4D Printing Shape-Morphing Hybrid Biomaterials for Advanced Bioengineering Applications
Abstract
:1. Introduction
2. Stimuli-Responsive Materials in 4D Printing
2.1. Temperature-Responsive Materials
2.2. Humidity-Responsive Materials
2.3. pH-Responsive Materials
2.4. Light-Responsive Materials
2.5. Electric Field Responsive Materials
2.6. Magnetic Field Responsive Materials
2.7. Shape Memory Polymers
3. Achievable 4D Transformations
4. Mathematical Modeling for 4D Printing
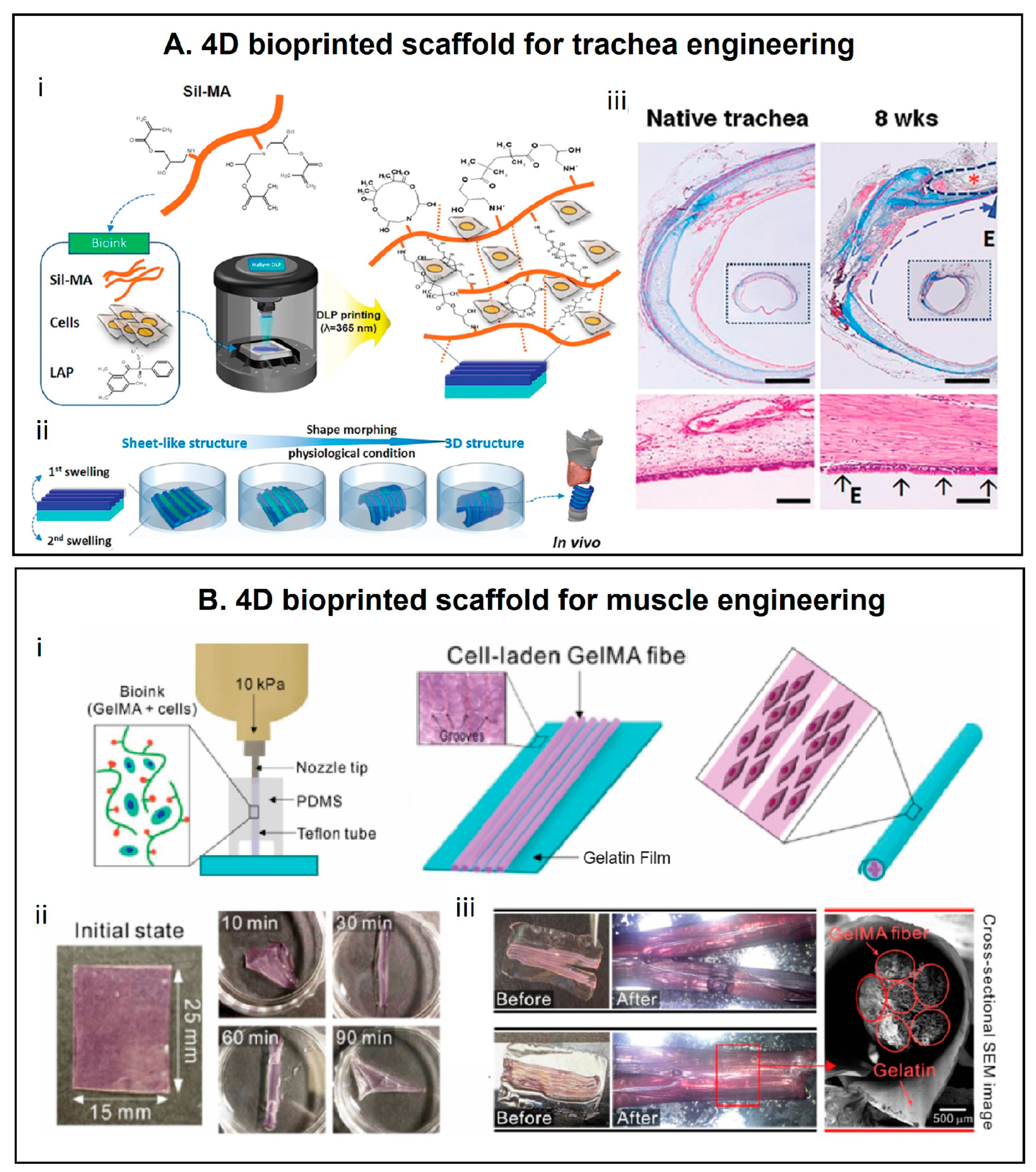
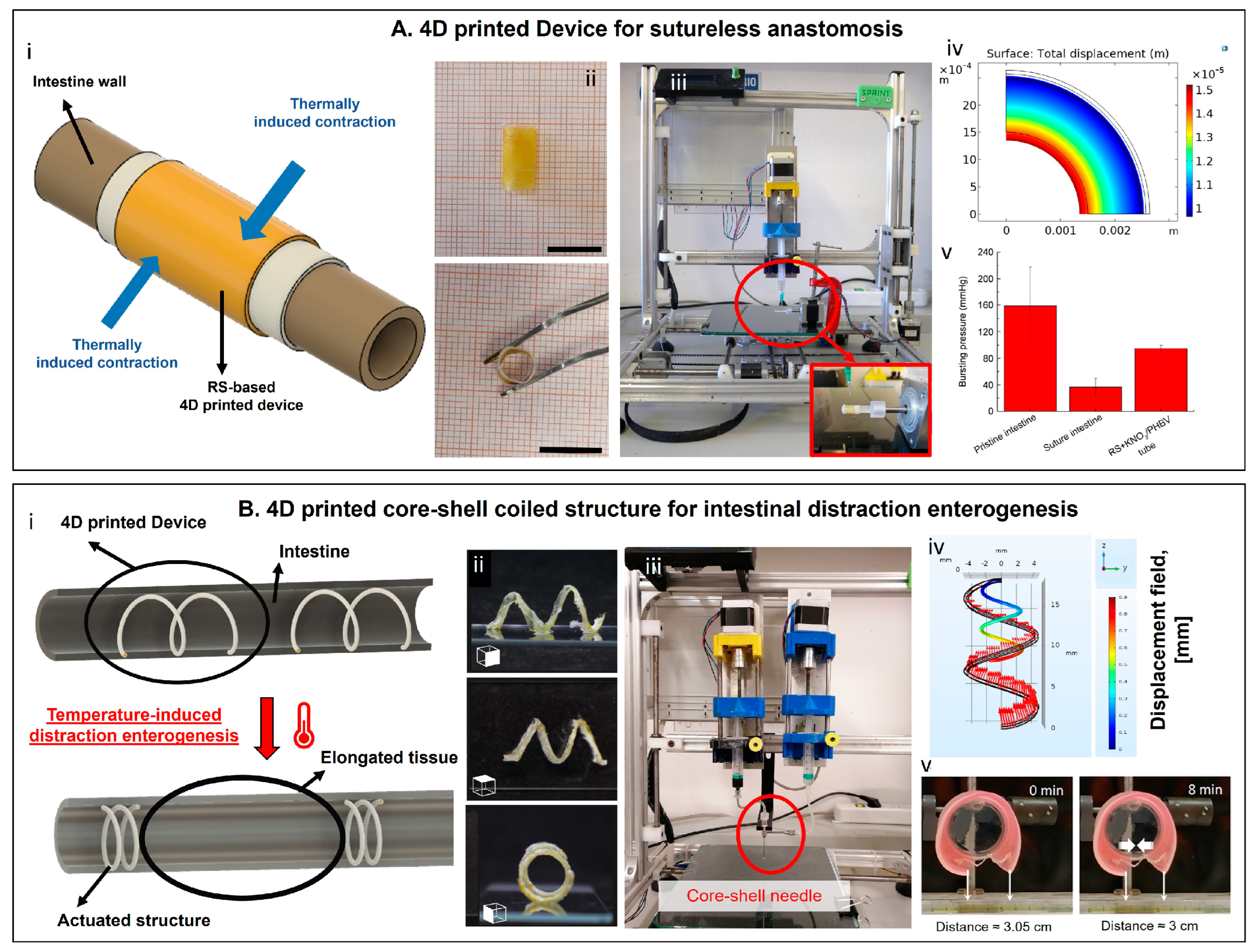
5. 4D Printing in the Biomedical Field
5.1. Bioactuators
5.2. Tissue Engineering
5.3. Medical Devices
5.4. Drug Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.-U.; Boydston, A.J.; Nelson, A. Stimuli-responsive materials in additive manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef]
- Bonatti, A.F.; Fortunato, G.M.; De Maria, C.; Vozzi, G. Bioprinting technologies: An overview. Bioprinting 2022, 19–49. [Google Scholar] [CrossRef]
- De Maria, C.; Vozzi, G.; Moroni, L. Multimaterial, Heterogeneous, and multicellular three-dimensional bioprinting. Trends Biotechnol. 2017, 42, 578–584. [Google Scholar] [CrossRef]
- Micalizzi, S.; Russo, L.; Giacomelli, C.; Montemurro, F.; De Maria, C.; Nencioni, M.; Marchetti, L.; Trincavelli, M.L.; Vozzi, G. Multimaterial and multiscale scaffold for engineering enthesis organ. Int. J. Bioprint. 2023, 9, 296–313. [Google Scholar] [CrossRef]
- Cendrero, A.; Fortunato, G.; Munoz-Guijosa, J.; De Maria, C.; Diaz Lantada, A. Benefits of Non-Planar Printing Strategies Towards Eco-Efficient 3D Printing. Sustainability 2021, 13, 1599. [Google Scholar] [CrossRef]
- Fortunato, G.M.; Batoni, E.; Bonatti, A.F.; Vozzi, G.; De Maria, C. Surface reconstruction and tissue recognition for robotic-based in situ ioprinting. Bioprinting 2022, 26, e00195. [Google Scholar] [CrossRef]
- Agarwal, T.; Hann, S.J.; Chiesa, I.; Cui, H.; Celikkin, N.; Micalizzi, S.; Barbetta, A.; Costantini, M.; Esworthy, T.; Zhang, L.G.; et al. 4D printing in biomedical applications: Emerging trends and technologies. J. Mater. Chem. B 2021, 9, 7608–7632. [Google Scholar] [CrossRef]
- Kuang, X.; Roach, D.J.; Wu, J.; Hamel, C.M.; Ding, Z.; Wang, T.; Dunn, M.L.; Qi, H.J. Advances in 4D printing: Materials and applications. Adv. Funct. Mater. 2019, 29, 1805290. [Google Scholar] [CrossRef]
- Tibbits, S. 4D printing: Multi-material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Bodaghi, M.; Noroozi, R.; Zolfagharian, A.; Fotouhi, M.; Norouzi, S. 4D Printing Self-Morphing Structures. Materials 2019, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.-j.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2019, 20, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.P.; Mertens, J.A.; Bahubalendruni, M.V.A.R. Auxetic mechanical metamaterials and their futuristic developments: A state-of-art review. Mater. Today Commun. 2023, 34, 105285. [Google Scholar] [CrossRef]
- Bonatti, A.F.; Chiesa, I.; Micalizzi, S.; Vozzi, G.; De Maria, C. Bioprinting for bone tissue engineering. Minerva Orthop. 2021, 72, 376–394. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D Printable Graphene. Composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cao, Q.; Venton, B. 3D printing for customized carbon electrodes. Curr. Opin. Electrochem. 2023, 9, 101228. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, S.; Luque, R.; Han, S.; Hu, L.; Xu, G. Recent development of carbon electrode materials and their bioanalytical and environmental applications. Chem. Soc. Rev. 2016, 45, 715–752. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Micalizzi, S.; Díaz Lantada, A.; De Maria, C. Shape-memory actuators manufactured by dual extrusion multimaterial 3d printing of conductive and non-conductive filaments. Smart Mater. Struct. 2019, 28, 105025. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications -A review. Eur. J. Pharm. Biopharm. 2011, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.; Sow, W.; Tan, L.; Wu, Y.; Lai, Y.; Li, H. 4D Printing and Stimuli-responsive Materials in Biomedical Aspects. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Hossain, M.; Ramakrishna, S.; Umer, R. 3D/4D printing of cellulose nanocrystals-based biomaterials: Additives for sustainable applications. Int. J. Biol. Macromol. 2023, 251, 126287. [Google Scholar] [CrossRef] [PubMed]
- Bora, L.V.; Vadaliya, K.S.; Bora, N.V. Sustainable feedstocks for 4D printing: A review of biodegradable polymers and natural resources for stimuli-responsive manufacturing. Green Mater. 2023, 1–14. [Google Scholar]
- Lantada, A.D.; De Maria, C. Sustainable Open-Source Medical Devices Manufactured with Green Biomaterials And Accessible Resources. Curr. Opin. Biomed. Eng. 2023, 28, 100500. [Google Scholar] [CrossRef]
- Costa, P.D.C.; Costa, D.C.S.; Correia, T.R.; Gaspar, V.M.; Mano, J.F. Natural Origin Biomaterials for 4D Bioprinting Tissue-Like Constructs. Adv. Mater. Technol. 2021, 6, 2100168. [Google Scholar] [CrossRef]
- Ramezani, M.; Mohd Ripin, Z. 4D Printing in Biomedical Engineering: Advancements, Challenges, and Future Directions. J. Funct. Biomater. 2023, 14, 347. [Google Scholar] [CrossRef]
- Barrera, G.; Bruno, J.; Barron, T.; Allan, N. Negative thermal expansion. J. Condens. Matter Phys. 2005, 17, R217–R252. [Google Scholar] [CrossRef]
- Chiesa, I.; De Maria, C.; Ceccarini, M.R.; Mussolin, L.; Coletta, R.; Morabito, A.; Tonin, R.; Calamai, M.; Morrone, A.; Beccari, T.; et al. 3D Printing Silk-Based Bioresorbable Piezoelectric Self-Adhesive Holey Structures for In Vivo Monitoring on Soft Tissues. ACS Appl. Mater. Interfaces 2022, 14, 19253–19264. [Google Scholar] [CrossRef]
- Ciabattini, S.; Raggi, V.; Valentini, L.; Morabito, A. Silk Fibroin Hybrids for Biological Scaffolds with Adhesive Surface and Adaptability to the Target Tissue Change. Eurobiotech J. 2023, 7, 75–86. [Google Scholar] [CrossRef]
- Li, L.; Shan, H.; Yue, C.; Lam, Y.C.; Tam, K.; Hu, X.M. Thermally Induced Association and Dissociation of Methylcellulose in Aqueous Solutions. Langmuir 2002, 18, 7291–7298. [Google Scholar] [CrossRef]
- Castro, N.J.; Meinert, C.; Levett, P.; Hutmacher, D.W. Current developments in multifunctional smart materials for 3D/4D bioprinting. Curr. Opin. Biomed. Eng. 2017, 2, 67–75. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Hydrogels. In Polymer Science and Nanotechnology Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Quesada-Pérez, M.; Maroto, A.; Forcada, J.; Hidalgo-Alvarez, R. Gel Swelling Theories: The Classical Formalism and Recent Approaches. Front. Med. Technol. 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Jamal, M.; Kadam, S.S.; Xiao, R.; Jivan, F.; Onn, T.-M.; Fernandes, R.; Nguyen, T.D.; Gracias, D.H. Bio-Origami Hydrogel Scaffolds Composed of Photocrosslinked PEG Bilayers. Adv. Healthc. Mater. 2013, 2, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, I.; Ligorio, C.; Bonatti, A.F.; De Acutis, A.; Smith, A.M.; Saiani, A.; Vozzi, G.; De Maria, C. Modeling the Three-Dimensional Bioprinting Process of β-Sheet Self-Assembling Peptide Hydrogel Scaffolds. Front. Med. Technol. 2020, 2, 1142–1150. [Google Scholar] [CrossRef]
- Dai, S.; Ravi, P.; Tam, K. pH-Responsive polymers: Synthesis, properties and applications. Front. Med. Technol. 2008, 4, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Peralta Ramos, M.L.; González, J.A.; Fabian, L.; Javier Pérez, C.; Villanueva, M.E.; Copello, G.J. Sustainable and smart keratin hydrogel with pH-sensitive swelling and enhanced mechanical properties. Mater. Sci. Eng. C 2017, 78, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Banwell, E.F.; Abelardo, E.S.; Adams, D.J.; Birchall, M.A.; Corrigan, A.; Donald, A.M.; Kirkland, M.; Serpell, L.C.; Butler, M.F.; Woolfson, D.N. Rational design and application of responsive α-helical peptide. Nat. Mater. 2009, 8, 596–600. [Google Scholar] [CrossRef]
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration. Gels 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.M.; Li, N.; Andrade, M.; Fang, S.; Oh, J.; Spinks, G.; Kozlov, M.; Haines, C.; Suh, D.; Foroughi, J.; et al. Electrically, Chemically, and Photonically Powered Torsional and Tensile Actuation of Hybrid Carbon Nanotube Yarn Muscles. Science 2012, 338, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ravi, P.; Tam, K.C. Thermo- and photo-responsive polymeric systems. Soft Matter 2009, 5, 2513–2533. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Sowan, N.; Tumbic, J.A.; Bowman, C.N.; Mather, P.T.; Qi, H.J. Photo-induced bending in a light-activated polymer laminated composite. Soft Matter 2015, 11, 2673–2682. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Chi-wei Lo, C.-W.; Jiang, H. Reversible white-light actuation of carbon nanotube incorporated liquid crystalline elastomer nanocomposites. Soft Matter 2011, 7, 7511–7516. [Google Scholar] [CrossRef]
- Jeong, H.J.; Woo, B.H.; Kim, N.; Jun, J.C. Multicolor 4D printing of shape-memory polymers for light-induced selective heating and remote actuation. Sci. Rep. 2020, 10, 6258. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Zhang, H.; Schweizerhof, S.; Schulte, M.F.; Mourran, A.; Möller, M. 4D Printing of a Light-Driven Soft Actuator with Programmed Printing Density. ACS Appl. Mater. Interfaces 2020, 12, 12176–12185. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive smart polymers for biomedical applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef]
- Borisova, O.V.; Billon, L.; Richter, R.P.; Reimhult, E.; Borisov, O.V. PH- and Electro-Responsive Properties of Poly(acrylic acid) and Poly(acrylic acid)-block-poly(acrylic acid-grad-styrene) Brushes Studied by Quartz Crystal Microbalance with Dissipation Monitoring. Langmuir 2015, 31, 7684–7694. [Google Scholar] [CrossRef] [PubMed]
- Morouço, P.; Azimi, B.; Milazzo, M.; Mokhtari, F.; Fernandes, C.; Reis, D.; Danti, S. Four-dimensional (Bio-)printing: A review on stimuli-responsive mechanisms and their biomedical suitability. Appl. Sci. 2020, 10, 9143. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Stimuli-responsive polymers and colloids under electric and magnetic fields. Polymer 2014, 6, 2803–2818. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, R.; Tang, W.; Yang, F.; Tian, H.; Peng, S.; Pan, H.; Shuai, C. Structural and Functional Adaptive Artificial Bone: Materials, Fabrications, and Properties. Adv. Funct. Mater. 2023, 33, 2214726. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, Z.; Huo, Y.; Sun, L.; Chen, S.; Liu, Z.; He, C.; Bi, X.; Fanc, X.; You, Z. A biodegradable functional water-responsive shape memory polymer for biomedical applications. J. Mater. Chem. 2019, 7, 123–132. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, Z.; Peng, S.; Shuai, Y.; Chen, Y.; Zeng, D.; Feng, P. Water-responsive shape memory thermoplastic polyurethane scaffolds triggered at body temperature for bone defect repair. Mater. Chem. Front. 2022, 6, 1456–1469. [Google Scholar] [CrossRef]
- Nam, S.; Pei, E. A taxonomy of shape-changing behavior for 4D printed parts using shape-memory polymers. Prog. Addit. Manuf. 2019, 4, 167–184. [Google Scholar] [CrossRef]
- Bittolo Bon, S.; Chiesa, I.; Morselli, D.; Degli Esposti, M.; Fabbri, P.; De Maria, C.; Foggi Viligiardi, T.; Morabito, A.; Giorgi, G.; Valentini, L. Printable smart 3D architectures of regenerated silk on poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Mater. Des. 2021, 201, 109492. [Google Scholar] [CrossRef]
- De Maria, C.; Chiesa, I.; Morselli, D.; Ceccarini, M.R.; Bittolo Bon, S.; Degli Esposti, M.; Fabbri, P.; Morabito, A.; Beccari, T.; Valentini, L. Biomimetic tendrils by four dimensional printing bimorph springs with torsion and contraction properties based on bio-compatible graphene/silk fibroin and poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Adv. Funct. Mater. 2021, 31, 2105665. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Hu, G. Smart three-dimensional light- weight structure triggered from a thin composite sheet via 3D printing technique. Sci. Rep. 2016, 6, 22431. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Kim, S.; Seo, Y.; Yeon, Y.; Lee, Y.J.; Park, H.; Sultan, M.; Lee, J.M.; Lee, J.; Lee, O.J.; Hong, H.; et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 2020, 260, 120281. [Google Scholar] [CrossRef]
- Yang, G.H.; Kim, W.; Kim, J.; Kim, G.H. A skeleton muscle model using GelMA-based cell-aligned bioink processed with an electric-field assisted 3D/4D bioprinting. Theranostics 2021, 11, 48–63. [Google Scholar] [CrossRef]
- Ge, Q.; Qi, J.H.; Dunn, M.L. Active materials by four-dimension printing. Appl. Phys. Lett. 2013, 103, 131901. [Google Scholar] [CrossRef]
- Trujillo-Miranda, M.; Apsite, I.; Agudo, J.A.R.; Constante, G.; Ionov, L. 4D Biofabrication of Mechanically Stable Tubular Constructs Using Shape Morphing Porous Bilayers for Vascularization Application. Macromol. Biosci. 2023, 23, e2200320. [Google Scholar] [CrossRef]
- Lai, J.; Ye, X.; Liu, J.; Wang, C.; Li, J.; Wang, X.; Ma, M.; Wang, M. 4D printing of highly printable and shape morphing hydrogels composed of alginate and methylcellulose. Mater. Des. 2021, 205, 109699. [Google Scholar] [CrossRef]
- Wang, W.; Yao, L.; Zhang, T.; Cheng, C.Y.; Levine, D.; Ishii, H. Transformative appetite: Shape-changing food transforms from 2D to 3D by water interaction through cooking. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems, Denver, CO, USA, 6–11 May 2017; Association for Computing Machinery: New York, NY, USA, 2017; pp. 6123–6132. [Google Scholar]
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.J.; Dunn, M.L. Direct 4D printing via active composite materials. Sci. Adv. 2017, 3, e1602890. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, J.N.; Shea, K. Redeployable, 4D printed wave spring actuators. Mater. Design 2023, 232, 112163. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, C.; Sattari, K.; Ling, Y.; Su, J.-W.; Yan, Z.; Lin, J. 4D Printing Elastic Composites for Strain-Tailored Multistable Shape Morphing. ACS Appl. Mater. Interfaces 2021, 13, 12719–12725. [Google Scholar] [CrossRef]
- Tibbits, S.; McKnelly, C.; Olguin, C.; Dikovsky, D.; Hirsch, S. 4D printing and universal transformation. In Proceedings of the 34th Annual Conference of the Association for Computer Aided Design in Architecture, Los Angeles, CA, USA, 23–25 October 2014; pp. 539–548. [Google Scholar]
- Chen, T.; Mueller, J.; Shea, K. Integrated Design and Simulation of Tunable, Multi-State Structures Fabricated Monolithically with Multi-Material 3D Printing. Sci. Rep. 2017, 7, 45671. [Google Scholar] [CrossRef]
- Pandini, S.; Inverardi, N.; Scalet, G.; Battini, D.; Bignotti, F.; Marconi, S.; Auricchio, F. Shape memory response and hierarchical motion capabilities of 4D printed auxetic structures. Mech. Res. Commun. 2020, 103, 103463. [Google Scholar] [CrossRef]
- Yamamura, S.; Iwase, E. Hybrid hinge structure with elastic hinge on self-folding of 4D printing using a fused deposition modeling 3D printer. Mater. Design 2021, 203, 109605. [Google Scholar] [CrossRef]
- Howell, L.L. Compliant Mechanisms. In 21st Century Kinematics: The 2012 NSF Workshop; McCarthy, J., Ed.; Springer: London, UK, 2013; pp. 189–216. [Google Scholar]
- Kim, S.W.; Kim, D.Y.; Roh, H.H.; Kim, H.S.; Lee, J.W.; Lee, K.Y. Three-Dimensional Bioprinting of Cell-Laden Constructs Using Polysaccharide-Based Self-Healing Hydrogels. Biomacromolecules 2019, 20, 1860–1866. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, K.; Dunn, C.K.; Wu, J.; Li, V.C.F.; Qi, H.J. 3D Printing of Highly Stretchable, Shape-Memory, and Self-Healing Elastomer toward Novel 4D Printing. Appl. Mater. Interfaces 2018, 10, 7381–7388. [Google Scholar] [CrossRef]
- Momeni, F.; Hassani, N.S.M.M.; Liu, X.; Ni, J. A review of 4D printing. Mater. Design 2017, 122, 42–79. [Google Scholar] [CrossRef]
- Yue, W.; Cui, H.; Esworthy, T.; Mei, D.; Wang, Y.; Zhang, L.G. Emerging 4D Printing Strategies for Next-Generation Tissue Regeneration and Medical. Adv. Mater. 2022, 34, 2109198. [Google Scholar]
- Biswas, M.C.; Chakraborty, S.; Bhattacharjee, A.; Mohammed, Z. 4D Printing of Shape Memory Materials for Textiles: Mechanism, Mathematical Modeling, and Challenges. Adv. Funct. Mater. 2021, 31, 2100257. [Google Scholar] [CrossRef]
- Yu, K.; Xie, T.; Leng, J.; Ding, Y.; Qi, H.J. Mechanisms of multi-shape memory effects and associated energy release in shape memory polymers. Soft Matter 2012, 8, 5687–5695. [Google Scholar] [CrossRef]
- Friedenthal, S.; Moore, A.; Steiner, R. Integrating SysML into a Systems Development Environment. In A Practical Guide to SysML; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Momeni, F.; Ni, J. Laws of 4D Printing. Engineering 2020, 6, 1035–1055. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M. Mechanisms of the multi-shape memory effect and temperature memory effect in shape memory polymers. Soft Matter 2010, 6, 4403–4406. [Google Scholar] [CrossRef]
- Sossou, G.; Demoly, F.; Belkebir, H.; Qi, H.J.; Gomes, S.; Montavon, G. Design for 4D printing: Modeling and computation of smart materials distributions. Mater. Des. 2019, 181, 108074. [Google Scholar] [CrossRef]
- Hiller, J.; Lipson, H. Dynamic Simulation of Soft Multimaterial 3D-Printed Objects. Soft Robot. 2014, 1, 88–101. [Google Scholar] [CrossRef]
- Dara, A.; Bahubalendruni, M.V.A.R.; Mertens, A.J.; Balamurali, G. Numerical and experimental investigations of novel nature inspired open lattice cellular structures for enhanced stiffness and specific energy absorption. Mater. Today Commun. 2022, 31, 103286. [Google Scholar]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef]
- Miriyev, A.; Stack, K.; Lipson, H. Soft material for soft actuators. Nat. Commun. 2017, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Carrico, J.D.; Tyler, T.; Leang, K.K. A comprehensive review of select smart polymeric and gel actuators for soft mechatronics and robotics applications: Fundamentals, freeform fabrication, and motion control. Int. J. Smart Nano Mater. 2017, 8, 144–213. [Google Scholar] [CrossRef]
- Tawk, C.; Panhuis, M.I.H.; Spinks, G.M.; Alici, G. Bioinspired 3d printable soft vacuum actuators for locomotion robots, grippers and artificial muscles. Soft Robot. 2018, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Pané, S.; Nelson, B.J. 4D printing and robotics. Sci. Robot. 2018, 3, 2–4. [Google Scholar] [CrossRef]
- Schaffner, M.; Faber, J.A.; Pianegonda, L.; Rühs, P.A.; Coulter, F.; Studart, A.R. 3D printing of robotic soft actuators with programmable bioinspired architectures. Nat. Commun. 2018, 9, 878. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Wang, M.; Liang, Y.; Ren, L.; Ren, L. Recent progress in 4D printing of stimuli-responsive polymeric materials. Sci. China Technol. Sci. 2020, 63, 532–544. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Erol, O.; Pantula, A.; Liu, W.; Jiang, Z.; Kobayashi, K.; Chatterjee, D.; Hibino, N.; Romer, L.H.; et al. Dual-Gel 4D Printing of Bioinspired Tubes. ACS Appl. Mater. Interfaces 2019, 11, 8492–8498. [Google Scholar] [CrossRef]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of miniaturized walking biological machines. Sci. Rep. 2012, 2, 857. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2017, 9, e012001. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, I.; De Maria, C.; Vozzi, G.; Gottardi, R. Three-dimensional and four-dimensional printing in otolaryngology. MRS Bull. 2023, 48, 676–687. [Google Scholar] [CrossRef]
- Cimmino, C.; Rossano, L.; Netti, P.A.; Ventre, M. Spatio-temporal control of cell adhesion: Toward programmable platforms to manipulate cell functions and fate. Front. Bioeng. Biotechnol. 2018, 6, 190. [Google Scholar] [CrossRef]
- Uto, K.; Tsui, J.H.; DeForest, C.A.; Kim, D.-H. Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog. Polym. Sci. 2017, 65, 53–82. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Chen, G.; Liu, C.; Ye, X.; Wang, S.; Dong, M.; Sun, M.; He, J.; Yu, X.; Ye, G.; et al. 4D printing of multi-responsive membrane for accelerated in vivo bone healing via remote regulation of stem cell fate. Adv. Funct. Mater. 2021, 31, 2103920. [Google Scholar] [CrossRef]
- Hwangbo, H.; Lee, H.; Roh, E.J.; Kim, W.; Joshi, H.P.; Kwon, S.Y.; Choi, U.Y.; Han, I.B.; Kim, G.H. Bone tissue engineering via application of a collagen/hydroxyapatite 4D-printed biomimetic scaffold for spinal fusion. Appl. Phys. Rev. 2021, 8, 021403. [Google Scholar] [CrossRef]
- Ding, A.; Lee, S.J.; Tang, R.; Gasvoda, K.L.; He, F.; Alsberg, E. 4D Cell-Condensate Bioprinting. Small 2022, 18, 2202196. [Google Scholar] [CrossRef]
- Luo, K.; Wang, L.; Wang, M.X.; Du, R.; Tang, L.; Yang, K.K.; Wang, Y.Z. 4D Printing of Biocompatible Scaffolds via In Situ Photo-crosslinking from Shape Memory Copolyesters. ACS Appl. Mater. Interfaces 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Study Group 1 of the Global Harmonization Task Force. In Definition of the Terms “Medical Device and In Vitro Diagnostic (IVD) Medical Device; GHTF, 2012; Available online: https://www.imdrf.org/sites/default/files/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n071-2012-definition-of-terms-120516.pdf (accessed on 6 October 2023).
- The European Parliament and the Council of the European Union. Regulation (Eu) 2017/745 of the European Parliament and Of the Council on Medical Devices; Official Journal of the European Union: Luxembourg, 2017. [Google Scholar]
- Javaid, M.; Haleem, A. 4D printing applications in medical field: A brief review. Clin. Epidemiol. Glob. Health 2019, 7, 317–321. [Google Scholar] [CrossRef]
- Lin, C.; Liu, L.; Liu, Y.; Leng, J. 4D Printing of Bioinspired Absorbable Left Atrial Appendage Occluders: A Proof-of-Concept Study. ACS Appl. Mater. Interfaces 2021, 13, 12668–12678. [Google Scholar] [CrossRef]
- Chiesa, I.; Bonatti, A.F.; De Acutis, A.; Fortunato, G.M.; Vozzi, G.; De Maria, C. 4D Printing in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Banerjee, S., Ed.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Masalehdan, T.; Kapsa, R.M.I.; Quigley, A.; Lalatsa, A.; Bruggeman, K.F.; Franks, S.J.; Williams, R.J.; Nisbet, D.R. Traction of 3D and 4D Printing in the Healthcare Industry: From Drug Delivery and Analysis to Regenerative Medicine. ACS Biomater. Sci. Eng. 2022, 8, 2764–2797. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Solis, D.M.; Czekanski, A. The effect of the printing temperature on 4D DLP printed pNIPAM hydrogels. Soft Matter. 2022, 18, 3422–3429. [Google Scholar] [CrossRef]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter. 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Wang, J.; Duggal, I.; Maniruzzaman, M.; Banerjee, S. Four-Dimensional Printed Construct from Temperature-Responsive Self-Folding Feedstock for Pharmaceutical Applications with Machine Learning Modeling. Pharmaceutics. 2023, 15, 1266. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Palazzi, V.; Salvati, R.; Chiesa, I.; De Maria, C.; Bonafoni, S.; Mezzanotte, P.; Codini, M.; Pacini, L.; Errante, F. Biomaterial Inks from Peptide-Functionalized Silk Fibers for 3D Printing of Futuristic Wound-Healing and Sensing Materials. Int. J. Mol. Sci. 2023, 24, 947. [Google Scholar] [CrossRef]
- Afami, M.E.; El Karim, I.; About, I.; Krasnodembskaya, A.D.; Laverty, G.; Lundy, F.T. Multicomponent peptide hydrogels as an innovative platform for cell-based tissue engineering in the dental pulp. Pharmaceutics 2021, 13, 1575. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R.; Kumar, L. Significance of 4D printing for dentistry: Materials, process, and potentials. J. Oral Biol. Craniofacial Res. 2022, 12, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ren, X.; Guo, T.; Cao, Z.; Sun, H.; Wang, C.; Wang, F.; Shu, Z.; Hao, J.; Gui, S. Controlled release of iodine from cross-linked cyclodextrin metal-organic frameworks for prolonged periodontal pocket therapy. Carbohydr. Polym. 2021, 267, 118187. [Google Scholar] [CrossRef]
- Suo, L.; Wu, H.; Wang, P.; Xue, Z.; Gao, J.; Shen, J. The improvement of periodontal tissue regeneration using a 3D–printed carbon nanotube/chitosan/sodium alginate composite scaffold. J. Biomed. Mater. Res. Part B 2023, 111, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.B.; Schulz-Siegmund, M. Utilizing 4D Printing to Design Smart Gastroretentive, Esophageal, and Intravesical Drug Delivery Systems. Adv. Healthc. Mater. 2023, 12, e2202631. [Google Scholar] [CrossRef]
- Chadwick, M.; Yang, C.; Liu, L.; Gamboa, C.M.; Jara, K.; Lee, H.; Sabaawy, H.E. Rapid Processing and Drug Evaluation in Glioblastoma Patient-Derived Organoid Models with 4D Bioprinted Arrays. iScience 2020, 23, 101365. [Google Scholar] [CrossRef]
- Hao, W.; Zheng, Z.; Zhu, L.; Pang, L.; Ma, J.; Zhu, S.; Du, L.; Jin, Y. 3D printing-based drug-loaded implanted prosthesis to prevent breast cancer recurrence post-conserving surgery. Asian J. Pharm. Sci. 2021, 16, 86–96. [Google Scholar] [CrossRef]
- Moroni, S.; Bingham, R.; Buckley, N.; Casettari, L.; Lamprou, D.A. 4D printed multipurpose smart implants for breast cancer management. Int. J. Pharm. 2023, 642, 123154. [Google Scholar] [CrossRef] [PubMed]

| Taxonomy | Description | Schematic Image | Refs. |
|---|---|---|---|
| Expansion and contraction | Description: changing in length, volume, and area. How to achieve: linear swelling and shrinking of thermo-responsive materials after immersion in cold and hot water. |  | [58,59] |
| Bending | Description: curvature of the entire structure. How to achieve: swelling/shrinkage mismatch between layered materials. |  | [60,61] |
| Folding | Description: sharp curvature along a crease on the construct. How to achieve: caused by a stress mismatch between rigid and active materials. |  | [62,63] |
| Rolling | Description: the structure moves by turning over and over on its own axis. Materials maintain the same orientation in the deformation direction. How to achieve: usually triggered by heat in the common shape memory cycles that foresee programming and recovery steps. |  | [64,65] |
| Helixing | Description: a curve traced on a cylinder by the rotation of the structure with a constant oblique angle. How to achieve: can be programmed to perform different deposition patterns and exploit the swelling/shrinkage mismatch between materials. |  | [12,66] |
| Twisting | Description: curvature created by a rotation of the structure around a stationary point. How to achieve: twisting can be programmed to perform different deposition patterns and exploit the swelling/shrinkage mismatch between materials. |  | [64,67] |
| Curving | Description: self-curving occurs when a flat structure performs local deviations from a plane. How to achieve: this activation can be programmed by exploiting the shear stresses at the interface between two different materials disposed in concentric circles. |  | [61,67] |
| Waving | Description: shape that has regular undulating features or a regular wavy up-and-down form. How to achieve: structures composed of three materials: two active materials inside a passive matrix. In function of the deposition pattern of the two active materials, different waving structures can be fabricated. |  | [68,69] |
| Curling | Description: shape that has irregular undulating features or an irregular wavy up-and-down form. How to achieve: structures composed of three materials: two active materials inside a passive matrix. The curling occurs due to the mismatch in swelling properties between passive and active materials. |  | [70,71] |
| Hierarchical structures | Description: assembling of different active elements and mechanisms that react, resulting in a uniform movement of the structure. How to achieve: actuatable units are co-joined into a complete system and later into a much larger system of systems. | 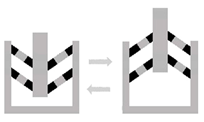 | [72,73] |
| Compliant mechanisms | Description: mechanism in which the motion is not only governed by the geometry and mass distribution but also by the forces. How to achieve: a compliant mechanism gains its mobility from the deformation of its flexible member. | 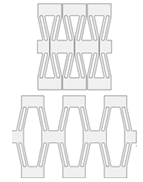 | [74,75] |
| Non-topologically equivalent changing | Description: non-topologically equivalent changes occur when a construct is able to self-fill holes or self-repair cuts. How to achieve: exploiting the self-healing properties of certain polymers. The increase in temperature is a possible method to trigger non-topologically equivalent changes. | 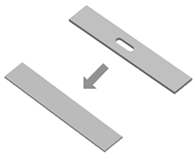 | [76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiesa, I.; Ceccarini, M.R.; Bittolo Bon, S.; Codini, M.; Beccari, T.; Valentini, L.; De Maria, C. 4D Printing Shape-Morphing Hybrid Biomaterials for Advanced Bioengineering Applications. Materials 2023, 16, 6661. https://doi.org/10.3390/ma16206661
Chiesa I, Ceccarini MR, Bittolo Bon S, Codini M, Beccari T, Valentini L, De Maria C. 4D Printing Shape-Morphing Hybrid Biomaterials for Advanced Bioengineering Applications. Materials. 2023; 16(20):6661. https://doi.org/10.3390/ma16206661
Chicago/Turabian StyleChiesa, Irene, Maria Rachele Ceccarini, Silvia Bittolo Bon, Michela Codini, Tommaso Beccari, Luca Valentini, and Carmelo De Maria. 2023. "4D Printing Shape-Morphing Hybrid Biomaterials for Advanced Bioengineering Applications" Materials 16, no. 20: 6661. https://doi.org/10.3390/ma16206661
APA StyleChiesa, I., Ceccarini, M. R., Bittolo Bon, S., Codini, M., Beccari, T., Valentini, L., & De Maria, C. (2023). 4D Printing Shape-Morphing Hybrid Biomaterials for Advanced Bioengineering Applications. Materials, 16(20), 6661. https://doi.org/10.3390/ma16206661












