Non-Thermal Plasma Treatment of Poly(tetrafluoroethylene) Dental Membranes and Its Effects on Cellular Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. NTP Treatment
2.2. Surface Characterization
2.2.1. Scanning Electron Microscopy (SEM) and Contact Angle Measurements
2.2.2. X-ray Photoelectron Spectroscopy (XPS)/Electron Spectroscopy for Chemical Analysis (ESCA)
2.3. In Vitro Characterization
2.3.1. Cell Passaging
2.3.2. Fluorescent Cell Membrane Labeling
2.3.3. Adhesion and Proliferation Experiments
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral. Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Bartee, B.K. Extraction site reconstruction for alveolar ridge preservation. Part 1: Rationale and materials selection. J. Oral. Implant. 2001, 27, 187–193. [Google Scholar] [CrossRef]
- Sclar, A.G. Strategies for management of single-tooth extraction sites in aesthetic implant therapy. J. Oral. Maxillofac. Surg. 2004, 62 (Suppl. S2), 90–105. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Marzola, A.; Bozzi, L.; Liljenberg, B.; Lindhe, J. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 2008, 35, 630–639. [Google Scholar] [CrossRef]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, E.T.P.; Balderrama, Í.F.; Ferreira, M.R.; Spielman, R.; Slavin, B.V.; Torroni, A.; Tovar, N.; Nayak, V.V.; Slavin, B.R.; Coelho, P.G.; et al. Osteogenic differentiation and reconstruction of mandible defects using a novel resorbable membrane: An in vitro and in vivo experimental study. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1966–1978. [Google Scholar] [CrossRef]
- Bassett, C.A.; Campbell, J.B.; Girado, J.M.; Rossi, J.P.; Seymour, R.J. Application of monomolecular filter tubes in bridging gaps in peripheral nerves and for prevention of neuroma formation; a preliminary report. J. Neurosurg. 1956, 13, 635–637. [Google Scholar] [CrossRef]
- Hurley, L.A.; Stinchfield, F.E.; Bassett, A.L.; Lyon, W.H. The role of soft tissues in osteogenesis. An experimental study of canine spine fusions. J. Bone Jt. Surg. Am. 1959, 41, 1243–1254. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Heynen, G.; Bosshardt, D.D.; Buser, D. Effect of two bioabsorbable barrier membranes on bone regeneration of standardized defects in calvarial bone: A comparative histomorphometric study in pigs. J. Periodontol. 2009, 80, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int. J. Oral. Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Heal. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants–a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. In Proceedings of Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 86–100. [Google Scholar]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral. Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef]
- Lech, A.; Butruk-Raszeja, B.A.; Ciach, T.; Lawniczak-Jablonska, K.; Kuzmiuk, P.; Bartnik, A.; Wachulak, P.; Fiedorowicz, H. Surface Modification of PLLA, PTFE and PVDF with Extreme Ultraviolet (EUV) to Enhance Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 9679. [Google Scholar] [CrossRef]
- Kameda, T.; Ohkuma, K.; Oka, S.J.D.M.J. Polytetrafluoroethylene (PTFE): A resin material for possible use in dental prostheses and devices. Dent. Mater. J. 2019, 38, 136–142. [Google Scholar] [CrossRef]
- Dorkhan, M.; Yücel-Lindberg, T.; Hall, J.; Svensäter, G.; Davies, J.R. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral. Health 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guda, T.; Walker, J.A.; Singleton, B.M.; Hernandez, J.W.; Son, J.-S.; Kim, S.-G.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Guided bone regeneration in long-bone defects with a structural hydroxyapatite graft and collagen membrane. Tissue Eng. Part. A 2012, 19, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, T.; Broggini, N.; Jensen, S.S.; Bornstein, M.M.; Schenk, R.K.; Buser, D. Membrane durability and tissue response of different bioresorbable barrier membranes: A histologic study in the rabbit calvarium. Int. J. Oral. Maxillofac. Implant. 2005, 20, 843–853. [Google Scholar]
- Köunönen, M.; Hormia, M.; Kivilahti, J.; Hautaniemi, J.; Thesleff, I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J. Biomed. Mater. Res. 1992, 26, 1325–1341. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X. Barrier membranes for guided bone regeneration (gbr): A focus on recent advances in collagen membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Brånemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone response to laser-induced micro-and nano-size titanium surface features. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 220–227. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. Part. B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R. Osseointegration of metallic devices: Current trends based on implant hardware design. Arch. Biochem. Biophys. 2014, 561, 99–108. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral. Maxillofac. Implant. 2005, 20, 425–431. [Google Scholar]
- Salapare III, H.S.; Guittard, F.; Noblin, X.; de Givenchy, E.T.; Celestini, F.; Ramos, H.J. Stability of the hydrophilic and superhydrophobic properties of oxygen plasma-treated poly (tetrafluoroethylene) surfaces. J. Colloid. Interface Sci. 2013, 396, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Danna, N.R.; Beutel, B.G.; Tovar, N.; Witek, L.; Marin, C.; Bonfante, E.A.; Granato, R.; Suzuki, M.; Coelho, P.G. Assessment of atmospheric pressure plasma treatment for implant osseointegration. BioMed Res. Int. 2015, 2015, 761718. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. Part. A 2013, 101, 98–103. [Google Scholar] [CrossRef]
- Sawase, T.; Jimbo, R.; Baba, K.; Shibata, Y.; Ikeda, T.; Atsuta, M. Photo-induced hydrophilicity enhances initial cell behavior and early bone apposition. Clin. Oral. Implant. Res. 2008, 19, 491–496. [Google Scholar] [CrossRef]
- Teixeira, H.S.; Marin, C.; Witek, L.; Freitas Jr, A.; Silva, N.R.; Lilin, T.; Tovar, N.; Janal, M.N.; Coelho, P.G. Assessment of a chair-side argon-based non-thermal plasma treatment on the surface characteristics and integration of dental implants with textured surfaces. J. Mech. Behav. Biomed. Mater. 2012, 9, 45–49. [Google Scholar] [CrossRef]
- Dmitriev, S.; Kravets, L.; Levkovich, N.; Sleptsov, V.; Elinson, V.; Potryasai, V. Surface modification of poly (ethylene terephthalate) track membranes in an allyl alcohol plasma. High. Energy Chem. C/C Khimiia Vysok. Energii 1998, 32, 275–279. [Google Scholar]
- Gancarz, I.; Bryjak, J.; Bryjak, M.; Poźniak, G.; Tylus, W. Plasma modified polymers as a support for enzyme immobilization 1.: Allyl alcohol plasma. Eur. Polym. J. 2003, 39, 1615–1622. [Google Scholar] [CrossRef]
- Whittle, J.D.; Short, R.D.; Douglas, C.W.I.; Davies, J. Differences in the aging of allyl alcohol, acrylic acid, allylamine, and octa-1,7-diene plasma polymers as studied by X-ray photoelectron spectroscopy. Chem. Mater. 2000, 12, 2664–2671. [Google Scholar] [CrossRef]
- Dekker, A.; Reitsma, K.; Beugeling, T.; Bantjes, A.; Feijen, J.; Van Aken, W. Adhesion of endothelial cells and adsorption of serum proteins on gas plasma-treated polytetrafluoroethylene. Biomaterials 1991, 12, 130–138. [Google Scholar] [CrossRef]
- Mavadat, M.; Ghasemzadeh-Barvarz, M.; Turgeon, S.p.; Duchesne, C.; Laroche, G.t. Correlation between the plasma characteristics and the surface chemistry of plasma-treated polymers through partial least-squares analysis. Langmuir 2013, 29, 15859–15867. [Google Scholar] [CrossRef]
- Ramires, P.; Mirenghi, L.; Romano, A.; Palumbo, F.; Nicolardi, G. Plasma-treated PET surfaces improve the biocompatibility of human endothelial cells. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 51, 535–539. [Google Scholar] [CrossRef]
- Sipehia, R.; Martucci, G.; Barbarosie, M.; Wu, C. Enhanced attachment and growth of human endothelial cells derived from umbilical veins on ammonia plasma modified surfaces of PTFE and ePTFE synthetic vascular graft biomaterials. Biomater. Artif. Cells Immobil. Biotechnol. 1993, 21, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kwok, D.T.; Xu, M.; Shi, H.; Wu, Z.; Zhang, W.; Chu, P.K. Tailoring of Mesenchymal Stem Cells Behavior on Plasma-Modified Polytetrafluoroethylene. Adv. Mater. 2012, 24, 3315–3324. [Google Scholar] [CrossRef]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. Part. A 2012, 100, 1901–1906. [Google Scholar] [CrossRef]
- Bartee, C.M.; Bartee, B.K.; Bartee Chaddick, M.; Bartee Barry, K. Hydrophilic High Density PTFE Medical Barrier. United States patent US 7,296,998, 20 November 2007. [Google Scholar]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Silva, N.R.F.A.; Coelho, P.G.; Valverde, G.B.; Becker, K.; Ihrke, R.; Quade, A.; Thompson, V.P. Surface characterization of Ti and Y-TZP following non-thermal plasma exposure. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2011, 99B, 199–206. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mutin, M.; George, F.; Lesaule, G.; Sampol, J. Reevaluation of trypsin-EDTA for endothelial cell detachment before flow cytometry analysis. Endothelium 1996, 4, 289–295. [Google Scholar] [CrossRef]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef]
- Ramkumar, M.C.; Navaneetha Pandiyaraj, K.; Arun Kumar, A.; Padmanabhan, P.V.A.; Cools, P.; De Geyter, N.; Morent, R.; Uday Kumar, S.; Kumar, V.; Gopinath, P.; et al. Atmospheric pressure non-thermal plasma assisted polymerization of poly (ethylene glycol) methylether methacrylate (PEGMA) on low density polyethylene (LDPE) films for enhancement of biocompatibility. Surf. Coat. Technol. 2017, 329, 55–67. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.; Brown, N. The surface oxidation of selected polymers using an atmospheric pressure air dielectric barrier discharge. Part I. Appl. Surf. Sci. 2004, 221, 203–214. [Google Scholar] [CrossRef]
- Akishev, Y.; Grushin, M.; Dyatko, N.; Kochetov, I.; Napartovich, A.; Trushkin, N.; Duc, T.M.; Descours, S. Studies on cold plasma–polymer surface interaction by example of PP-and PET-films. J. Phys. D Appl. Phys. 2008, 41, 235203. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid. Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Lee, H.-U.; Jeong, Y.-S.; Jeong, S.-Y.; Park, S.-Y.; Bae, J.-S.; Kim, H.-G.; Cho, C.-R. Role of reactive gas in atmospheric plasma for cell attachment and proliferation on biocompatible poly ε-caprolactone film. Appl. Surf. Sci. 2008, 254, 5700–5705. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H.; Irani, S. Plasma surface modification of poly (l-lactic acid) and poly (lactic-co-glycolic acid) films for improvement of nerve cells adhesion. Radiat. Phys. Chem. 2008, 77, 280–287. [Google Scholar] [CrossRef]
- Slepička, P.; Kasálková, N.S.; Stránská, E.; Bačáková, L.; Švorčík, V. Surface characterization of plasma treated polymers for applications as biocompatible carriers. Express Polym. Lett. 2013, 7, 535–545. [Google Scholar] [CrossRef]
- Sipehia, R.; Liszowski, M.; Lu, A. In vivo evaluaton of ammonia plasma modified ePTFE grafts for small diameter blood vessel replacement: A preliminary report. J. Cardiovasc. Surg. 2001, 42, 537. [Google Scholar]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization-a review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Kasaj, A.; Reichert, C.; Götz, H.; Röhrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head. Face Med. 2008, 4, 1–8. [Google Scholar] [CrossRef]
- Ertel, S.I.; Ratner, B.D.; Horbett, T.A. Radiofrequency plasma deposition of oxygen-containing films on polystyrene and poly (ethylene terephthalate) substrates improves endothelial cell growth. J. Biomed. Mater. Res. 1990, 24, 1637–1659. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, W.; Hertl, W.; Nowlan, E.; Binkowski, N. Surface treatments and cell attachment. In Vitro 1984, 20, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; Khang, D.; Webster, T.J. Nanometer polymer surface features: The influence on surface energy, protein adsorption and endothelial cell adhesion. Nanotechnology 2008, 19, 505103. [Google Scholar] [CrossRef] [PubMed]
- Comelles, J.; Estévez, M.; Martínez, E.; Samitier, J. The role of surface energy of technical polymers in serum protein adsorption and MG-63 cells adhesion. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Absolom, D.; Thomson, C.; Hawthorn, L.; Zingg, W.; Neumann, A. Kinetics of cell adhesion to polymer surfaces. J. Biomed. Mater. Res. 1988, 22, 215–229. [Google Scholar] [CrossRef]
- Grausova, L.; Vacik, J.; Vorlicek, V.; Svorcik, V.; Slepicka, P.; Bilkova, P.; Vandrovcova, M.; Lisa, V.; Bacakova, L. Fullerene C60 films of continuous and micropatterned morphology as substrates for adhesion and growth of bone cells. Diam. Relat. Mater. 2009, 18, 578–586. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Vacik, J.; Svorcik, V.; Slepicka, P.; Kasalkova, N.; Vorlicek, V.; Lavrentiev, V.; Vosecek, V.; Grausova, L.; Lisa, V. Fullerene C60 and hybrid C60/Ti films as substrates for adhesion and growth of bone cells. Phys. Status Solidia 2008, 205, 2252–2261. [Google Scholar] [CrossRef]
- Hoornaert, A.; d’Arros, C.; Heymann, M.-F.; Layrolle, P.J.B.M. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Meemusaw, M.; Magaraphan, R. Surface and bulk properties improvement of HDPE by a batch plasma treatment. J. Appl. Polym. Sci. 2016, 133, 43011. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Choi, D.M.; Park, C.K.; Cho, K.; Park, C.E. Adhesion improvement of epoxy resin/polyethylene joints by plasma treatment of polyethylene. Polymer 1997, 38, 6243–6249. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
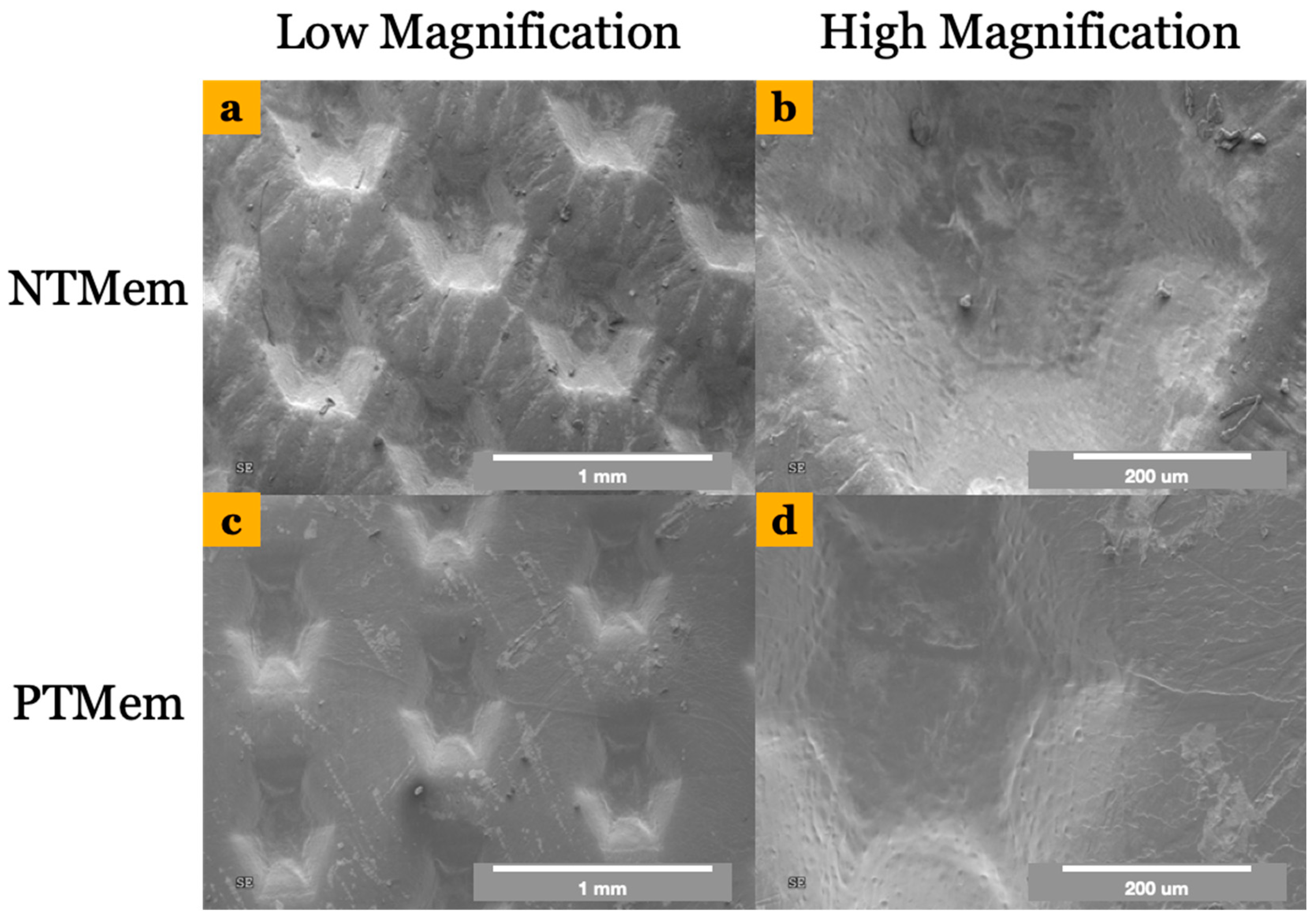
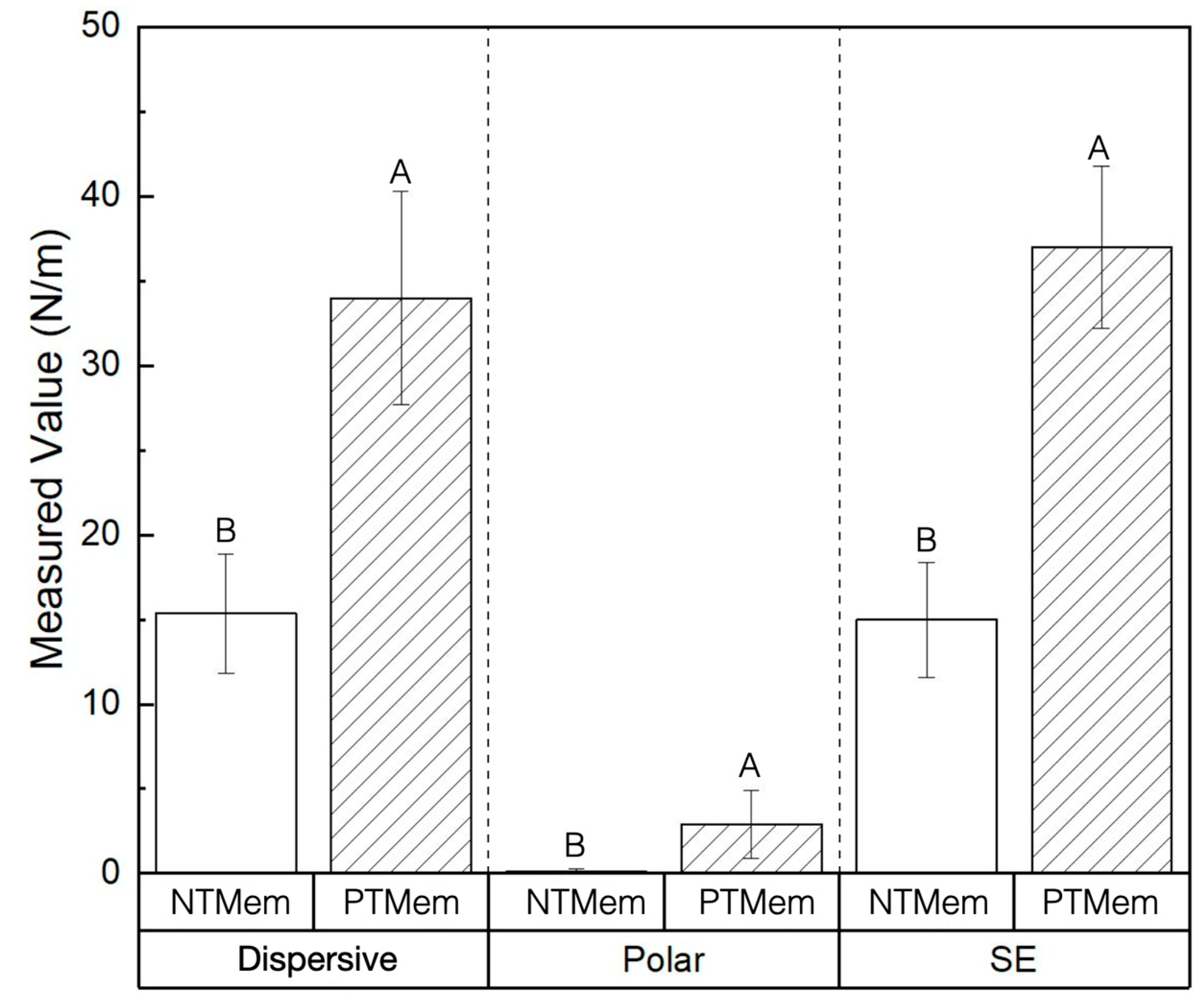

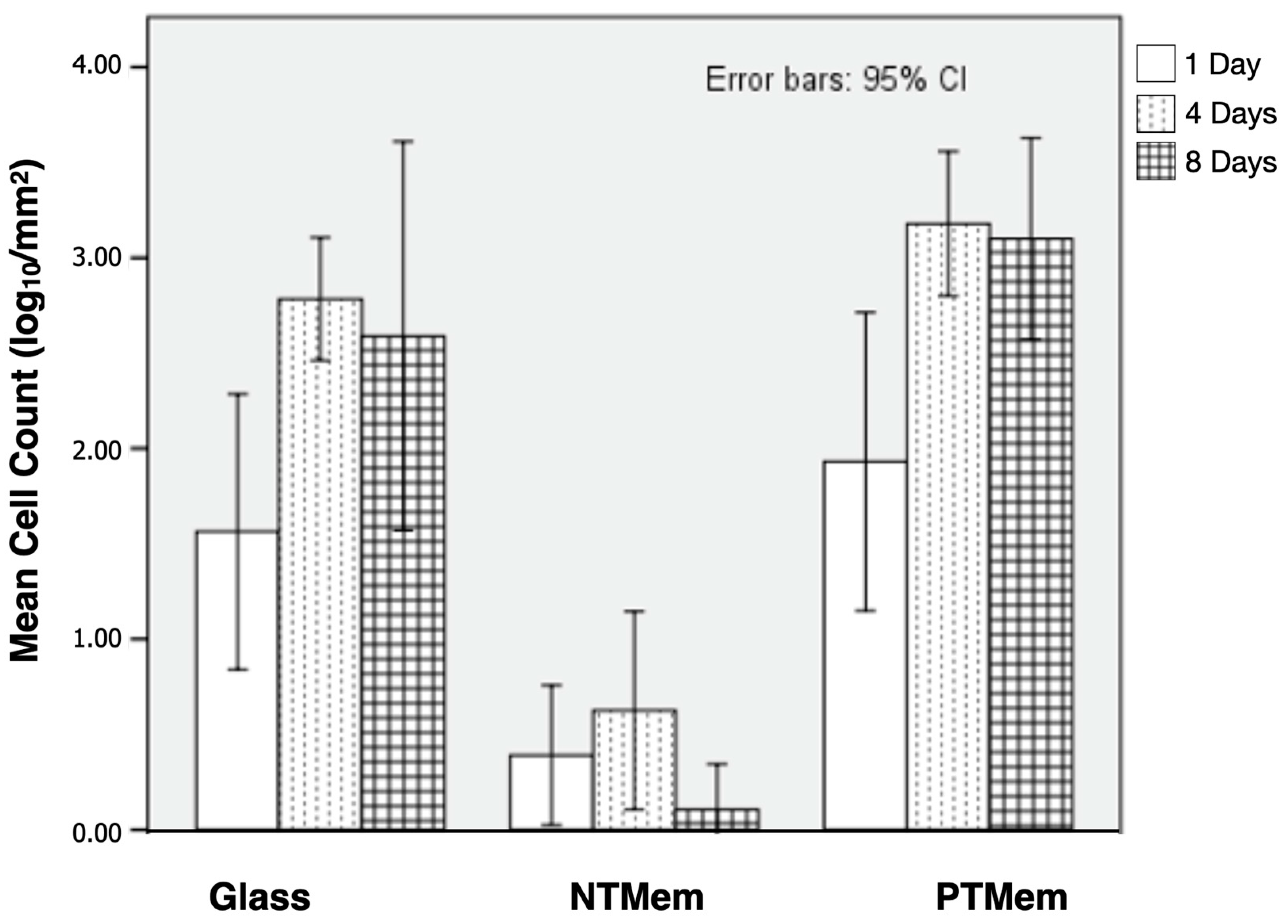
| Experimental Group | Parameter | Measured Value (Mean ± 95% Confidence Interval (CI)) (All Values in N/m) |
|---|---|---|
| NTMem | SE | 15.0 ± 3.4 |
| Dispersive | 15.0 ± 3.5 | |
| Polar | 0.11 ± 0.2 | |
| PTMem | SE | 37.0 ± 4.8 |
| Dispersive | 34.0 ± 6.3 | |
| Polar | 2.9 ± 2.0 |
| Group | Sample | C | O | F | Si | Cl |
|---|---|---|---|---|---|---|
| NTMem | 1 | 33.7 | 0.4 | 65.8 | 0.1 | <0.05 |
| 2 | 31.0 | 0.2 | 68.9 | - | <0.05 | |
| 3 | 30.9 | - | 69.0 | - | <0.05 | |
| PTMem | 1 | 73.0 | 19.7 | 7.1 | 0.1 | <0.05 |
| 2 | 66.3 | 17.0 | 16.7 | - | <0.05 | |
| 3 | 75.1 | 21.2 | 3.4 | 0.3 | <0.05 |
| Group | Sample | C-C | C-O | C=O | O-C=O | CF | CF2 | CF3 |
|---|---|---|---|---|---|---|---|---|
| NTMem | 1 | 2.6 | 0.4 | 0.3 | 0.0 | 0.5 | 28.0 | 1.9 |
| 2 | 0.8 | 0.3 | 0.2 | 0.0 | 0.4 | 27.2 | 2.1 | |
| 3 | 0.3 | 0.2 | 0.2 | 0.0 | 0.5 | 27.9 | 1.9 | |
| PTMem | 1 | 49.2 | 17.7 | 3.4 | 0.8 | 0.0 | 1.8 | 0.0 |
| 2 | 41.1 | 15.7 | 2.9 | 0.7 | 0.0 | 6.0 | 0.0 | |
| 3 | 50.6 | 19.1 | 3.7 | 1.1 | 0.0 | 0.7 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, V.V.; Mirsky, N.A.; Slavin, B.V.; Witek, L.; Coelho, P.G.; Tovar, N. Non-Thermal Plasma Treatment of Poly(tetrafluoroethylene) Dental Membranes and Its Effects on Cellular Adhesion. Materials 2023, 16, 6633. https://doi.org/10.3390/ma16206633
Nayak VV, Mirsky NA, Slavin BV, Witek L, Coelho PG, Tovar N. Non-Thermal Plasma Treatment of Poly(tetrafluoroethylene) Dental Membranes and Its Effects on Cellular Adhesion. Materials. 2023; 16(20):6633. https://doi.org/10.3390/ma16206633
Chicago/Turabian StyleNayak, Vasudev Vivekanand, Nicholas Alexander Mirsky, Blaire V. Slavin, Lukasz Witek, Paulo G. Coelho, and Nick Tovar. 2023. "Non-Thermal Plasma Treatment of Poly(tetrafluoroethylene) Dental Membranes and Its Effects on Cellular Adhesion" Materials 16, no. 20: 6633. https://doi.org/10.3390/ma16206633
APA StyleNayak, V. V., Mirsky, N. A., Slavin, B. V., Witek, L., Coelho, P. G., & Tovar, N. (2023). Non-Thermal Plasma Treatment of Poly(tetrafluoroethylene) Dental Membranes and Its Effects on Cellular Adhesion. Materials, 16(20), 6633. https://doi.org/10.3390/ma16206633







