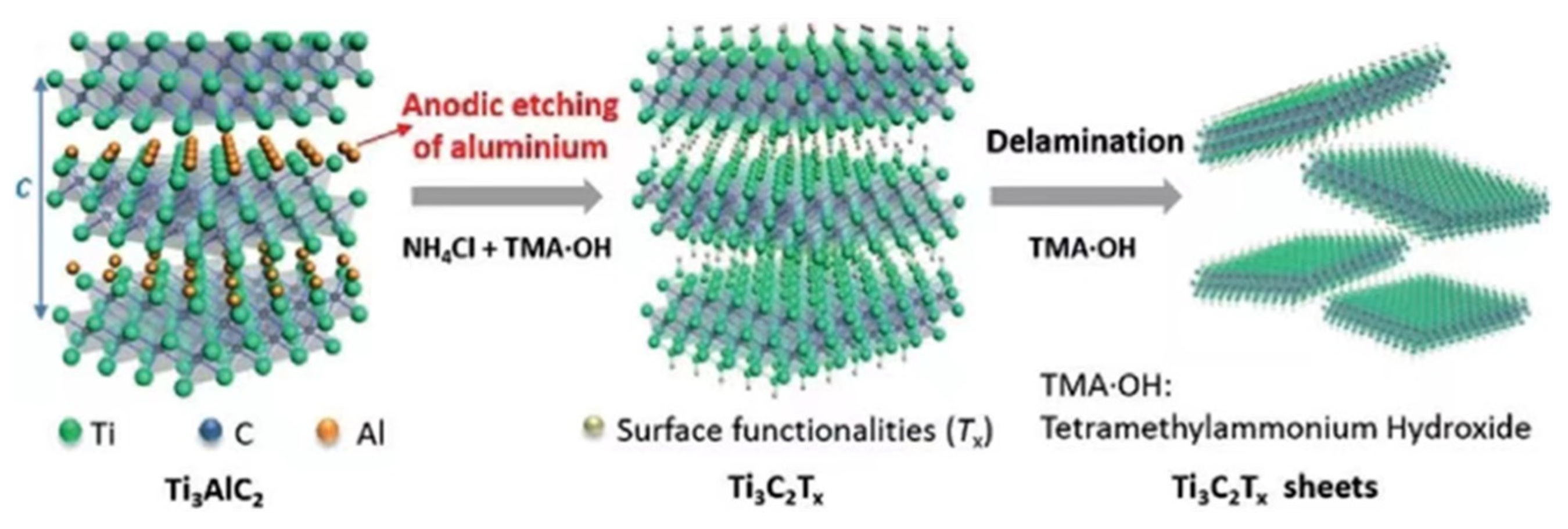

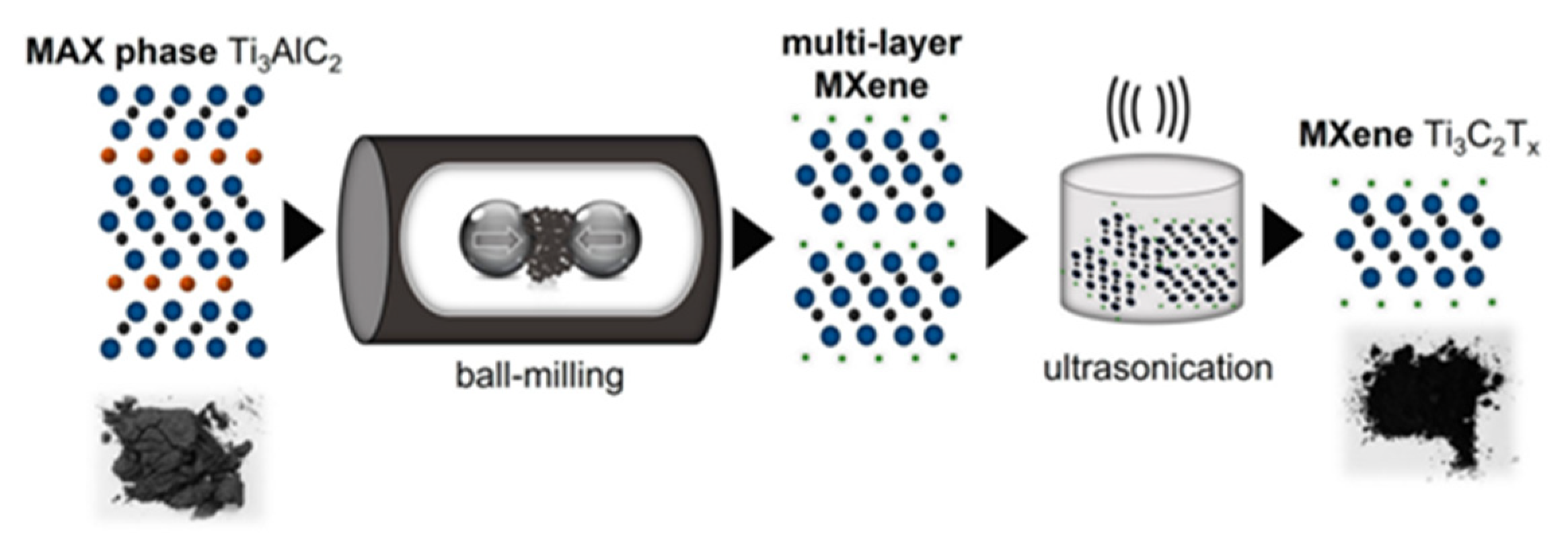
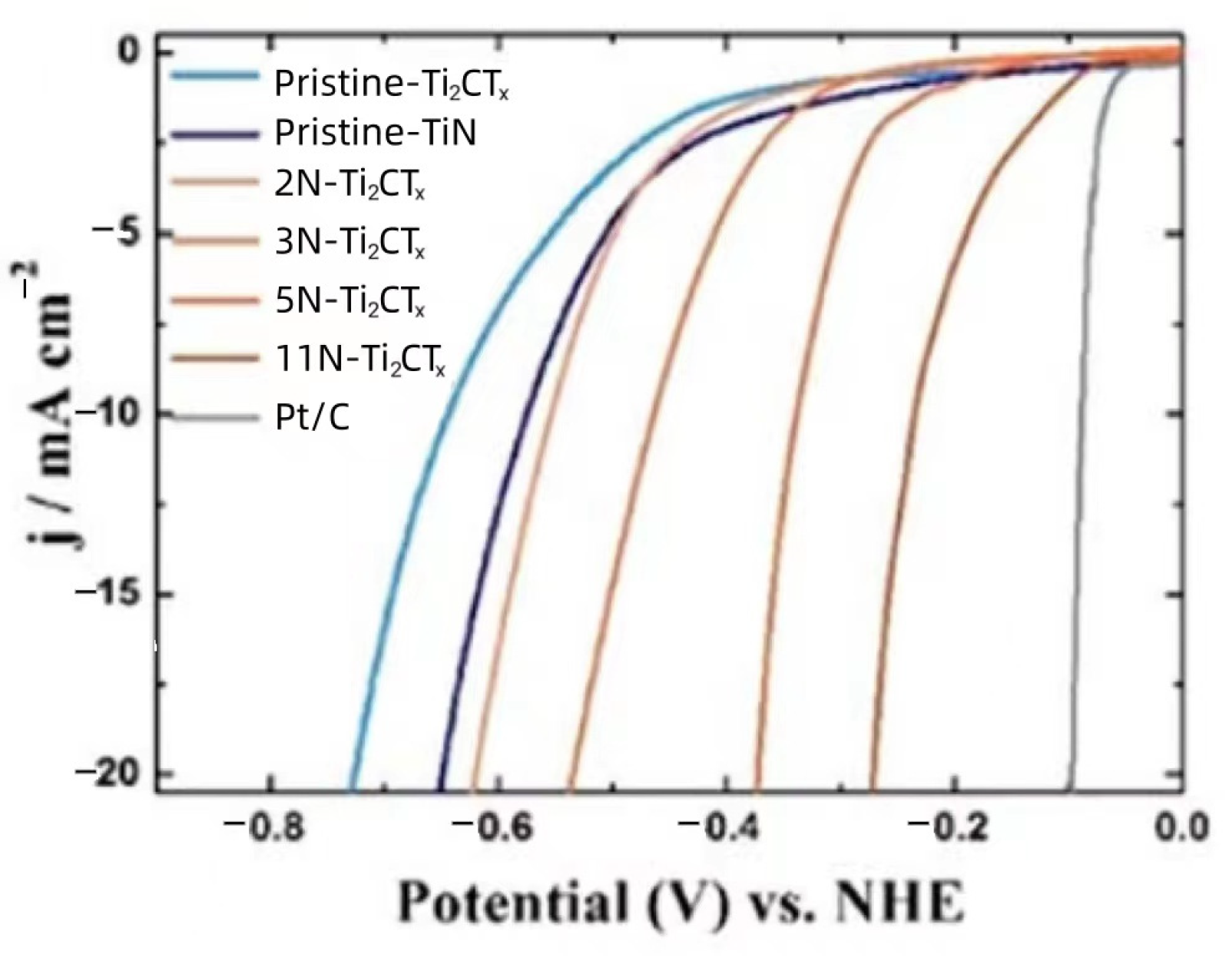
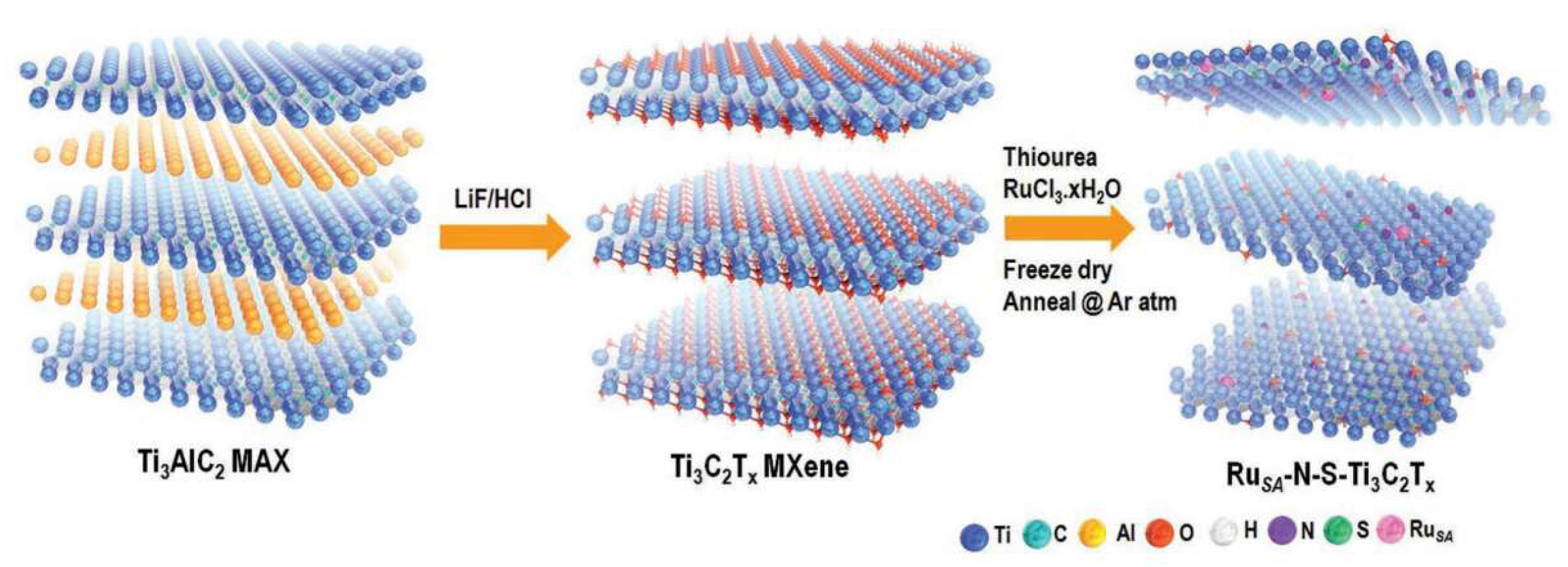

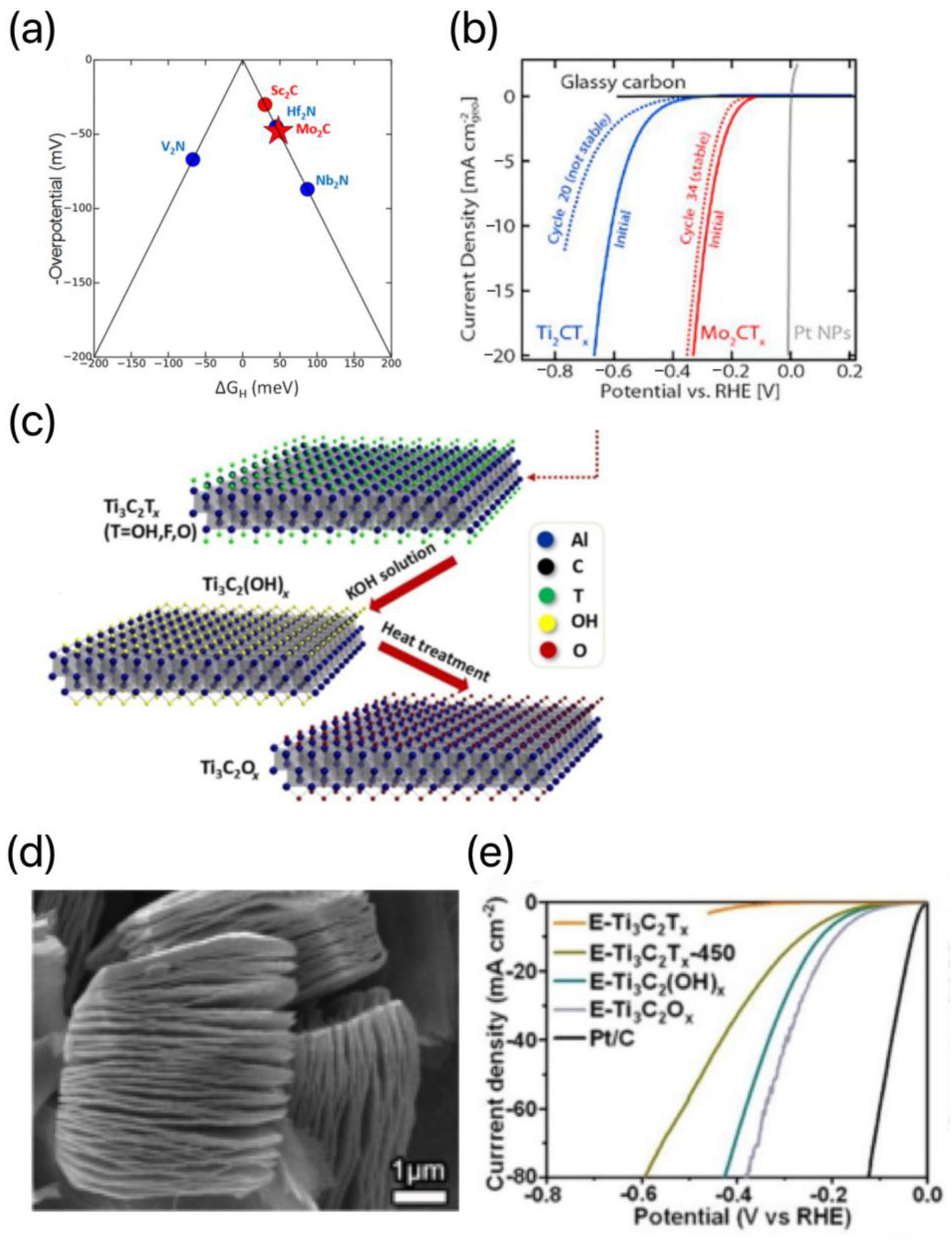
| Title | Focus | Ref. |
|---|---|---|
| Progress in the synthesis process and electrocatalytic application of MXene materials | The preparation methods of xylene are reviewed in detail. The research progress in electrocatalysis and the key factors affecting the properties of the materials, such as the functional groups, electrical conductivity, and interface, are summarized. The main challenges and opportunities facing MXene materials in basic research and practical applications as a next generation electrocatalytic platform are highlighted. | This work |
| Recent advances in noble metal MXene-based catalysts for electrocatalysis | The paper reviewed the strategies for the synthesis of noble-metal MXene-based catalysts, focusing on the application of noble-metal MXene-based catalysts in the field of electrocatalysis and highlighting the strategies for improving the electrocatalytic performance of noble-metal MXene-based catalysts. | [19] |
| Recent advances in structural engineering of MXene electrocatalysts | Representative advances in MXenes as electrocatalysts for hydrogen evolution reactions were reviewed both experimentally and theoretically. | [20] |
| 2D MXene Nanomaterials as Electrocatalysts for Hydrogen Evolution Reaction (HER): A Review | Recent advances in the synthesis and HER performance of MXene-based electrocatalysts were summarized from both theoretical and experimental perspectives. The advantages of MXene-based catalysts over conventional Pt/C catalysts in terms of HER kinetics, Tafel slopes, overpotentials, and stability in acidic and alkaline electrolytic environments were systematically evaluated. | [21] |
| A Review on MXene as Promising Support Materials for Oxygen Evolution Reaction Catalysts | The role of MXenes as support materials in improving the performance of OER catalysts was emphasized. | [22] |
| Recent Advances on MXene-Based Electrocatalysts toward Oxygen Reduction Reaction: A Focused Review | Current research on MXenes for ORR was discussed, focusing on synthesis strategies, ORR activity, and factors responsible for improving electrocatalytic performance. Several strategies for further development of efficient and durable ORR-based MXene catalysts were also presented. | [23] |
| Recent advances of MXene as promising catalysts for electrochemical nitrogen reduction reaction | Recent advances on MXene-based catalysts for electrochemical N2 reduction reactions (NRRs) were emphasized. With respect to providing guidelines for exploring more efficient MXene-based catalysts for NRR, the preparation and surface modification of MXene were discussed. In addition, the shortcomings and challenges of current research were summarized, and future research directions were envisioned. | [24] |
| Photocatalytic and electrocatalytic reduction of CO2 by MXene-based nanomaterials: A review | A comprehensive review of the current findings on the photocatalytic and electrocatalytic reduction of CO2 by various MXene-based nanomaterials was presented. The review focused on the (i) photocatalytic reduction of CO2 by functionalized Ti3C2, TiO2/Ti3C2, g-C3 N4/Ti3C2, and other/Ti3C2 catalysts, (ii) electrocatalytic CO2 reduction, (iii) CO2 reduction associated with photothermal catalysis and hydrogenation, and (iv) the stability of MXene-based photocatalysts. | [25] |
| Applications of 2D Mxenes for Electrochemical Energy Conversion and Storage | This paper highlighted the preparation methods and special features of MXenes in terms of electrode materials, conductive substrates, surface modification, heteroatom doping, wrinkling, and protective layers against dendrite growth. | [26] |
| MXenes: Emerging 2D materials for hydrogen storage | In this paper, the application status, challenges, and future prospects of hydrogen storage materials based on MXene were reviewed. | [27] |
| Electrocatalyst | Substrate | Mass Loading [mg cm−2] | Overpotential η [mV] | Tafel Slope [mV dec−1] | Solution | Ref. |
|---|---|---|---|---|---|---|
| Pt/Ti3C2Tx | GCE | 0.38 | 55 | 65 | Acidic | [24] |
| Pt/3D Ti3C2 | GCE | 0.2 | 27 | 41 | Alkaline | [86] |
| Pt–SnS2 | — | — | 117 | 69 | Acidic | [87] |
| Ti3C2Tx/Ni3S2 | NF | 4.9 | 72 | 45 | Alkaline | [88] |
| TiOF2/Ti3C2Tx | GCE | 0.18 | 197 | 56.2 | Acidic | [89] |
| Co/Mo2CTx | GCE | 0.1 | 180 | 59 | Acidic | [90] |
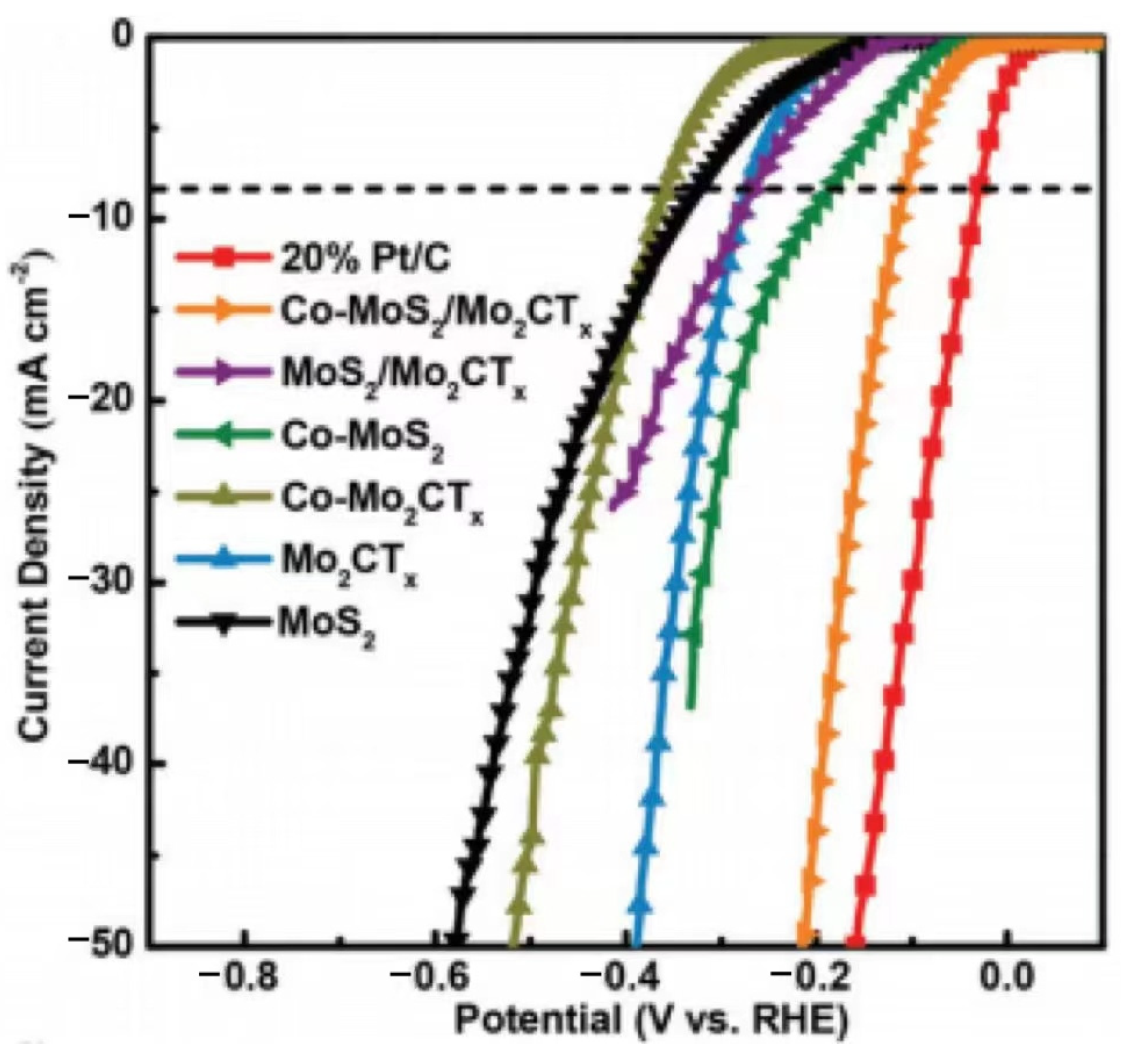
| Co–MoS2/Mo2CTx | GCE | 0.35 | 112 | 82 | Acidic | [84] |
| CoMoS | FTO | — | 282 | — | Neutral | [91] |
| Electrocatalyst | Substrate | Mass Loading [mg cm−2] | Overpotential η [mV] | Tafel Slope [mV dec−1] | Solution | Ref. |
|---|---|---|---|---|---|---|
| CoP/Ti3C2Tx | CFP | 1.5 | 230 | 50 | Alkaline | [104] |
| Co3+/Ti2CTx | GCE | 0.1 | — | 132 | Alkaline | [105] |
| Co-LDH@Ti3C2Tx | GEC | 0.35 | 330 | 32 | — | [106] |
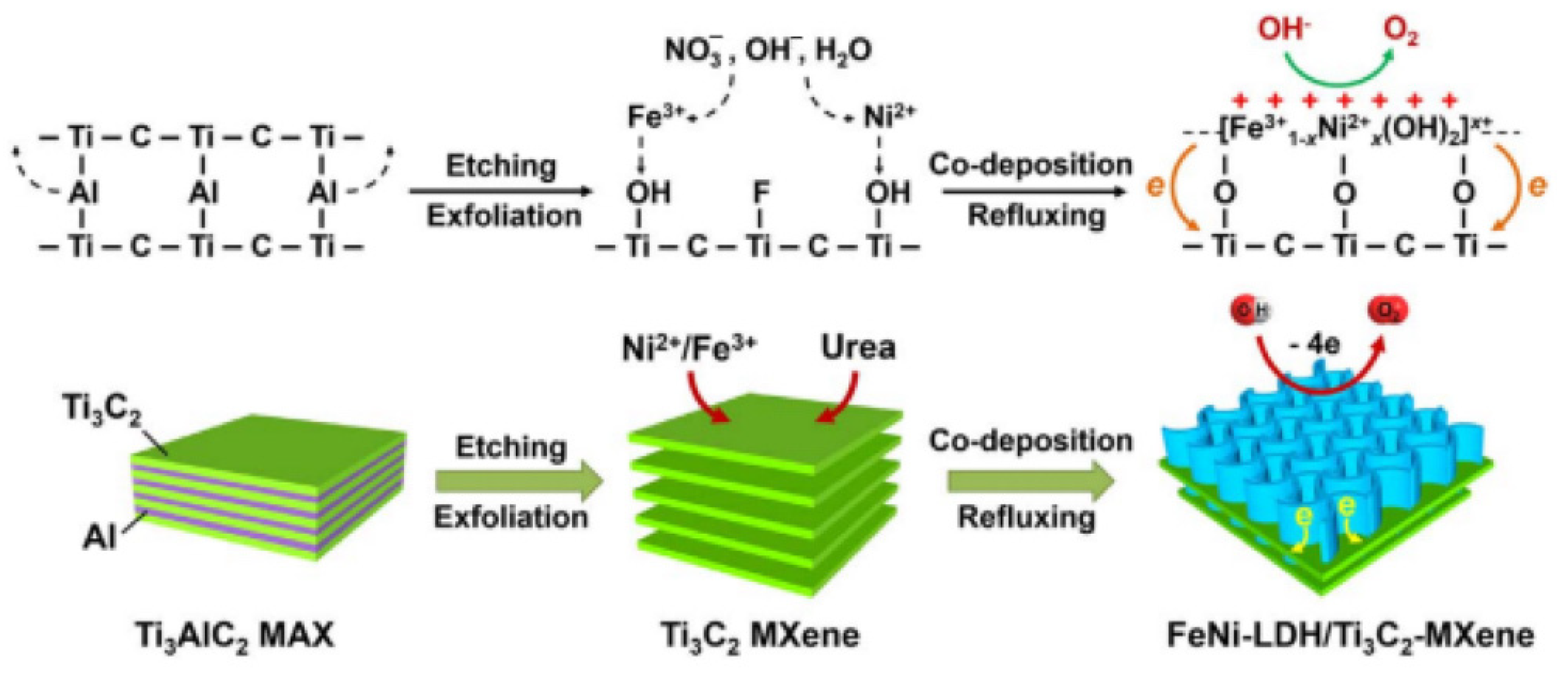
| FeCo-LDH/Ti3C2Tx | GEC | 0.357 | 268 | 85 | — | [107] |
| NiFe/Ti3C2Tx | — | 0.36 | 260 | — | Alkaline | [108] |
| FeNi2Se4–NrGO | CFP | — | 170 | 62.1 | Alkaline | [109] |
| Ni3Se2 | Au | — | 290 | 97.1 | Alkaline | [110] |
| NiFe LDH/rGO | Ni foam | — | 200/210 | 40 | Alkaline | [111] |
| Electrocatalyst | Half-Wave Potential (V) | Mass Loading [mg cm−2] | Onset Potential (V vs. RHE) | Tafel Slope [mV dec−1] | Solution | Ref. |
|---|---|---|---|---|---|---|
| FeNC/Ti3C2Tx | 0.814 | 0.1 | −1 | 30 | — | [117] |
| Fe–N–C/Ti3C2Tx | 0.84 | 0.1 | −0.92 | — | Alkaline | [118] |
| FeNxC/C–F | 0.76 | 0.8 | −0.88 | — | Acidic | [119] |
| mNC–Fe3O4@rGO-2 | 0.83 | 0.24 | −0.96 | — | Alkaline | [120] |
| MoS2–Ti3C2Tx/MWCNTs | 0.75 | — | −0.87 | 90 | Alkaline | [121] |
| Co–CNTs/Ti3C2Tx | 0.82 | — | — | 63 | — | [122] |
| g-C3N4/Ti3C2Tx | 0.79 | 0.4 | −0.92 | — | Alkaline | [14] |
| CPANI/Mn2O3 | 0.68 | 0.28 | −0.83 | — | Alkaline | [123] |
| Electrocatalyst | Substrate | Mass Loading [mg cm−2] | Potential η [mV] | Electrolyte | Product Yield [μg h−1 mgcat−1] | Faradic Efficiency [%] | Ref. |
|---|---|---|---|---|---|---|---|
| Ti3C2Tx (T = O, OH) | Carbon cloth | 0.8 | −0.3 | 0.01 M HCl | 36.9 | 9.1 | [126] |
| Ti3C2Tx | CP | 0.2 | −0.4 | 0.01 M HCl | 20.4 | 9.30 | [127] |
| TiO2/Ti3C2Tx | CP | 0.1 | −0.55 | 0.01 M HCl | 32.17 | 8 | [125] |
| Ru/Mo2CTx | CP | 0.3 | −0.3 | 0.5 M K2SO4 | 40.57 | 25.77 | [128] |
| Ru/Ti3C2Tx | GCE | 1.02 | −0.4 | 0.1 M KOH | 38.33 | 13.13 | [125] |
| Electrocatalyst | Substrate | Method(s) | Electrolyte | Faradic Efficiency [%] | Ref. |
|---|---|---|---|---|---|
| Pd/NbN | — | Heteroatom doping | 0.5 m NaHCO3 | 38.4 | [133] |
| Ti2CTx | — | Termination engineering | 0.1 m KHCO3 | 56.1 | [134] |
| SA-Cu-MXene | GCE | Heteroatom doping | 0.1 m KHCO3 | 59.1 | [131] |
| NTC-VTi | CP | Heteroatom doping/defect engineering | Seawater | 92 | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, B.; Wang, R. Progress in the Synthesis Process and Electrocatalytic Application of MXene Materials. Materials 2023, 16, 6816. https://doi.org/10.3390/ma16206816
Wang P, Wang B, Wang R. Progress in the Synthesis Process and Electrocatalytic Application of MXene Materials. Materials. 2023; 16(20):6816. https://doi.org/10.3390/ma16206816
Chicago/Turabian StyleWang, Peng, Bingquan Wang, and Rui Wang. 2023. "Progress in the Synthesis Process and Electrocatalytic Application of MXene Materials" Materials 16, no. 20: 6816. https://doi.org/10.3390/ma16206816
APA StyleWang, P., Wang, B., & Wang, R. (2023). Progress in the Synthesis Process and Electrocatalytic Application of MXene Materials. Materials, 16(20), 6816. https://doi.org/10.3390/ma16206816