One-Pot Synthesis and Characterization of CuCrS2/ZnS Core/Shell Quantum Dots as New Blue-Emitting Sources
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Characterization
2.3. Synthesis of CuCrS2/ZnS QDs in the One-Pot System
3. Results and Discussion
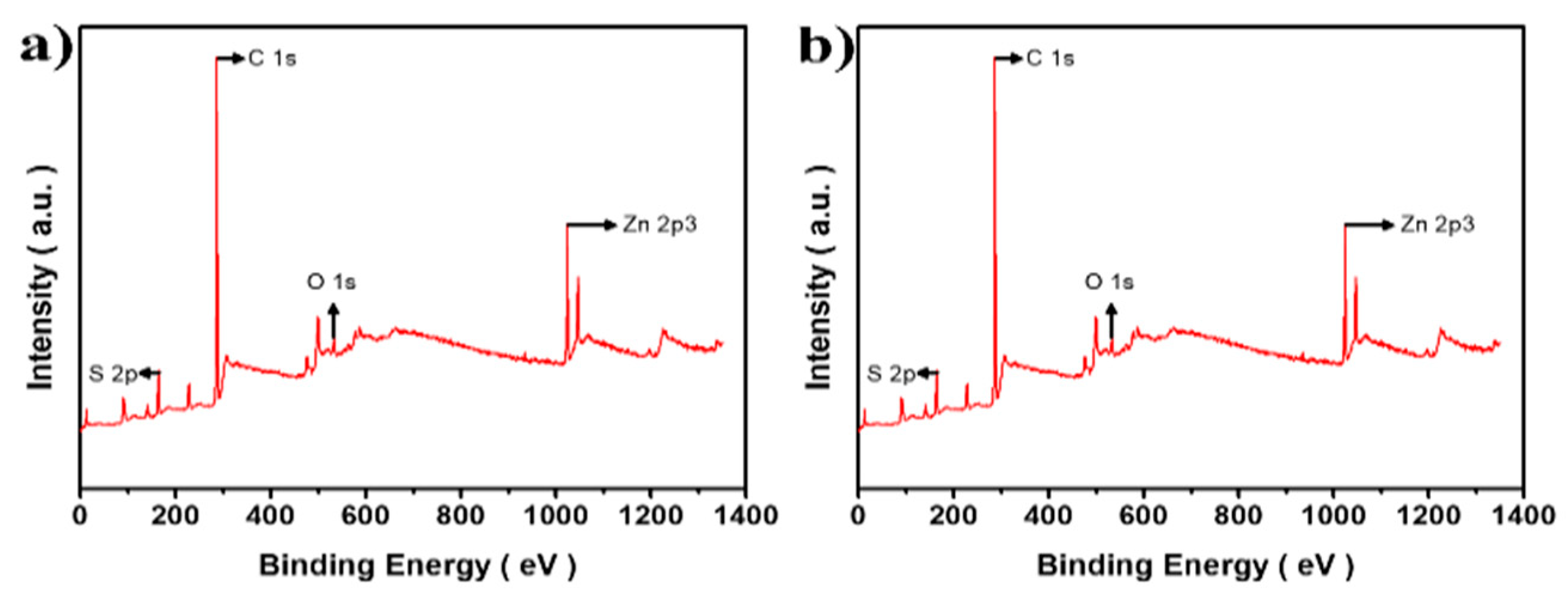
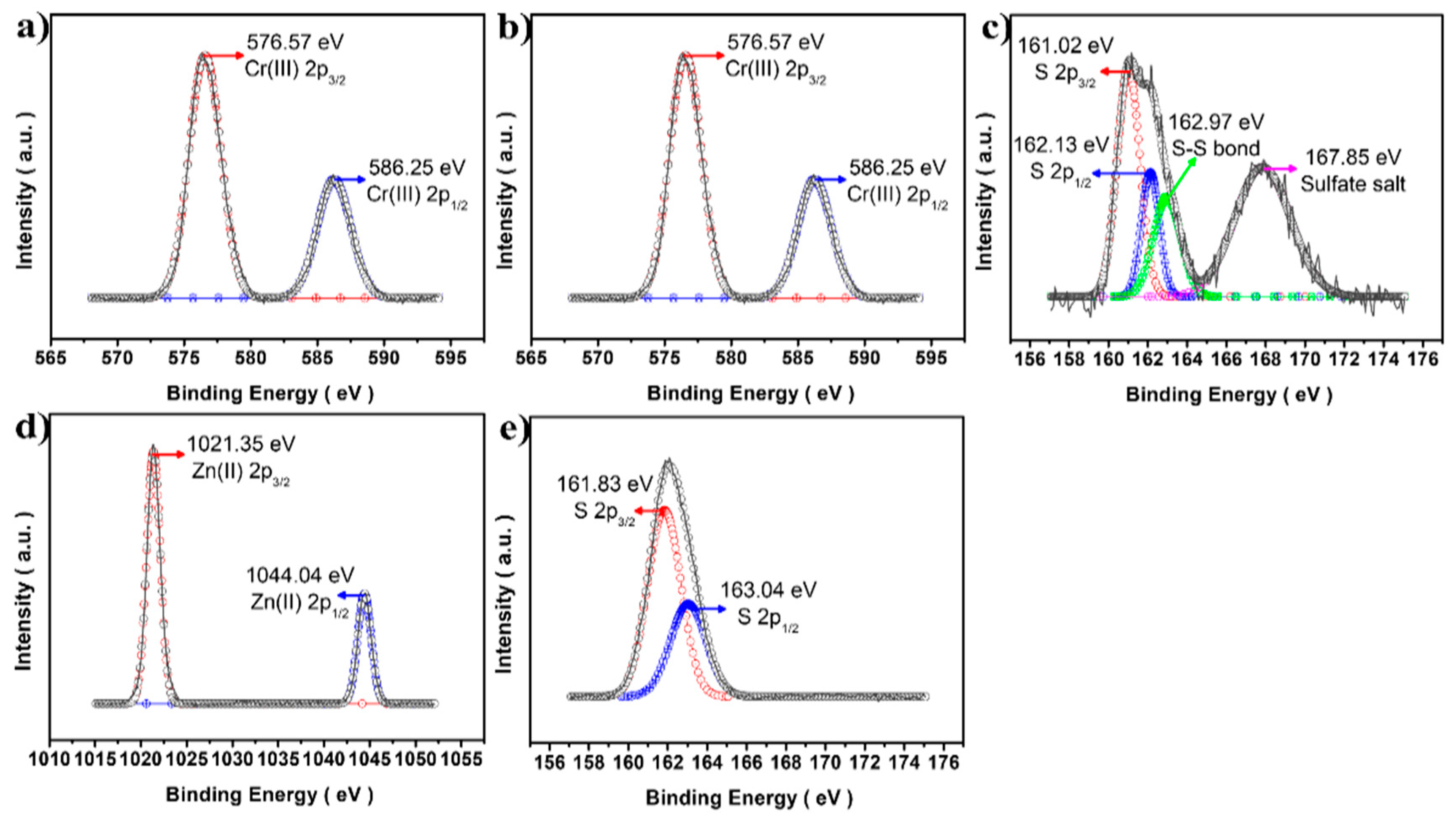
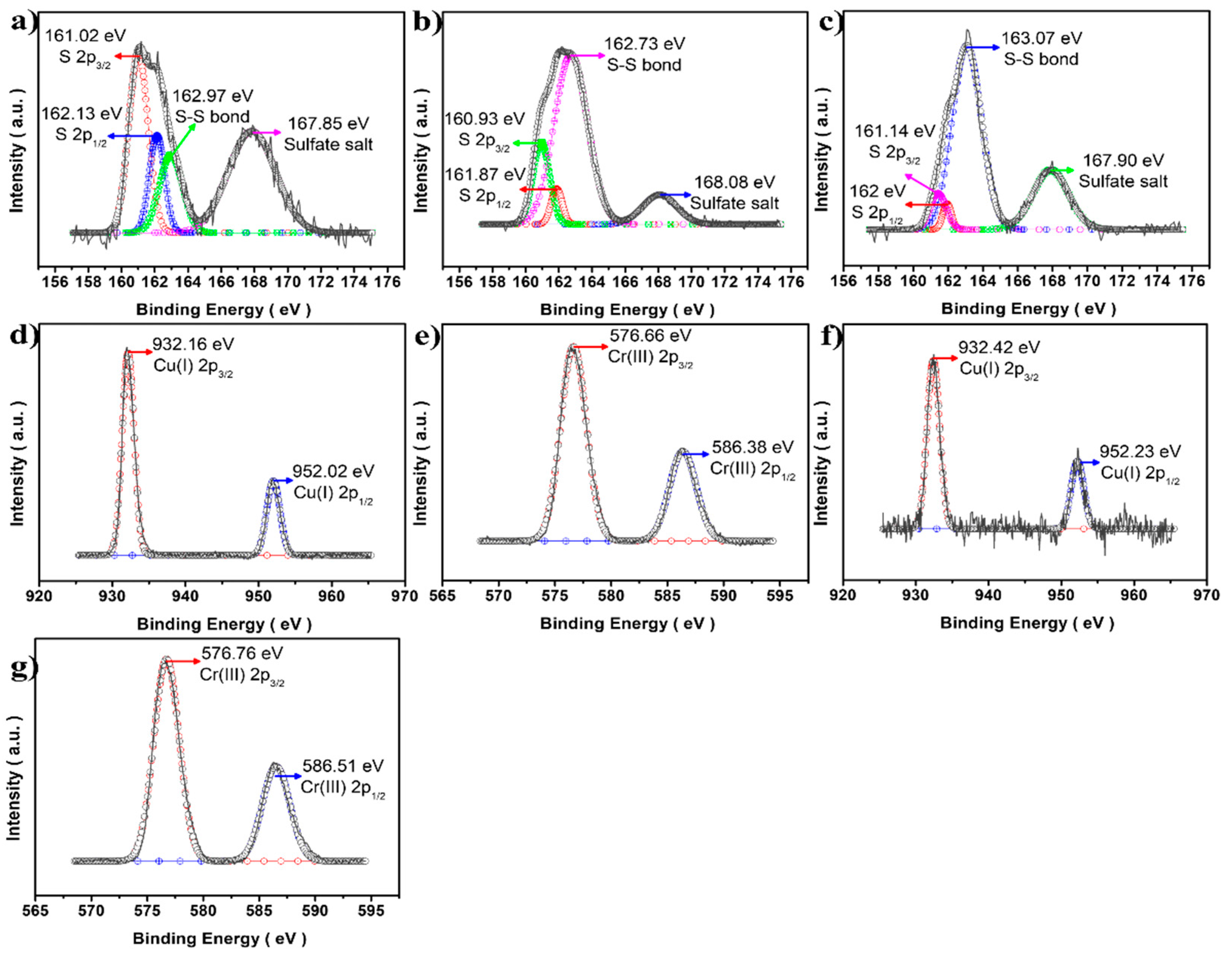
3.1. Morphology and Crystal Structure of CuCrS2/ZnS QDs
3.2. Band Gap Engineering of CuCrS2/ZnS QDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse Cde (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; RodriguezViejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Nam, D.E.; Song, W.S.; Yang, H. Noninjection, one-pot synthesis of Cu-deficient CuInS2/ZnS core/shell quantum dots and their fluorescent properties. J. Colloid Interface Sci. 2011, 361, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Foda, M.F.; Huang, L.; Shao, F.; Han, H.Y. Biocompatible and Highly Luminescent Near-Infrared CuInS2/ZnS Quantum Dots Embedded Silica Beads for Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2014, 6, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Shi, F.P.; Zhao, X.J.; Chen, L.; Su, X.G. 3-Aminophenyl boronic acid-functionalized CuInS2 quantum dots as a near-infrared fluorescence probe for the determination of dopamine. Biosens. Bioelectron. 2013, 47, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Pang, S.; Na, W.D.; Su, X.G. Near-infrared fluorescence probe for the determination of alkaline phosphatase. Biosens. Bioelectron. 2014, 55, 249–254. [Google Scholar] [CrossRef]
- Zhang, F.M.; Ma, P.Y.; Deng, X.Y.; Sun, Y.; Wang, X.H.; Song, D.Q. Enzymatic determination of uric acid using water-soluble CuInS/ZnS quantum dots as a fluorescent probe. Microchim. Acta 2018, 185, 499. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, D.E.; Tian, S.Q.; Qin, Z.Y.; Zeng, D.W.; Xie, C.S. CuInS2 QDs decorated ring-like NiO for significantly enhanced room-temperature NO2 sensing performances via effective interfacial charge transfer. Sens. Actuators B-Chem. 2018, 256, 1001–1010. [Google Scholar] [CrossRef]
- Park, S.H.; Hong, A.; Kim, J.H.; Yang, H.; Lee, K.; Jang, H.S. Highly Bright Yellow-Green-Emitting CuInS2 Colloidal Quantum Dots with Core/Shell/Shell Architecture for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 6764–6771. [Google Scholar] [CrossRef]
- Ilaiyaraja, P.; Rakesh, B.; Das, T.K.; Mocherla, P.S.V.; Sudakar, C. CuInS2 quantum dot sensitized solar cells with high V-OC approximate to 0.9 V achieved using microsphere-nanoparticulate TiO2 composite photoanode. Sol. Energy Mater. Sol. Cells 2018, 178, 208–222. [Google Scholar] [CrossRef]
- Song, W.S.; Yang, H. Efficient White-Light-Emitting Diodes Fabricated from Highly Fluorescent Copper Indium Sulfide Core/Shell Quantum Dots. Chem. Mater. 2012, 24, 1961–1967. [Google Scholar] [CrossRef]
- Uehara, M.; Watanabe, K.; Tajiri, Y.; Nakamura, H.; Maeda, H. Synthesis of CuInS2 fluorescent nanocrystals and enhancement of fluorescence by controlling crystal defect. J. Chem. Phys. 2008, 129, 134709. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Kim, J.H.; Jang, E.P.; Lee, S.H.; Jo, D.Y.; Kim, Y.; Do, Y.R.; Yang, H. Systematic and Extensive Emission Tuning of Highly Efficient Cu-In-S-Based Quantum Dots from Visible to Near Infrared. Chem. Mater. 2019, 31, 2627–2634. [Google Scholar] [CrossRef]
- Lesnyak, V.; Dubavik, A.; Plotnikov, A.; Gaponik, N.; Eychmuller, A. One-step aqueous synthesis of blue-emitting glutathione-capped ZnSe1-xTex alloyed nanocrystals. Chem. Commun. 2010, 46, 886–888. [Google Scholar] [CrossRef]
- Gao, M.; Yang, H.W.; Shen, H.B.; Zeng, Z.P.; Fan, F.J.; Tang, B.B.; Min, J.J.; Zhang, Y.; Hua, Q.Z.; Li, L.S.; et al. Bulk-like ZnSe Quantum Dots Enabling Efficient Ultranarrow Blue Light-Emitting Diodes. Nano Lett. 2021, 21, 7252–7260. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Q.; Wang, M.Q.; Yang, Z.; Wang, H.; Padhiar, M.A.; Qiu, H.W.; Dang, J.L.; Miao, Y.R.; Zhou, Y.; Bhatti, A.S. Strong violet emission from ultra-stable strontium-doped CsPbCl3 superlattices. Nanoscale 2022, 14, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, C.; Kim, B.; Choi, Y.; Chae, H. Synthesis of Blue-Emissive InP/GaP/ZnS Quantum Dots via Controlling the Reaction Kinetics of Shell Growth and Length of Capping Ligands. Nanomaterials 2020, 10, 2171. [Google Scholar] [CrossRef]
- Tewari, G.C.; Tripathi, T.S.; Kumar, P.; Rastogi, A.K.; Pasha, S.K.; Gupta, G. Increase in the Thermoelectric Efficiency of the Disordered Phase of Layered Antiferromagnetic CuCrS2. J. Electron. Mater. 2011, 40, 2368–2373. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Zvereva, V.V. Magnetic Properties of Novel Layered Disulfides CuCr0.99Ln0.01S2 (Ln = La horizontal ellipsis Lu). Materials 2021, 14, 5101. [Google Scholar] [CrossRef]
- Mantella, V.; Varandili, S.B.; Pankhurst, J.R.; Buonsanti, R. Colloidal Synthesis of Cu-M-S (M = V, Cr, Mn) Nanocrystals by Tuning the Copper Precursor Reactivity. Chem. Mater. 2020, 32, 9780–9786. [Google Scholar] [CrossRef]
- Xie, B.B.; Hu, B.B.; Jiang, L.F.; Li, G.; Du, Z.L. The phase transformation of CuInS2 from chalcopyrite to wurtzite. Nanoscale Res. Lett. 2015, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; An, D.; Sun, S.A.; Gao, J.Y.; Qian, L.P. Reduction and Removal of Chromium VI in Water by Powdered Activated. Carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Krylova, V. Deposition and characterization of silver sulfide layers on the polypropylene film surface. Chemija 2013, 24, 203–209. [Google Scholar]
- Lin, X.Y.; Liu, R.T.; Lin, W.; Li, Z.Z.; Xiang, X.; Ning, L.; Jie, C. The synergistic effect of carbon materials on properties of copper-based friction materials. Ind. Lubr. Tribol. 2021, 73, 170–176. [Google Scholar]
- Fichtner, T.; Fischer, A.R.; Dornack, C. Nitrate consumption by the oxidation of sulfides during an enhanced natural attenuation project at a contaminated site in Berlin, Germany. Environ. Sci. Eur. 2021, 33, 103. [Google Scholar] [CrossRef]
- Morselli, G.; Villa, M.; Fermi, A.; Critchley, K.; Ceroni, P. Luminescent copper indium sulfide (CIS) quantum dots for bioimaging applications. Nanoscale Horiz. 2021, 6, 676–695. [Google Scholar] [CrossRef]
- Chen, B.K.; Zhong, H.Z.; Zhang, W.Q.; Tan, Z.A.; Li, Y.F.; Yu, C.R.; Zhai, T.Y.; Bando, Y.S.; Yang, S.Y.; Zou, B.S. Highly Emissive and Color-Tunable CuInS2-Based Colloidal Semiconductor Nanocrystals: Off-Stoichiometry Effects and Improved Electroluminescence Performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Chuang, P.H.; Lin, C.C.; Liu, R.S. Emission-Tunable CuInS2/ZnS Quantum Dots: Structure, Optical Properties, and Application in White Light-Emitting Diodes with High Color Rendering Index. ACS Appl. Mater. Interfaces 2014, 6, 15379–15387. [Google Scholar] [CrossRef]
- Han, C.G.; Zhang, B.P.; Ge, Z.H.; Zhang, L.J.; Liu, Y.C. Thermoelectric properties of p-type semiconductors copper chromium disulfide CuCrS2+x. J. Mater. Sci. 2013, 48, 4081–4087. [Google Scholar] [CrossRef]
- Koroteev, V.O.; Bulushev, D.A.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Nanometer-Sized MoS2 Clusters on Graphene Flakes for Catalytic Formic Acid Decomposition. ACS Catal. 2014, 4, 3950–3956. [Google Scholar] [CrossRef]
- Koroteev, V.O.; Bulusheva, L.G.; Asanov, I.P.; Shlyakhova, E.V.; Vyalikh, D.V.; Okotrub, A.V. Charge Transfer in the MoS2/Carbon Nanotube Composite. J. Phys. Chem. C 2011, 115, 21199–21204. [Google Scholar] [CrossRef]
- Turo, M.J.; Macdonald, J.E. Crystal-Bound vs Surface-Bound Thiols on Nano crystals. ACS Nano 2014, 8, 10205–10213. [Google Scholar] [CrossRef] [PubMed]


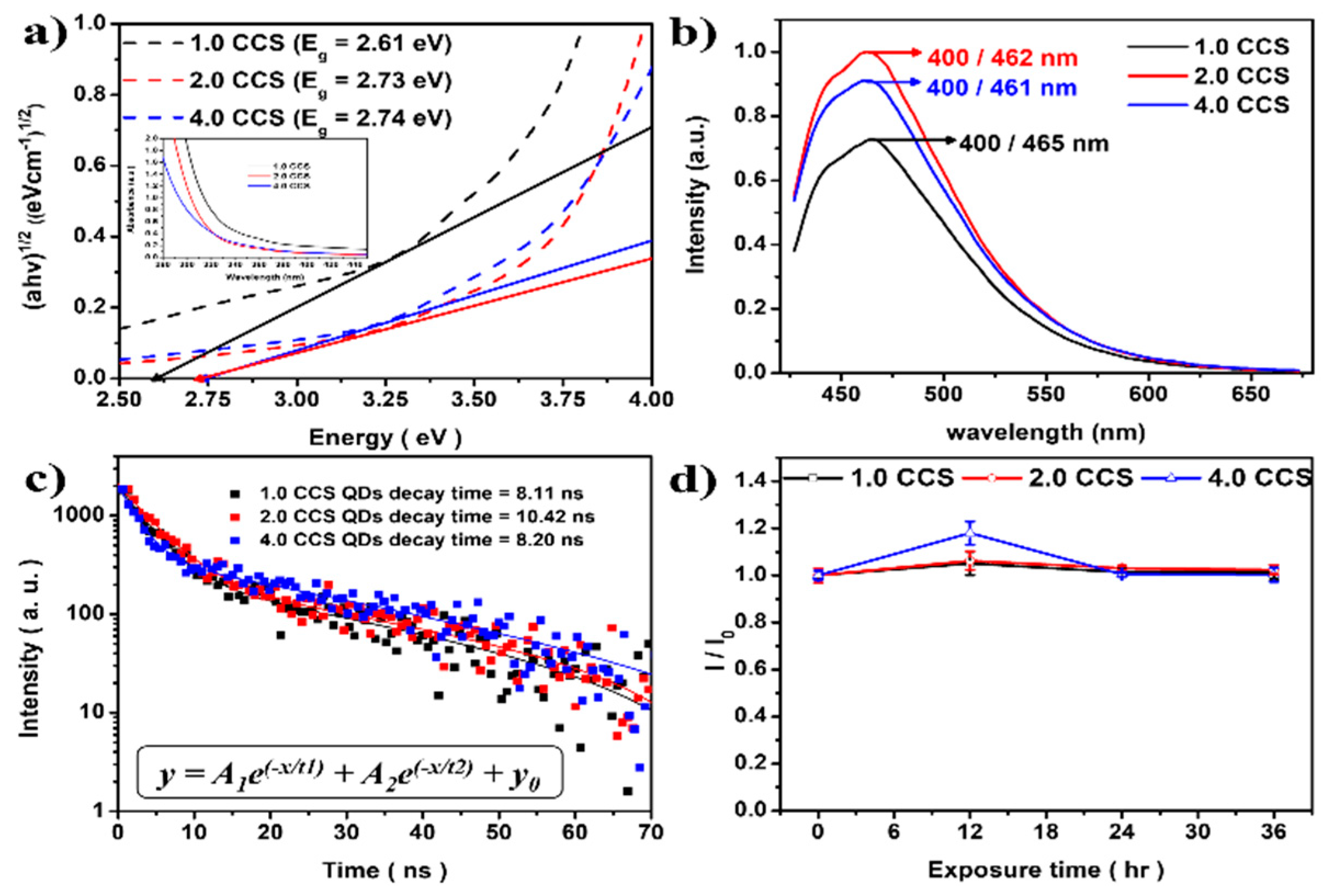

| Samples | [Cr3+]/[Cu+] Molar Ratio | PLE/PL | PL Lifetime Decay | Relative PLQY |
|---|---|---|---|---|
| 1.0 CCS | 0.375/0.375 (1.0) | 400 nm/465 nm | 8.11 ns | 5% |
| 2.0 CCS | 0.5/0.25 (2.0) | 400 nm/462 nm | 10.42 ns | 10% |
| 4.0 CCS | 0.6/0.15 (4.0) | 400 nm/461 nm | 8.2 ns | 4.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-K.; Ban, Y.-J.; Lee, H.-J.; Kim, J.-H.; Park, S.-J. One-Pot Synthesis and Characterization of CuCrS2/ZnS Core/Shell Quantum Dots as New Blue-Emitting Sources. Materials 2023, 16, 762. https://doi.org/10.3390/ma16020762
Lee H-K, Ban Y-J, Lee H-J, Kim J-H, Park S-J. One-Pot Synthesis and Characterization of CuCrS2/ZnS Core/Shell Quantum Dots as New Blue-Emitting Sources. Materials. 2023; 16(2):762. https://doi.org/10.3390/ma16020762
Chicago/Turabian StyleLee, Ho-Kyung, Ye-Jun Ban, Hyun-Jong Lee, Ji-Hyeon Kim, and Sang-Joon Park. 2023. "One-Pot Synthesis and Characterization of CuCrS2/ZnS Core/Shell Quantum Dots as New Blue-Emitting Sources" Materials 16, no. 2: 762. https://doi.org/10.3390/ma16020762
APA StyleLee, H.-K., Ban, Y.-J., Lee, H.-J., Kim, J.-H., & Park, S.-J. (2023). One-Pot Synthesis and Characterization of CuCrS2/ZnS Core/Shell Quantum Dots as New Blue-Emitting Sources. Materials, 16(2), 762. https://doi.org/10.3390/ma16020762








