Nanocrystalline CaWO4 and ZnWO4 Tungstates for Hybrid Organic–Inorganic X-ray Detectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterization
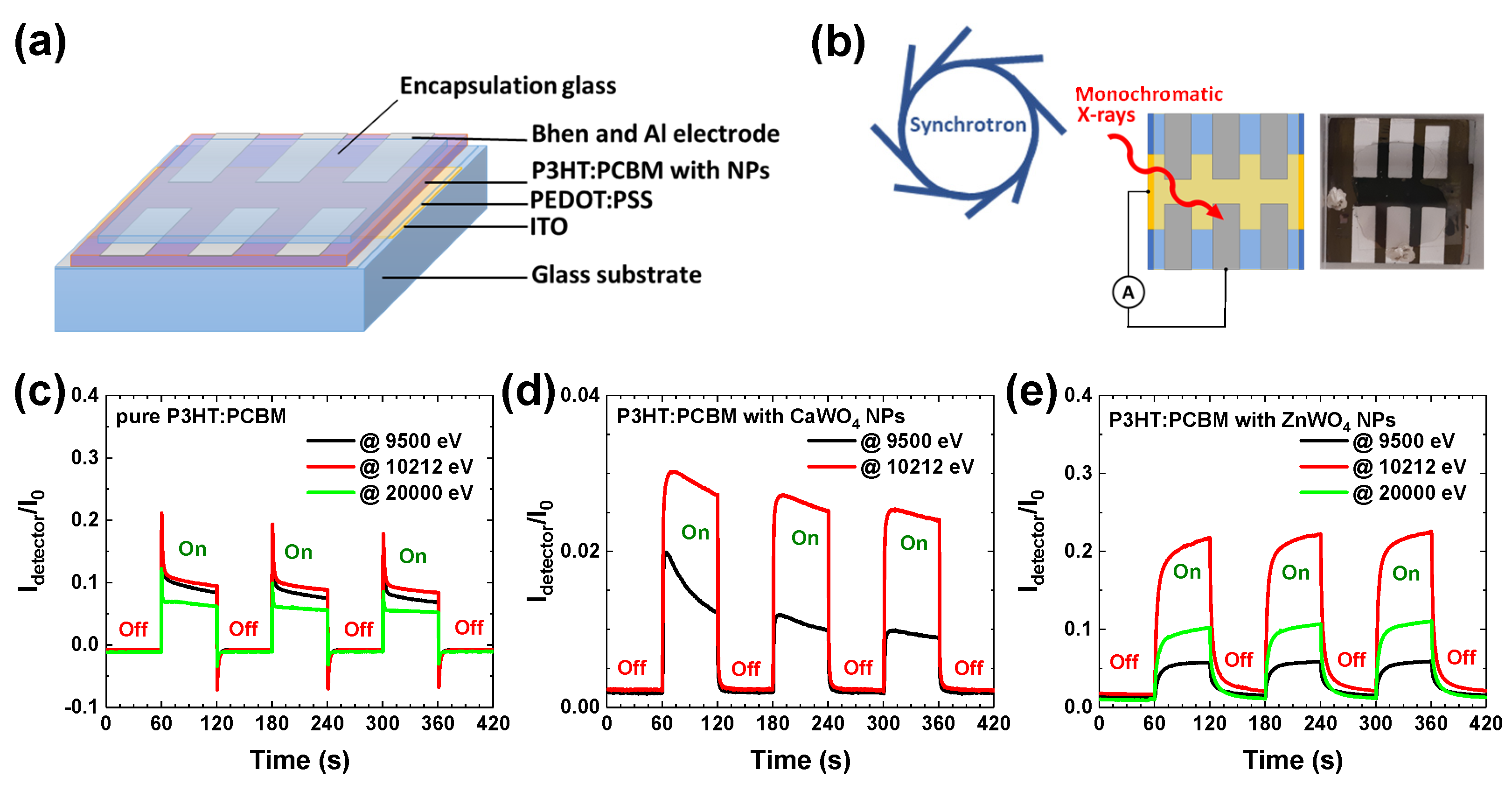
2.2. Hybrid Organic–Inorganic X-ray Detector Fabrication and Measurements
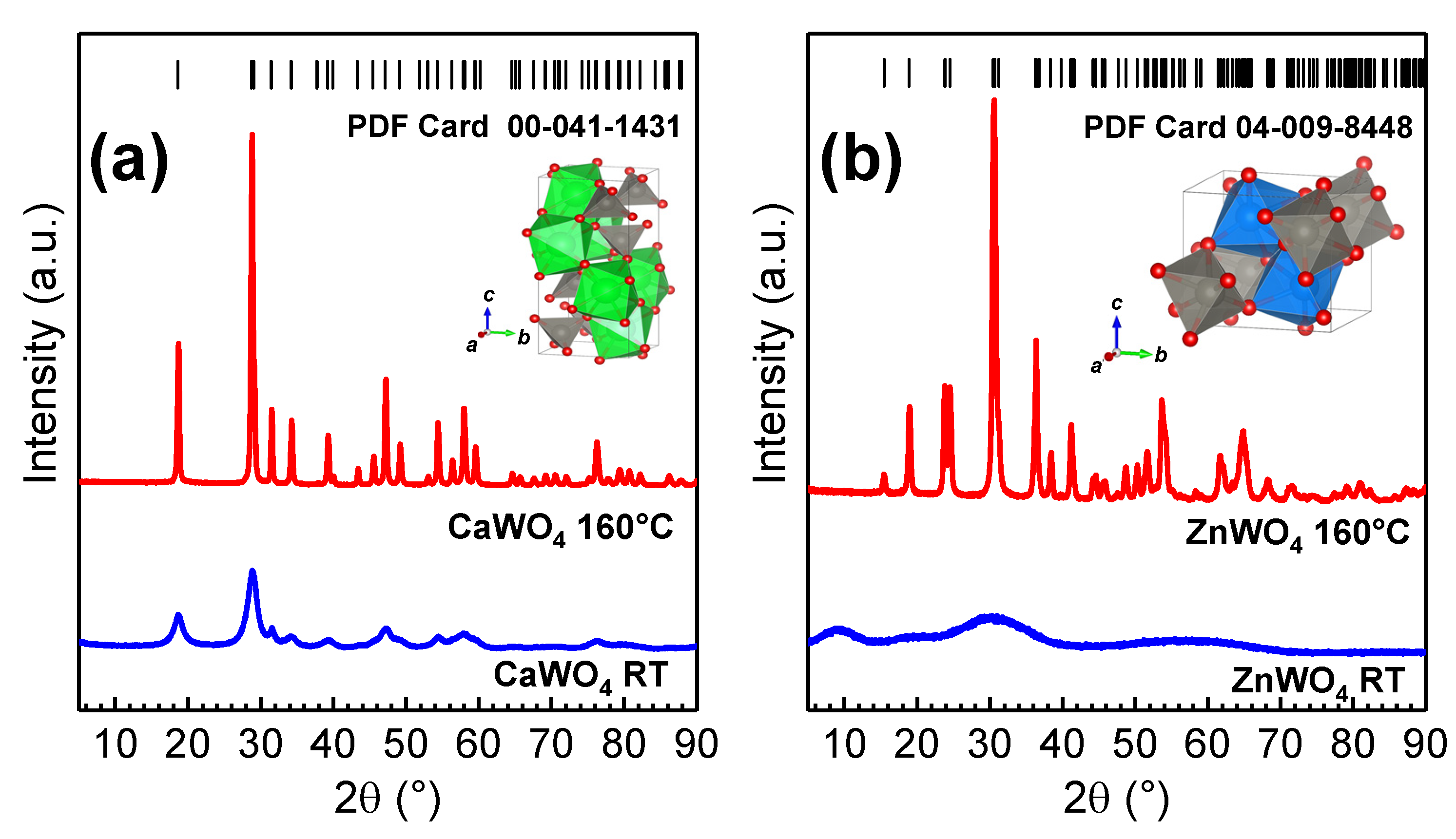
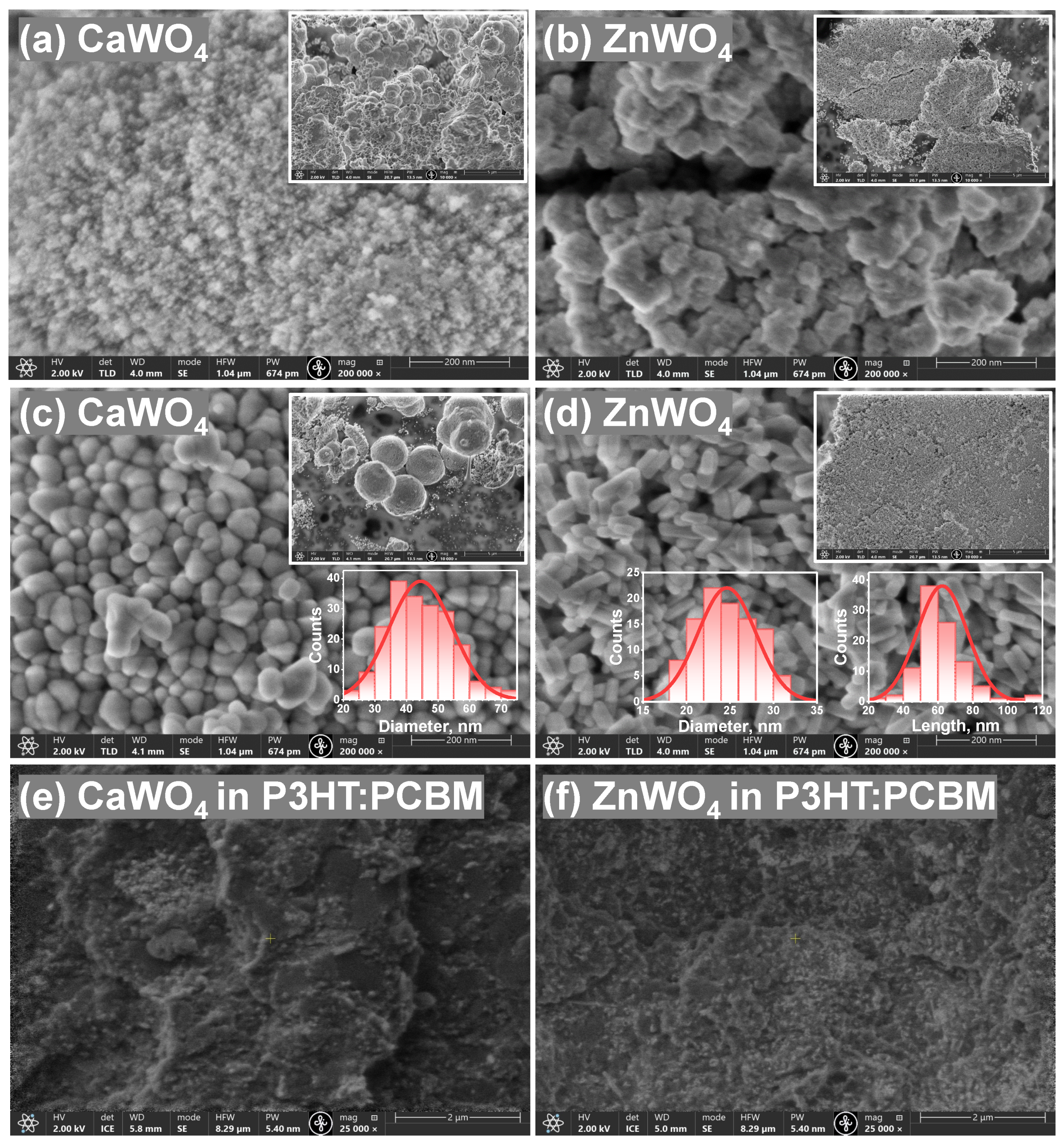
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EXAFS | Extended X-ray absorption fine structure |
| NP | Nanoparticle |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) |
| P3HT:PCBM | Poly(3-hexylthiophene-2,5-diyl):Phenyl-C61-butyric acid methyl ester |
| RT | Room temperature |
| SEM | Scanning electron microscopy |
| WL | White line |
| XANES | X-ray absorption near edge structure |
| XRD | X-ray diffraction |
References
- Luo, Z.; Moch, J.G.; Johnson, S.S.; Chen, C.C. A review on X-ray detection using nanomaterials. Curr. Nanosci. 2017, 13, 364–372. [Google Scholar] [CrossRef]
- Thirimanne, H.; Jayawardena, K.D.G.I.; Parnell, A.J.; Bandara, R.M.I.; Karalasingam, A.; Pani, S.; Huerdler, J.E.; Lidzey, D.G.; Tedde, S.F.; Nisbet, A.; et al. High sensitivity organic–inorganic hybrid X-ray detectors with direct transduction and broadband response. Nat. Commun. 2018, 9, 2926. [Google Scholar] [CrossRef]
- Fraboni, B.; Ciavatti, A.; Merlo, F.; Pasquini, L.; Cavallini, A.; Quaranta, A.; Bonfiglio, A.; Fraleoni-Morgera, A. Organic semiconducting single crystals as next generation of low-cost, room-temperature electrical X-ray detectors. Adv. Mater. 2012, 24, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyata, H.; Katsumata, M.; Nakano, S.; Matsuda, K.; Tamura, M. Organic semiconductors as real-time radiation detectors. Nucl. Instrum. Methods A 2014, 763, 304–307. [Google Scholar] [CrossRef]
- Basirico, L.; Ciavatti, A.; Cramer, T.; Cosseddu, P.; Bonfiglio, A.; Fraboni, B. Direct X-ray photoconversion in flexible organic thin film devices operated below 1V. Nat. Commun. 2016, 7, 13063. [Google Scholar] [CrossRef]
- Liu, J. Flexible, printable soft-X-ray detectors based on all-inorganic perovskite quantum dots. Adv. Mater. 2019, 31, 1901644. [Google Scholar] [CrossRef]
- Mescher, H.; Schackmar, F.; Eggers, H.; Abzieher, T.; Zuber, M.; Hamann, E.; Baumbach, T.; Richards, B.S.; Hernandez-Sosa, G.; Paetzold, U.W.; et al. Flexible inkjet-printed triple cation perovskite X-ray detectors. ACS Appl. Mater. Interfaces 2020, 12, 15774. [Google Scholar] [CrossRef]
- Sleight, A.W. Accurate cell dimensions for ABO4 molybdates and tungstates. Acta Cryst. B 1972, 28, 2899–2902. [Google Scholar] [CrossRef]
- Lee, J.; Rancilio, N.J.; Poulson, J.M.; Won, Y.Y. Block copolymer-encapsulated CaWO4 nanoparticles: Synthesis, formulation, and characterization. ACS Appl. Mater. Interfaces 2016, 8, 8608–8619. [Google Scholar] [CrossRef]
- Su, Y.; Li, G.; Xue, Y.; Li, L. Tunable physical properties of CaWO4 nanocrystals via particle size control. J. Phys. Chem. C 2007, 111, 6684–6689. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Caliebe, W.A.; Murzin, V.; Kalinko, A.; Gorlitz, M. High-flux XAFS-beamline P64 at PETRA III. AIP Conf. Proc. 2019, 2054, 060031. [Google Scholar] [CrossRef]
- Gürmen, E.; Daniels, E.; King, J.S. Crystal structure refinement of SrMoO4, SrWO4, CaMoO4, and BaWO4 by neutron diffraction. J. Chem. Phys. 1971, 55, 1093–1097. [Google Scholar] [CrossRef]
- Schofield, P.F.; Knight, K.S.; Cressey, G. Neutron powder diffraction study of the scintillator material. J. Mater. Sci. A 1996, 31, 2873. [Google Scholar] [CrossRef]
- Liu, S.; Tian, S.; Xing, R. CaWO4 hierarchical nanostructures: Hydrothermal synthesis, growth mechanism and photoluminescence properties. CrystEngComm 2011, 13, 7258–7261. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Gouveia, A.F.; Assis, M.; de Oliveira, R.C.; Pinatti, I.M.; Penha, M.; Goncalves, R.F.; Gracia, L.; Andres, J.; Longo, E. ZnWO4 nanocrystals: Synthesis, morphology, photoluminescence and photocatalytic properties. Phys. Chem. Chem. Phys. 2018, 20, 1923–1937. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lam, W.Y.; Luo, J.D.; Ho, Y.L.; Tang, B.Z.; Zhu, D.B.; Wong, M.; Kwok, H.S. Highly efficient organic light-emitting diodes with a silole-based compound. Appl. Phys. Lett. 2002, 81, 574–576. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, J.; Wang, X.; Yu, J. Effect of bulk and planar heterojunctions based charge generation layers on the performance of tandem organic light-emitting diodes. Org. Electron. 2016, 30, 136–142. [Google Scholar] [CrossRef]
- Ameri, T.; Khoram, P.; Min, J.; Brabec, C.J. Organic ternary solar cells: A review. Adv. Mater. 2013, 25, 4245–4266. [Google Scholar] [CrossRef]
- X-ray Data Booklet, 3rd ed.; Lawrence Berkeley National Laboratory, University of California: Davis, CA, USA, 2009.
- Balerna, A.; Bernieri, E.; Burattini, E.; Kuzmin, A.; Lusis, A.; Purans, J.; Cikmach, P. XANES studies of MeO3-x (Me = W, Re, Ir) crystalline and amorphous oxides. Nucl. Instrum. Methods Phys. Res. A 1991, 308, 240–242. [Google Scholar] [CrossRef]
- Kalinko, A.; Kuzmin, A. Static and dynamic structure of ZnWO4 nanoparticles. J. Non-Cryst. Solids 2011, 357, 2595–2599. [Google Scholar] [CrossRef]
- Cai, W.; Gong, X.; Cao, Y. Polymer solar cells: Recent development and possible routes for improvement in the performance. Sol. Energy Mater. Sol. Cells 2010, 94, 114–127. [Google Scholar] [CrossRef]
- Jayawardena, K.D.G.I.; Thirimanne, H.M.; Tedde, S.F.; Huerdler, J.E.; Parnell, A.J.; Bandara, R.M.I.; Mills, C.A.; Silva, S.R.P. Millimeter-scale unipolar transport in high sensitivity organic–inorganic semiconductor X-ray detectors. ACS Nano 2019, 13, 6973–6981. [Google Scholar] [CrossRef]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.D. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Butanovs, E.; Zolotarjovs, A.; Kuzmin, A.; Polyakov, B. Nanoscale X-ray detectors based on individual CdS, SnO2 and ZnO nanowires. Nucl. Instrum. Methods Phys. Res. A 2021, 1014, 165736. [Google Scholar] [CrossRef]
- Nanayakkara, M.P.A.; Matjacic, L.; Wood, S.; Richheimer, F.; Castro, F.A.; Jenatsch, S.; Zufle, S.; Kilbride, R.; Parnell, A.J.; Masteghin, M.G.; et al. Ultra-low dark current organic–inorganic hybrid X-ray detectors. Adv. Funct. Mater. 2020, 31, 2008482. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, X.; Wang, Y.; Xin, Z.; Chai, G.; Xu, Y.; Wang, K.; Chen, J.; Liu, C.; Wang, X.; et al. X-ray Sensitive hybrid organic photodetectors with embedded CsPbBr3 perovskite quantum dots. Org. Electron. 2021, 98, 106306. [Google Scholar] [CrossRef]
- Rehr, J.J.; Albers, R.C. Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 2000, 72, 621–654. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudza, I.; Pudzs, K.; Tokmakovs, A.; Strautnieks, N.R.; Kalinko, A.; Kuzmin, A. Nanocrystalline CaWO4 and ZnWO4 Tungstates for Hybrid Organic–Inorganic X-ray Detectors. Materials 2023, 16, 667. https://doi.org/10.3390/ma16020667
Pudza I, Pudzs K, Tokmakovs A, Strautnieks NR, Kalinko A, Kuzmin A. Nanocrystalline CaWO4 and ZnWO4 Tungstates for Hybrid Organic–Inorganic X-ray Detectors. Materials. 2023; 16(2):667. https://doi.org/10.3390/ma16020667
Chicago/Turabian StylePudza, Inga, Kaspars Pudzs, Andrejs Tokmakovs, Normunds Ralfs Strautnieks, Aleksandr Kalinko, and Alexei Kuzmin. 2023. "Nanocrystalline CaWO4 and ZnWO4 Tungstates for Hybrid Organic–Inorganic X-ray Detectors" Materials 16, no. 2: 667. https://doi.org/10.3390/ma16020667
APA StylePudza, I., Pudzs, K., Tokmakovs, A., Strautnieks, N. R., Kalinko, A., & Kuzmin, A. (2023). Nanocrystalline CaWO4 and ZnWO4 Tungstates for Hybrid Organic–Inorganic X-ray Detectors. Materials, 16(2), 667. https://doi.org/10.3390/ma16020667









