The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials
Abstract
1. Introduction
- There is no significant difference in the DoC between tested materials.
- There is no significant effect of the irradiation time on the Vickers hardness numbers and DoC of each individual material.
- There is no significant difference in mass change, water sorption or solubility among tested materials after 4 months of water storage.
2. Materials and Methods
2.1. Study Design
2.2. Depth of Cure
2.3. Water Sorption and Solubility
2.4. Statistical Analysis
3. Results
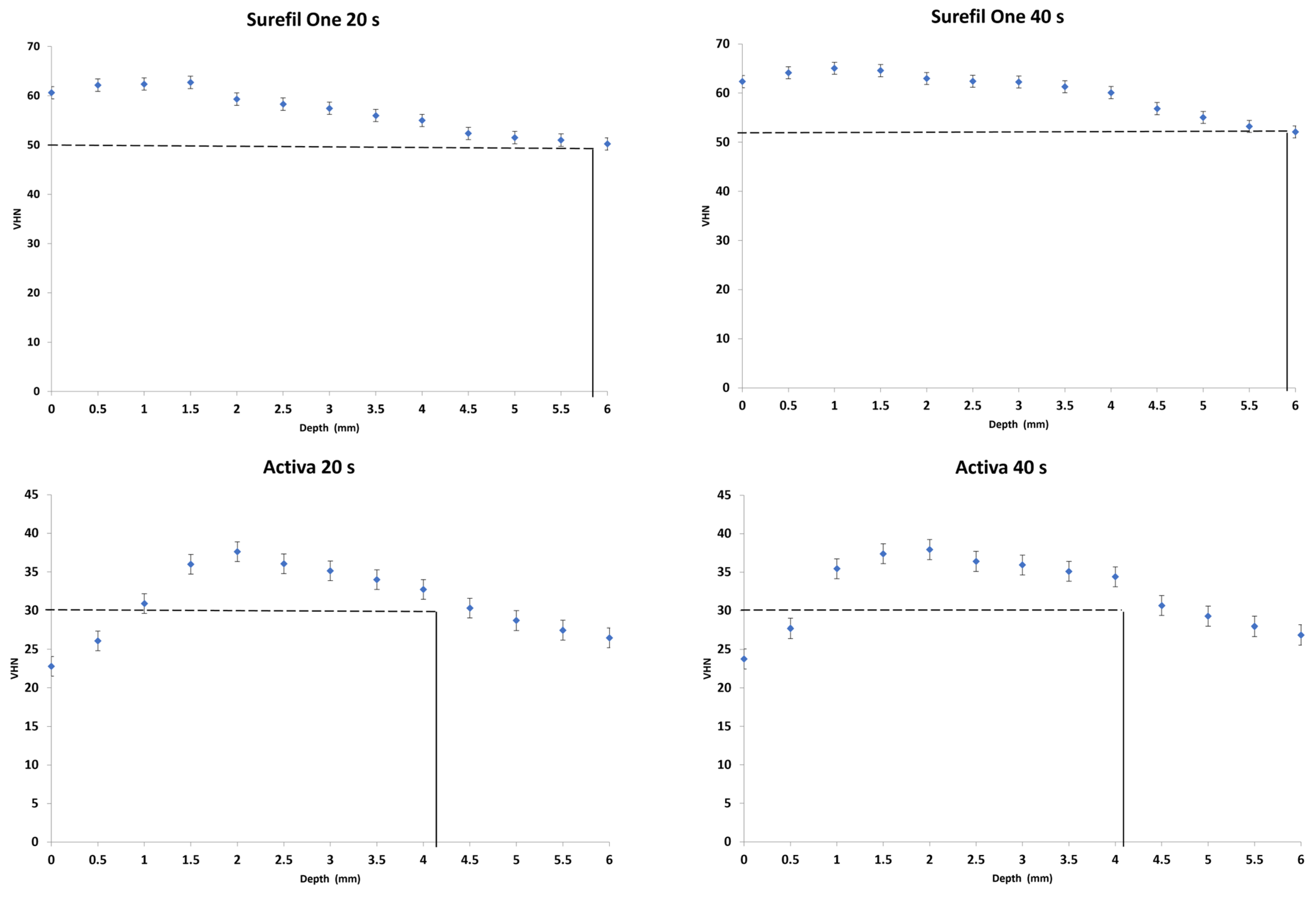
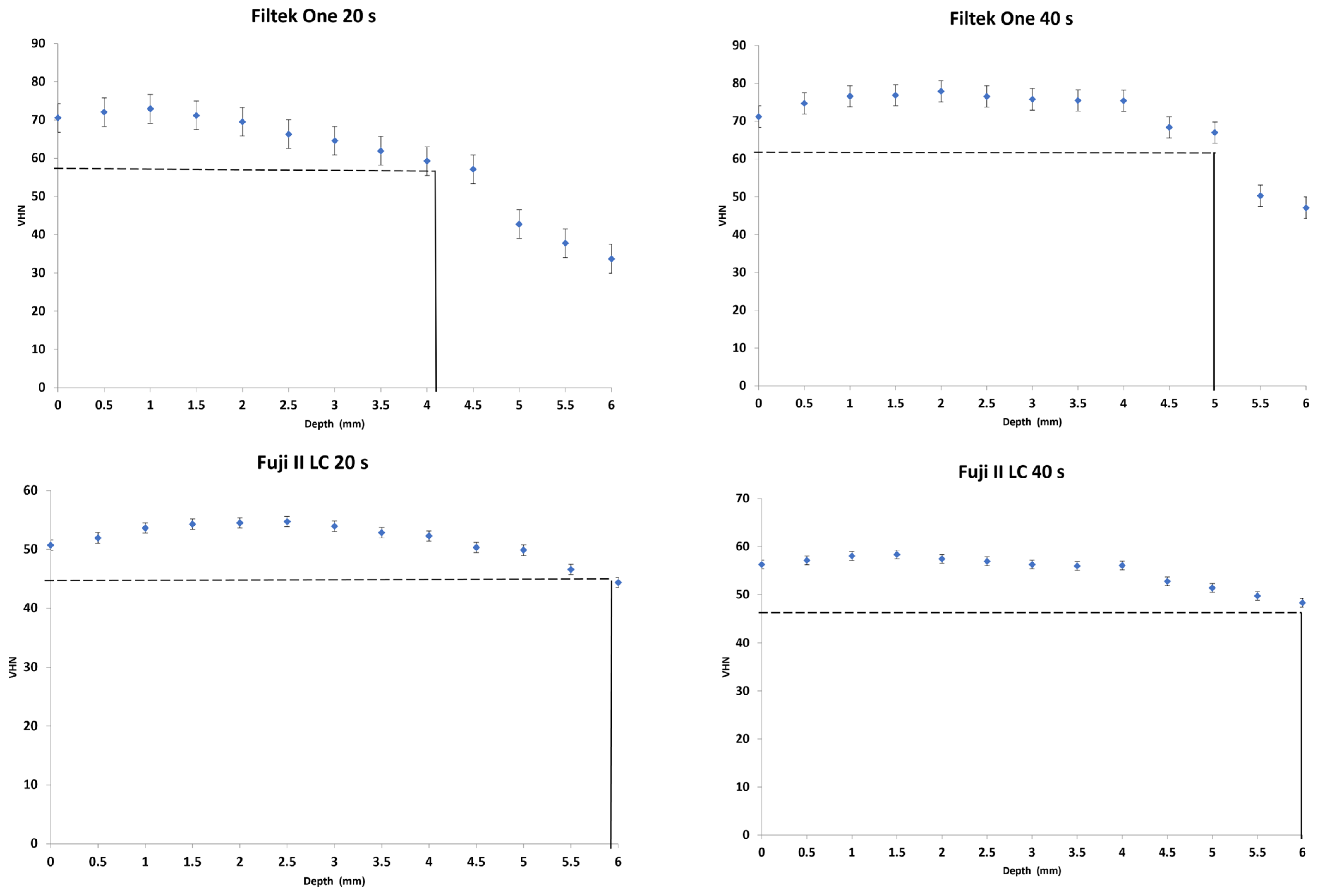
3.1. Depth of Cure
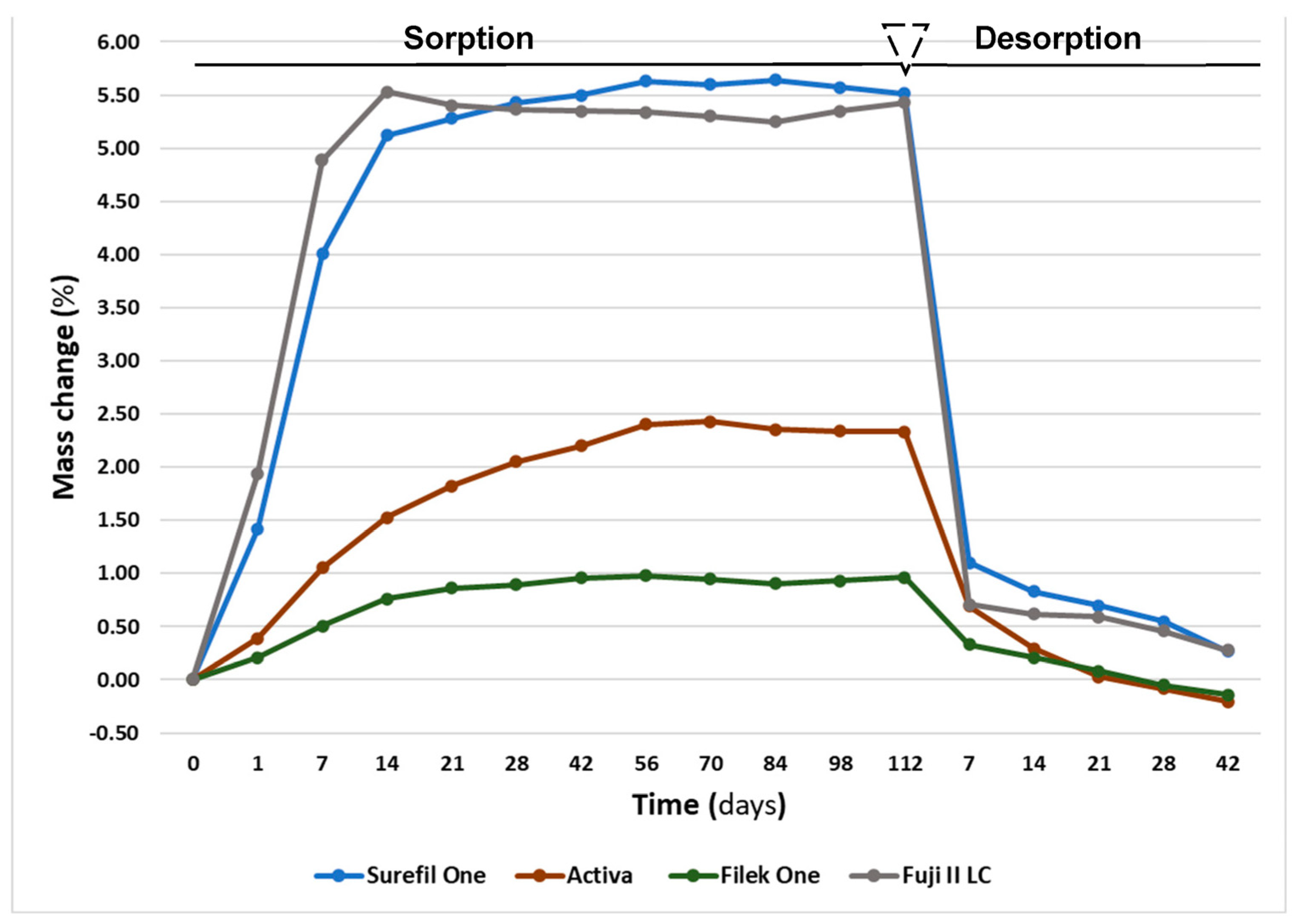
3.2. Sorption and Solubility
4. Discussion
4.1. Depth of Cure
4.2. Water Sorption and Solubility
5. Conclusions
- Surefil One achieved a 6 mm depth of cure whereas Activa showed 4 mm.
- Increasing irradiation time produced significantly higher VHN values yet showed no effect on the depth of cure of dual-cured bulk-fill materials.
- Both dual-cured bulk-fill materials exhibited higher mass change and water sorption values compared to the light-cured material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Rosa Rodolpho, P.A.; Rodolfo, B.; Collares, K.; Correa, M.B.; Demarco, F.F.; Opdam, N.J.; Cenci, M.S.; Moraes, R.R. Clinical performance of posterior resin composite restorations after up to 33 years. Dent. Mater. 2022, 38, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sarcev, B.P.; Balos, S.; Markovic, D.; Sarcev, I.; Vukcevic, M.; Zlatanovic, D.L.; Miletic, V. Effect of the Degree of Conversion on Mechanical Properties and Monomer Elution from Self-, Dual- and Light-Cured Core Composites. Materials 2021, 14, 5642. [Google Scholar] [CrossRef]
- Van Ende, A.; De Munck, J.; Lise, D.P.; Van Meerbeek, B. Bulk-fill composites: A review of the current literature. J. Adhes. Dent. 2017, 19, 95–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yap, A.U.J.; Pandya, M.; Toh, W.S. Depth of cure of contemporary bulk-fill resin-based composites. Dent. Mater. J. 2016, 35, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.F.; Vestphal, M.; Amaral, R.C.D.; Rodrigues, J.A.; Roulet, J.-F.; Roscoe, M.G. Efficiency of polymerization of bulk-fill composite resins: A systematic review. Braz. Oral Res. 2017, 31, 37–48. [Google Scholar] [CrossRef]
- Ilie, N.; Bucuta, S.; Draenert, M. Bulk-fill resin-based composites: An in vitro assessment of their mechanical performance. Oper. Dent. 2013, 38, 618–625. [Google Scholar] [CrossRef]
- Eweis, A.; Yap, A.; Yahya, N. Comparison of Flexural Properties of Bulk-fill Restorative/Flowable Composites and Their Conventional Counterparts. Oper. Dent. 2020, 45, 41–51. [Google Scholar] [CrossRef]
- Veloso, S.R.M.; Lemos, C.A.A.; de Moraes, S.L.; Vasconcelos, B.C.D.E.; Pellizzer, E.P.; Monteiro, G.Q.D.M. Clinical performance of bulk-fill and conventional resin composite restorations in posterior teeth: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Vandewalker, J.P.; Casey, J.A.; Lincoln, T.A.; Vandewalle, K.S. Properties of dual-cure, bulk-fill composite resin restorative materials. Gen. Dent. 2016, 64, 68–73. [Google Scholar] [PubMed]
- Wang, R.; Liu, H.; Wang, Y. Different depth-related polymerization kinetics of dual-cure, bulk-fill composites. Dent. Mater. 2019, 35, 1095–1103. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y. Depth-dependence of degree of conversion and microhardness for dual-cure and light-cure composites. Oper. Dent. 2020, 45, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Nakano, E.; de Souza, A.; Boaro, L.; Catalani, L.; Braga, R.; Gonçalves, F. Polymerization Stress and Gap Formation of Self-adhesive, Bulk-fill and Flowable Composite Resins. Oper. Dent. 2020, 45, E308–E316. [Google Scholar] [CrossRef]
- Koutroulis, A.; Kuehne, S.A.; Cooper, P.R.; Camilleri, J. The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci. Rep. 2019, 9, 19019. [Google Scholar] [CrossRef]
- ISO 4049:2009; Dentistry Polymer Based Restorative Materials. International Organization for Standarization: Geneva, Switzerland, 2009.
- Ferracane, J.; Hilton, T.; Stansbury, J.; Watts, D.; Silikas, N.; Ilie, N.; Heintze, S.; Cadenaro, M.; Hickel, R. Academy of Dental Materials guidance—Resin composites: Part II—Technique sensitivity (handling, polymerization, dimensional changes). Dent. Mater. 2017, 33, 1171–1191. [Google Scholar] [CrossRef] [PubMed]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Silikas, N.; Watts, D. Post-cure depth of cure of bulk fill dental resin-composites. Dent. Mater. 2014, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Miletic, V.; Pongprueksa, P.; De Munck, J.; Brooks, N.R.; Van Meerbeek, B. Curing characteristics of flowable and sculptable bulk-fill composites. Clin. Oral Investig. 2017, 21, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Alshabib, A.; Silikas, N.; Watts, D.C. Hardness and fracture toughness of resin-composite materials with and without fibers. Dent. Mater. 2019, 35, 1194–1203. [Google Scholar] [CrossRef]
- Al Sunbul, H.; Silikas, N.; Watts, D.C. Surface and bulk properties of dental resin- composites after solvent storage. Dent. Mater. 2016, 32, 987–997. [Google Scholar] [CrossRef]
- Al Sunbul, H.; Silikas, N.; Watts, D.C. Resin-based composites show similar kinetic profiles for dimensional change and recovery with solvent storage. Dent. Mater. 2015, 31, e201–e217. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Silikas, N.; Zhang, Z.-T.; Watts, D.C. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dent. Mater. 2011, 27, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Pulpdent Corp. ACTIVA BioACTIVE A Closer Look at BioACTIVE Materials. Available online: www.pulpdent.com (accessed on 7 April 2023).
- Dentsply Sirona. Dentsply Sirona. Surefil OneTM. Inf Sheet [Internet]. pp. 1–59. Available online: www.dentsplysirona.com (accessed on 13 March 2023).
- AlShaafi, M.M. Factors affecting polymerization of resin-based composites: A literature review. Saudi Dent. J. 2017, 29, 48–58. [Google Scholar] [CrossRef]
- Al Nahedh, H.; Al-Senan, D.; Alayad, A. The Effect of Different Light-curing Units and Tip Distances on the Polymerization Efficiency of Bulk-fill Materials. Oper. Dent. 2022, 47, E197–E210. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, M.Q.; Michaud, P.L.; Sullivan, B.; Labrie, D.; AlShaafi, M.M.; Price, R.B. Effect of high irradiance on depth of cure of a conventional and a bulk fill resin-based composite. Oper. Dent. 2015, 40, 662–672. [Google Scholar] [CrossRef]
- Zorzin, J.; Maier, E.; Harre, S.; Fey, T.; Belli, R.; Lohbauer, U.; Petschelt, A.; Taschner, M. Bulk-fill resin composites: Polymerization properties and extended light curing. Dent. Mater. 2015, 31, 293–301. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef]
- Shah, P.K.; Stansbury, J.W. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent. Mater. 2014, 30, 586–593. [Google Scholar] [CrossRef]
- Romano, B.d.C.; Soto-Montero, J.; Rueggeberg, F.A.; Giannini, M. Effects of extending duration of exposure to curing light and different measurement methods on depth-of-cure analyses of conventional and bulk-fill composites. Eur. J. Oral Sci. 2020, 128, 336–344. [Google Scholar] [CrossRef] [PubMed]
- GC Corporation. GC America|GC Fuji II LC® CAPSULE-Light-Cured Restorative n.d. Available online: www.gc.dental.com (accessed on 17 March 2023).
- Daabash, R.; Alshabib, A.; Alqahtani, M.Q.; Price, R.B.; Silikas, N.; Alshaafi, M.M. Ion releasing direct restorative materials: Key mechanical properties and wear. Dent. Mater. 2022, 38, 1866–1877. [Google Scholar] [CrossRef] [PubMed]
- Mount, G.; Patel, C.; Makinson, O. Resin modified glass-ionomers: Strength, cure depth and translucency. Aust. Dent. J. 2002, 47, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, K.-H.; Kwon, T.-Y. Setting Reaction of Dental Resin-Modified Glass Ionomer Restoratives as a Function of Curing Depth and Postirradiation Time. J. Spectrosc. 2015, 2015, 462687. [Google Scholar] [CrossRef]
- Yli-Urpo, H.; Lassila, L.V.; Närhi, T.; Vallittu, P.K. Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent. Mater. 2005, 21, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Makanjuola, J.; Deb, S. Chemically Activated Glass-Ionomer Cements as Bioactive Materials in Dentistry: A Review. Prosthesis 2023, 5, 327–345. [Google Scholar] [CrossRef]
- de Mendonça, B.C.; Soto-Montero, J.R.; de Castro, E.F.; Pecorari, V.G.A.; Rueggeberg, F.A.; Giannini, M. Flexural strength and microhardness of bulk-fill restorative materials. J. Esthet. Restor. Dent. 2021, 33, 628–635. [Google Scholar] [CrossRef]
- Hughes, K.; Powell, K.; Hill, A.; Tantbirojn, D.; Versluis, A. Delayed photoactivation of dual-cure composites: Effect on cuspal flexure, depth-of-cure, and mechanical properties. Oper. Dent. 2019, 44, E97–E104. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Bjelovucic, R.; Skenderovic, H.; Gamulin, O.; Tarle, Z. Curing potential of experimental resin composites with systematically varying amount of bioactive glass: Degree of conversion, light transmittance and depth of cure. J. Dent. 2018, 75, 113–120. [Google Scholar] [CrossRef]
- da Silva, E.M.; Gonçalves, L.; Guimarães, J.G.A.; Poskus, L.T.; Fellows, C.E. The diffusion kinetics of a nanofilled and a midifilled resin composite immersed in distilled water, artificial saliva, and lactic acid. Clin. Oral Investig. 2011, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2009, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Par, M.; Macan, M.; Klarić, N.; Plazonić, I.; Tarle, Z. Aging-Dependent Changes in Mechanical Properties of the New Generation of Bulk-Fill Composites. Materials 2022, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Latta, M.A.; Tsujimoto, A.; Takamizawa, T.; Barkmeier, W.W. Enamel and Dentin Bond Durability of Self-Adhesive Restorative Materials. J. Adhes. Dent. 2020, 22, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Ahmed, M.; Zhang, F.; Mercelis, B.; Van Landuyt, K.L.; Huang, C.; Van Meerbeek, B. Structural/Chemical Characterization and Bond Strength of a New Self-Adhesive Bulk-fill Restorative. J. Adhes. Dent. 2020, 22, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-J.; Silikas, N.; Zhang, Z.-T.; Watts, D.C. Diffusion and concurrent solubility of self-adhering and new resin–matrix composites during water sorption/desorption cycles. Dent. Mater. 2010, 27, 197–205. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; de Oliveira Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Müller, J.A.; Rohr, N.; Fischer, J. Evaluation of ISO 4049: Water sorption and water solubility of resin cements. Eur. J. Oral Sci. 2017, 125, 141–150. [Google Scholar] [CrossRef]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Bjelovucic, R.; Marovic, D.; Schmalz, G.; Gamulin, O.; Tarle, Z. Long-term water sorption and solubility of experimental bioactive composites based on amorphous calcium phosphate and bioactive glass. Dent. Mater. J. 2019, 38, 555–564. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M. Sorption of water, ethanol or ethanol/water solutions by light-cured dental dimethacrylate resins. Dent. Mater. 2011, 27, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- de Brito, O.; de Oliveira, I.; Monteiro, G. Hydrolytic and Biological Degradation of Bulk-fill and Self-adhering Resin Composites. Oper. Dent. 2019, 44, E223–E233. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.; Berzins, D.; Nicholson, J. Long-Term Water Balance Evaluation in Glass Ionomer Restorative Materials. Materials 2022, 15, 807. [Google Scholar] [CrossRef] [PubMed]
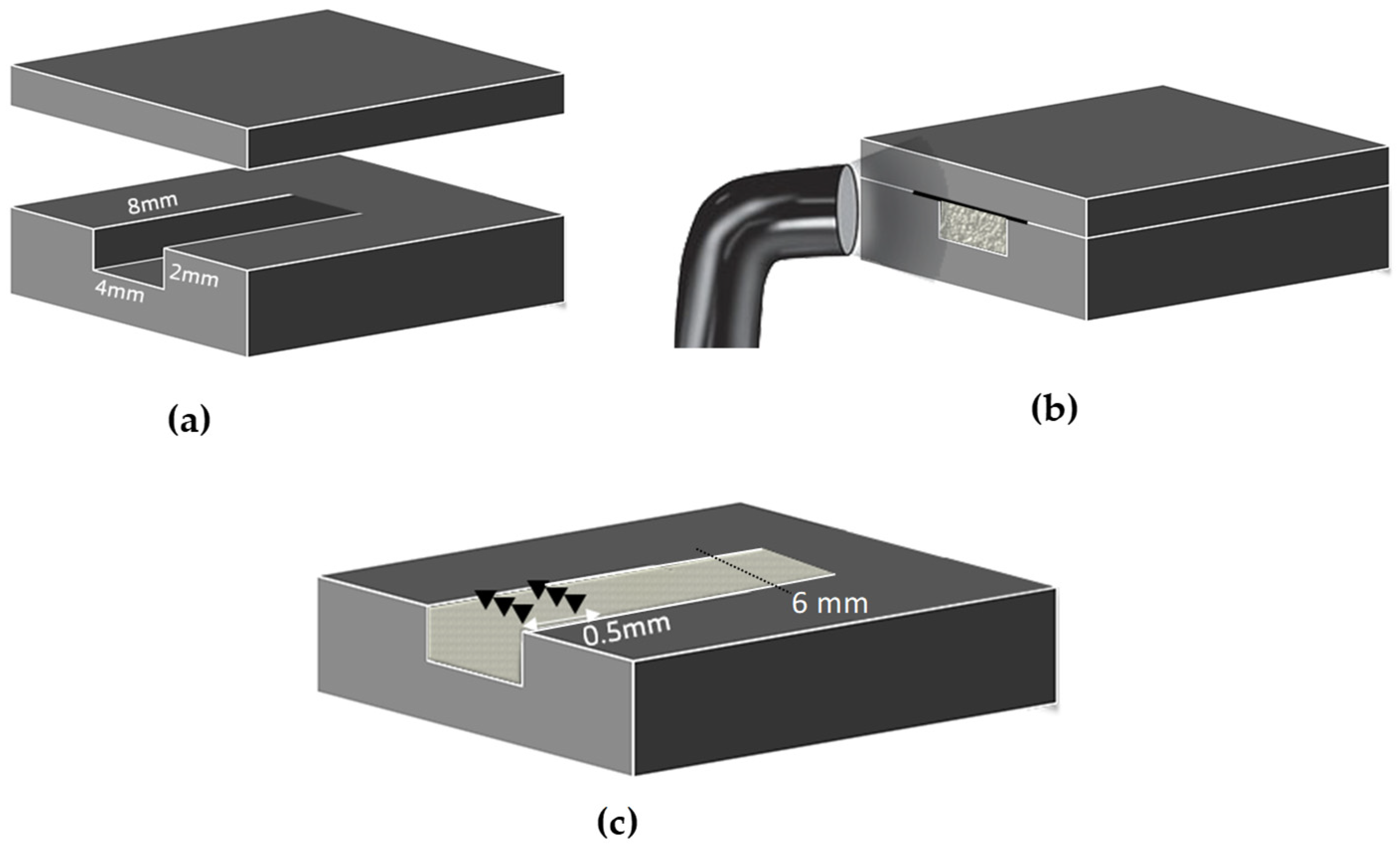
| Material | Manufacturer | Lot Number | Resin Matrix | Filler Type | Filler Load wt% |
|---|---|---|---|---|---|
| Surefil One (Dual-cured) | Dentsply Sirona; Konstanz, Germany | 2129000667 | Modified polyacids (MOPOS), bifunctional acrylate, acrylic acid, BADEP, camphorquinone, self-cure initiator, stabilizer | Aluminum-phoshor-strontium-sodium- fluoro-silicate glass, highly dispersed silicon dioxide, ytterbium fluoride | 77% |
| Activa Bioactive-Restorative (Dual-cured) | Pulpdent, Watertown, MA, USA | 215573 | Diurethane modified with hydrogenated polybutadiene, methacrylate monomers, modified polyacrylic acid | Bioactive glass, silica, sodium fluoride | 56% |
| Filtek One Bulk-fill (Light-cured) | 3M ESPE, St. Paul, MN, USA | NE19225 | AUDMA, UDMA, diurethane-DMA, and DDDMA, AFM, camphorquinone | 20 nm silica, 4–11 nm zirconia, cluster Zr-silica, 100 nm ytterbium trifluoride | 76.5% |
| Fuji II LC (Dual-cured) | GC, Tokyo, Japan | 225481A | HEMA, polyacrylic acid, UDMA, dimethacrylate | Alumino-fluoro-silicate glass | 58% |
| Material | Irradiation Time | |||||
|---|---|---|---|---|---|---|
| 20 s | 40 s | |||||
| Max. VHN Mean (SD) | VHN at 80% of Max. VHN Mean (SD) | Depth (mm) at 80% of Max. VHN Mean (SD) | Max. VHN Mean (SD) | VHN at 80% of Max. VHN Mean (SD) | Depth (mm) at 80% of Max. VHN Mean (SD) | |
| Surefil One | 63.3 (0.5) a,A | 50.6 (0.4) a,A | 5.8 (0.4) a,A | 65.1 (0.6) a,B | 52.1 (0.5) a,B | 5.9 (0.4) a,B |
| Activa | 37.8 (0.7) b,A | 30.3 (0.6) b,A | 4.2 (0.2) b,A | 38.4 (0.8) b,A | 30.7 (0.6) b,A | 4.2 (0.3) b,A |
| Filtek One | 72.8 (0.9) c,A | 58.2 (0.7) c,A | 4.1 (0.2) b,A | 78.1 (0.7) c,B | 62.5 (0.5) c,B | 5.0 (0.0) c,B |
| Fuji II LC | 55.4 (0.4) d,A | 44.3 (0.3) d,A | 5.9 (0.3) a,A | 58.6 (0.5) d,B | 46.9 (0.4) d,B | 6.0 (0.0) a,A |
| Materials | Mass Change % | Wso (µg/mm3) | Sol (µg/mm3) |
|---|---|---|---|
| Surefil One | 5.5 (0.3) a | 101.9 (1.5) a | −5.1 (1.0) a |
| Activa | 2.3 (0.1) b | 44.2 (1.3) b | 5.4 (0.4) b |
| Filtek One | 1.0 (0.0) c | 21.6 (0.8) c | 2.8 (0.9) c |
| Fuji II LC | 5.4 (0.1) a | 108.7 (1.5) d | −5.8 (1.6) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, B.; Alshabib, A.; Awliya, W. The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials. Materials 2023, 16, 6673. https://doi.org/10.3390/ma16206673
Alzahrani B, Alshabib A, Awliya W. The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials. Materials. 2023; 16(20):6673. https://doi.org/10.3390/ma16206673
Chicago/Turabian StyleAlzahrani, Bashayer, Abdulrahman Alshabib, and Wedad Awliya. 2023. "The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials" Materials 16, no. 20: 6673. https://doi.org/10.3390/ma16206673
APA StyleAlzahrani, B., Alshabib, A., & Awliya, W. (2023). The Depth of Cure, Sorption and Solubility of Dual-Cured Bulk-Fill Restorative Materials. Materials, 16(20), 6673. https://doi.org/10.3390/ma16206673







