Crystallization Kinetics and Structure Evolution during Annealing of Ni-Co-Mn-In Powders Obtained by Mechanical Alloying
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
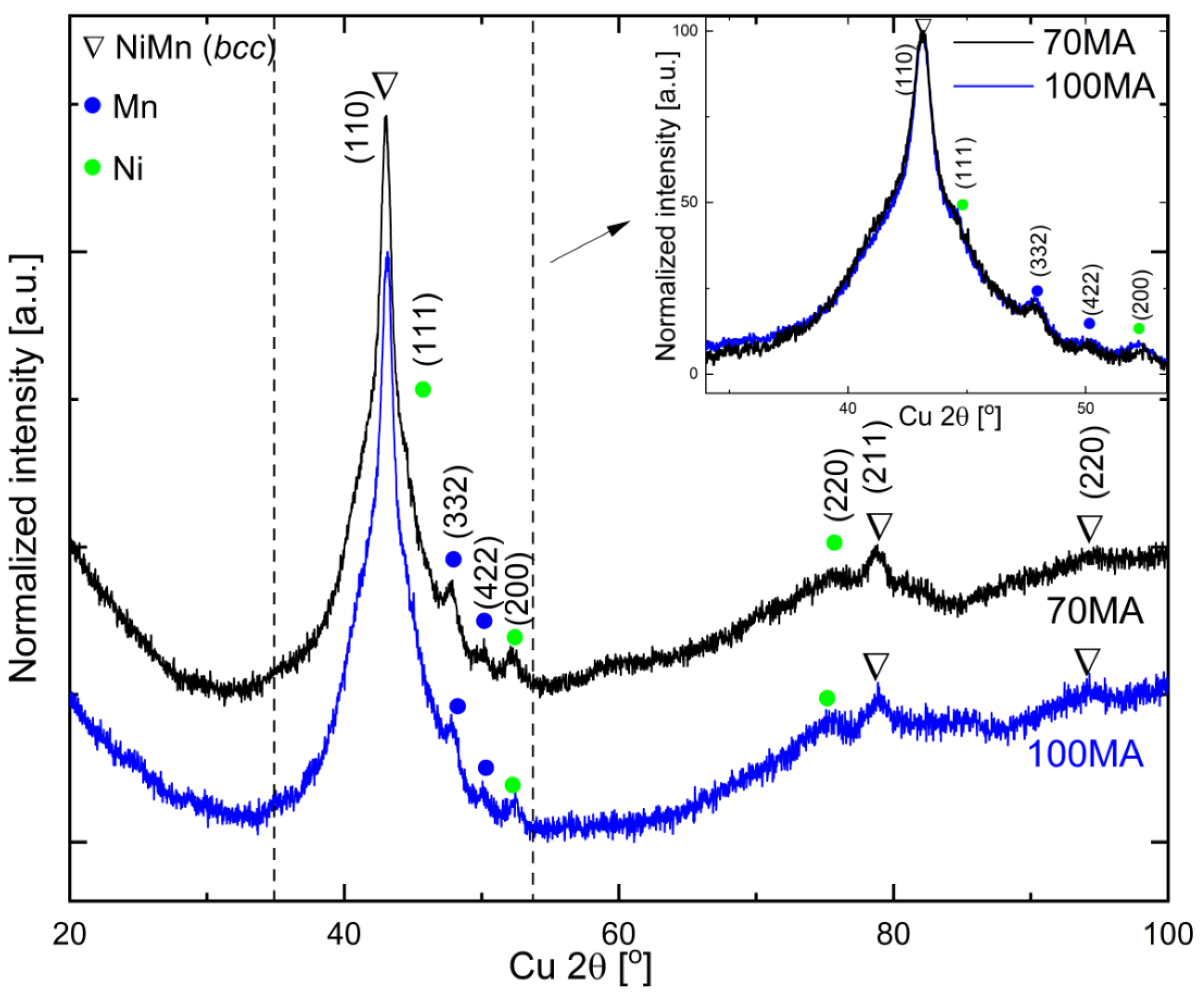
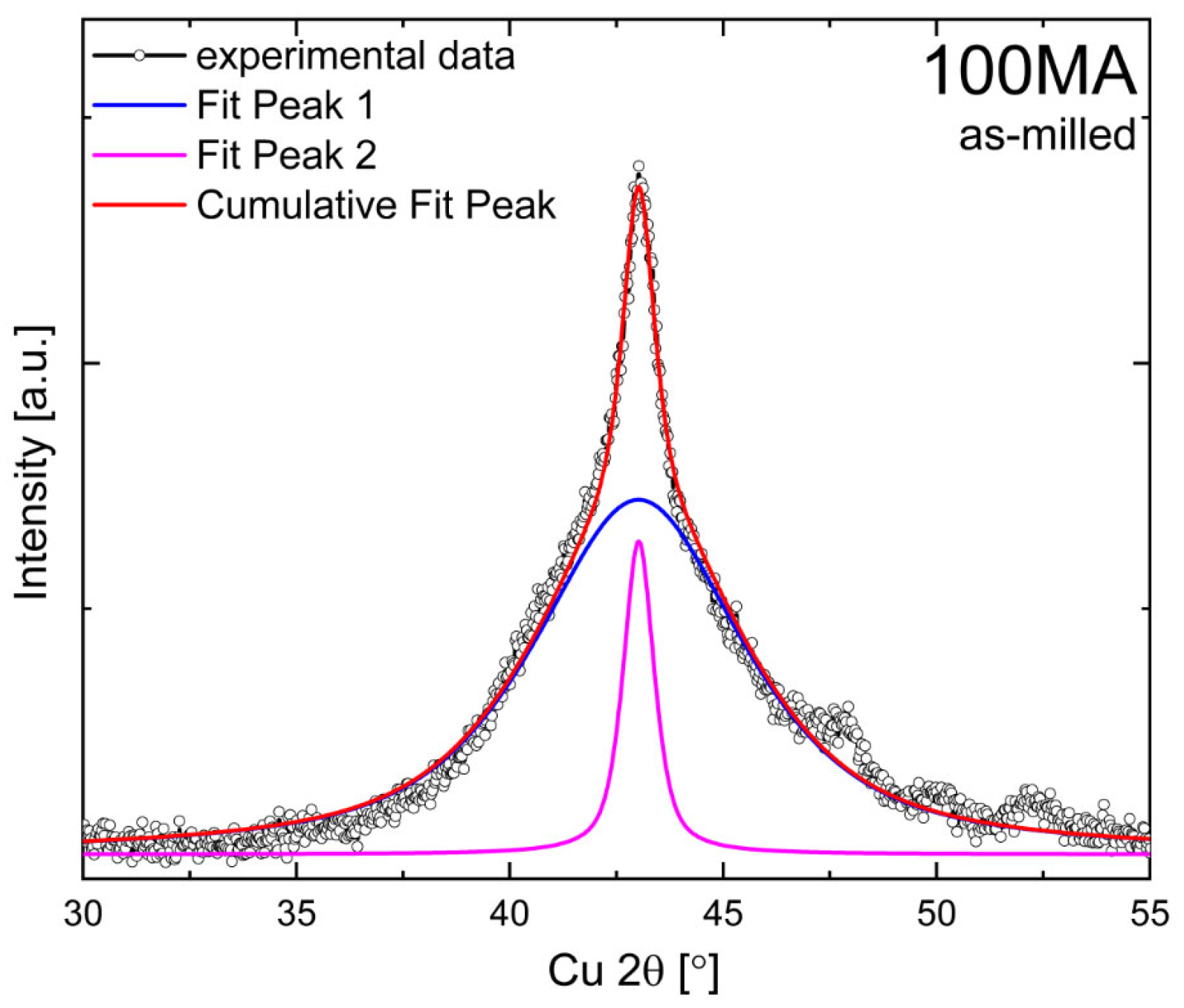
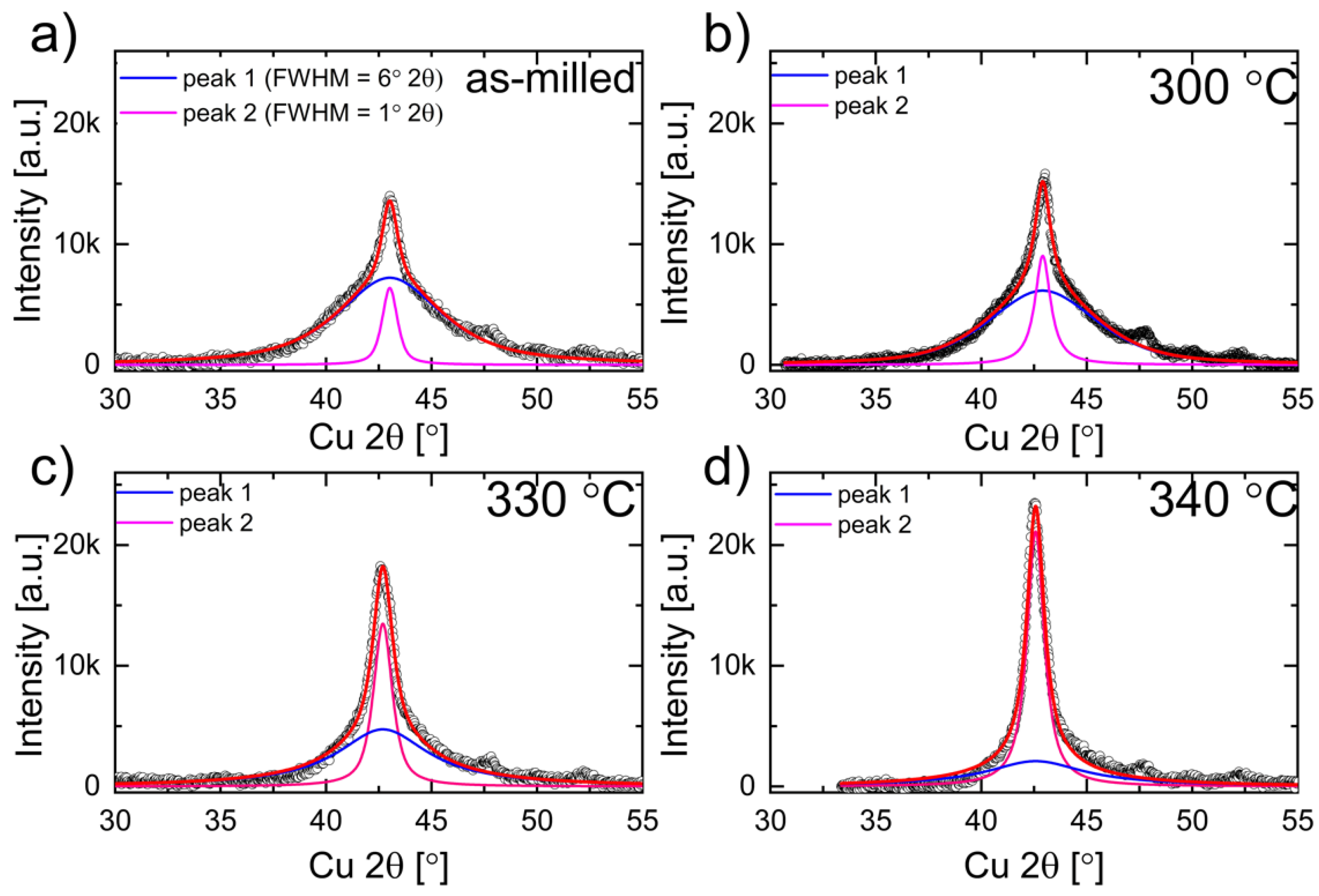
3.1. Structure of As-Milled Powders
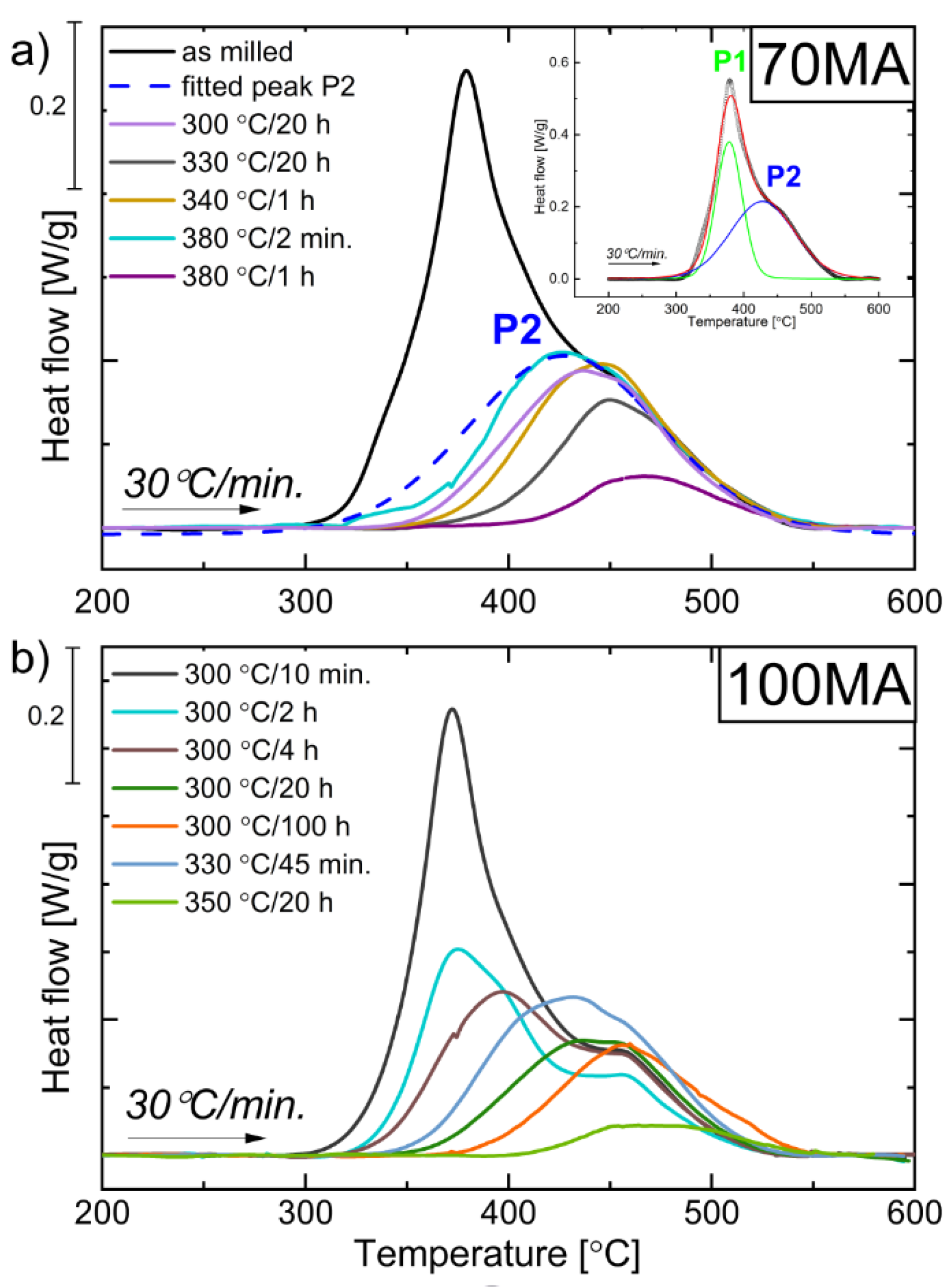
3.2. DSC Measurements
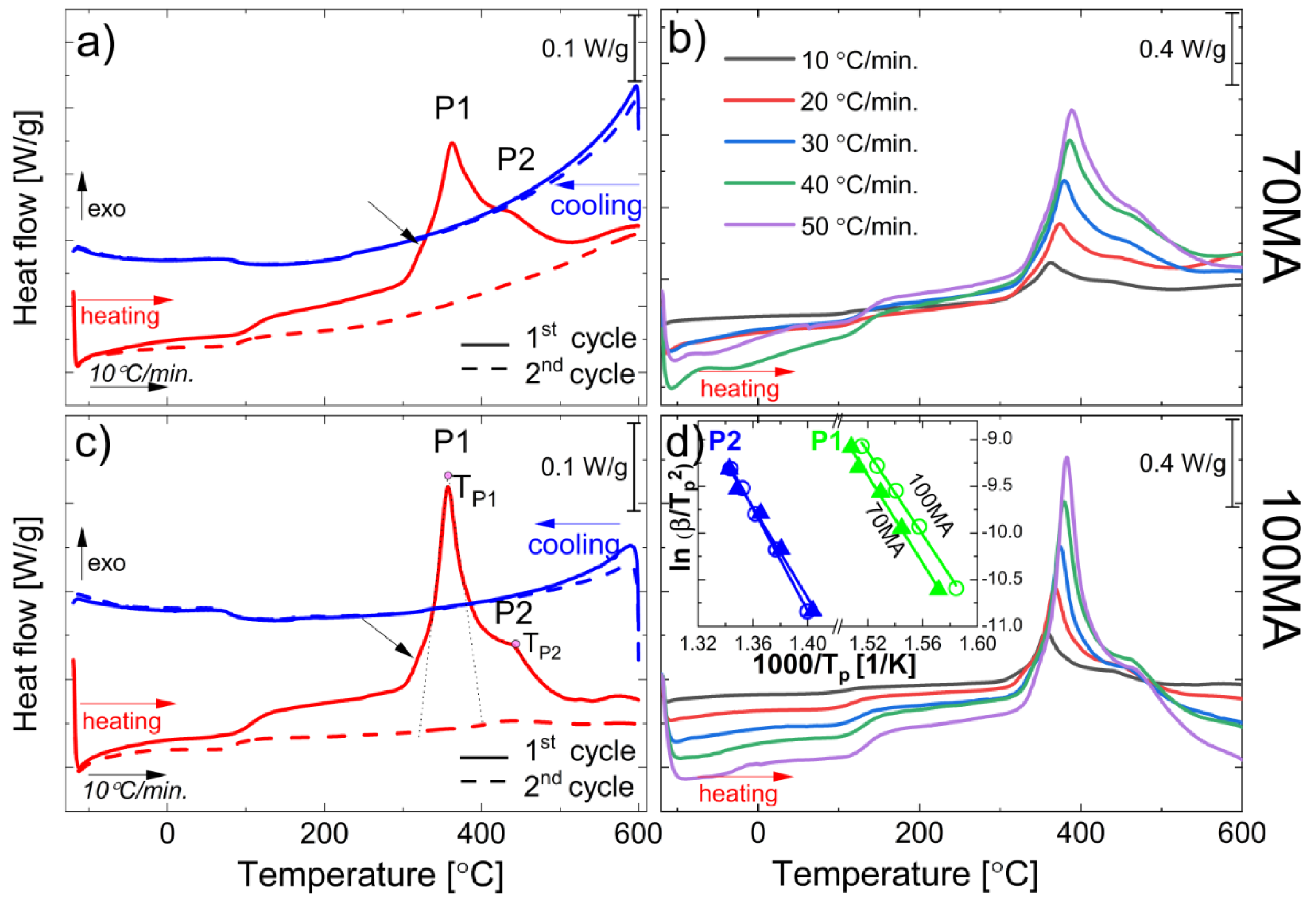
3.2.1. DSC Non-Isothermal Measurements
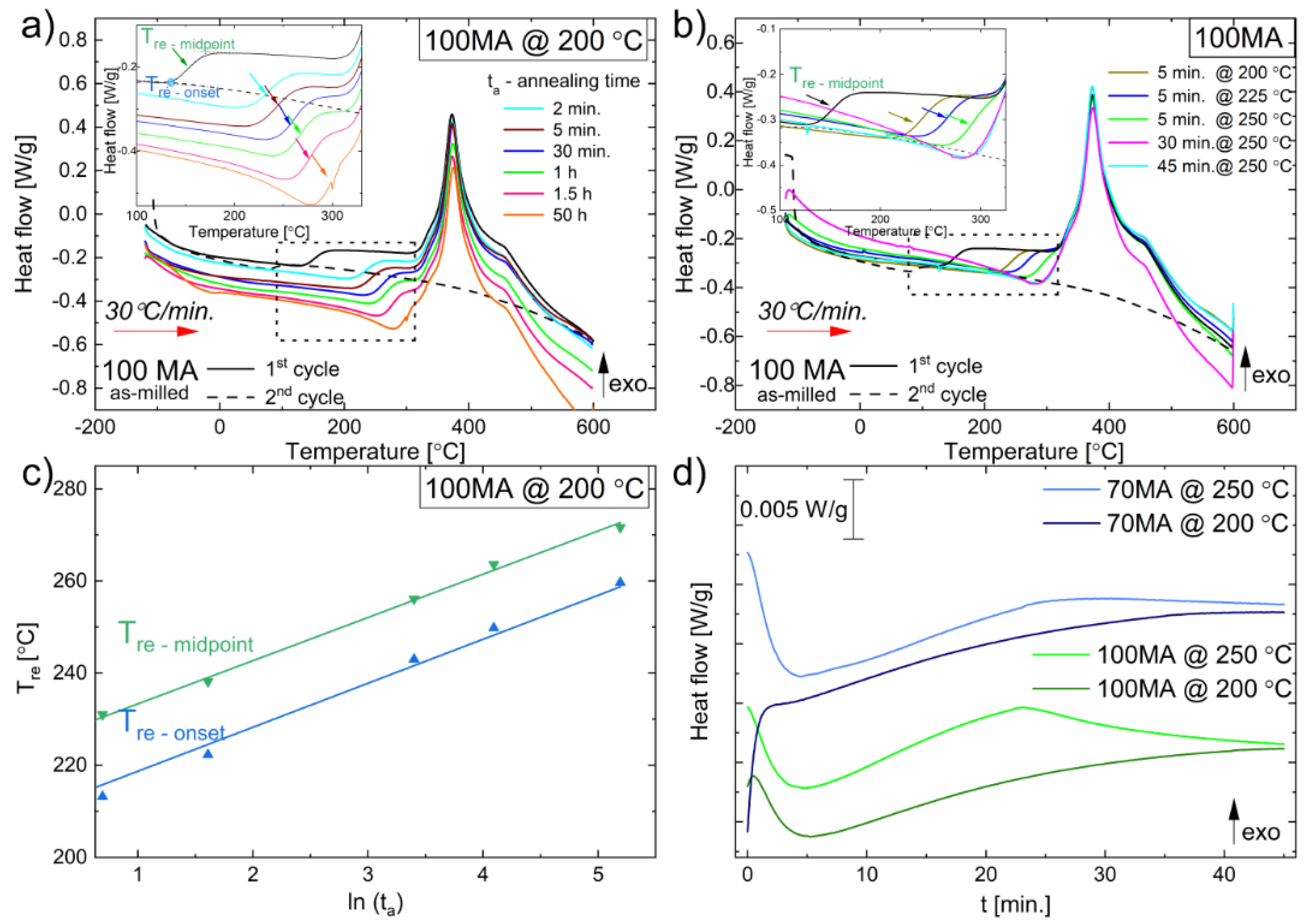
3.2.2. Relaxation Process
3.2.3. Activation Energy of P1 and P2 Thermal Processes
3.2.4. DSC Isothermal Measurements
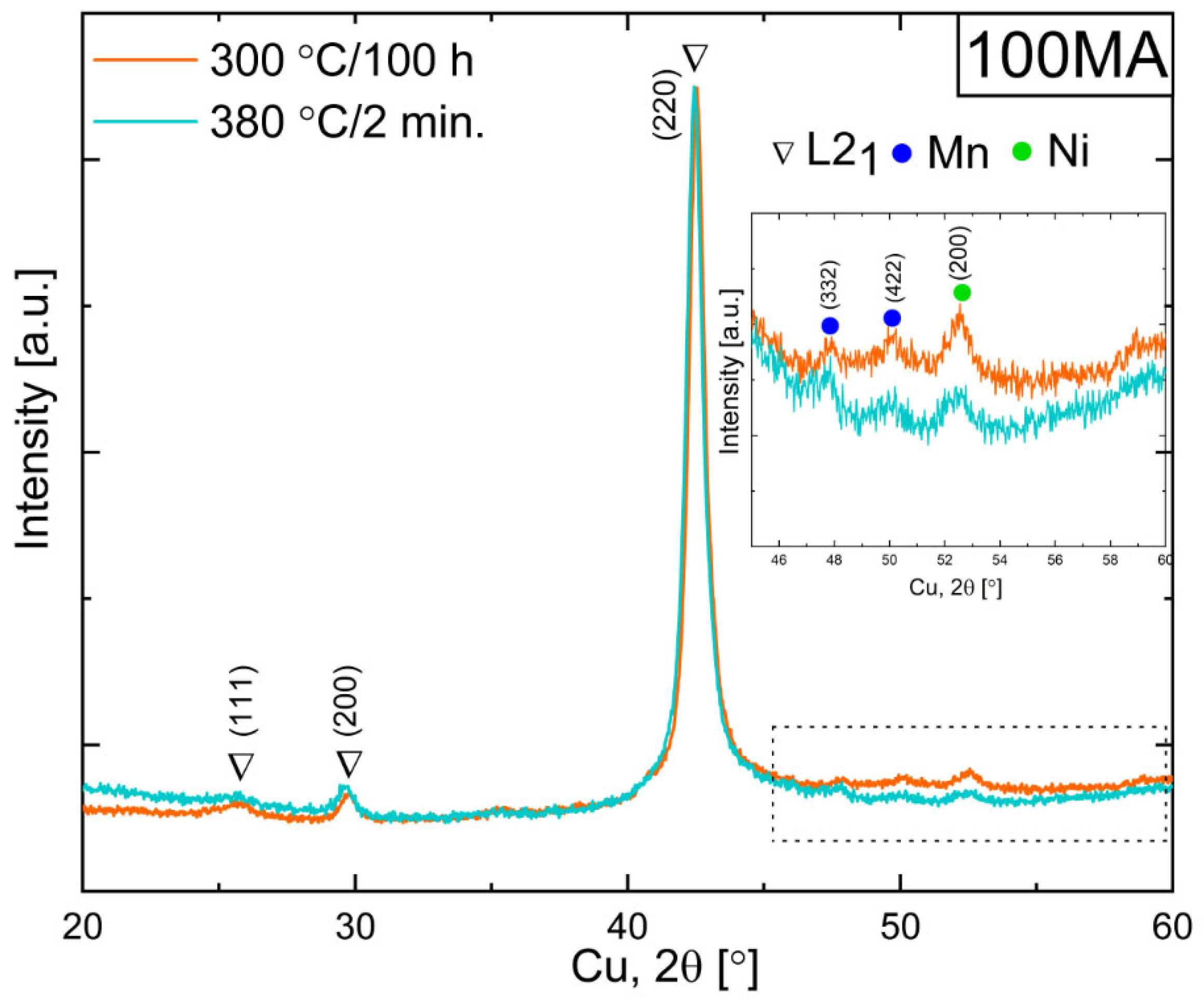
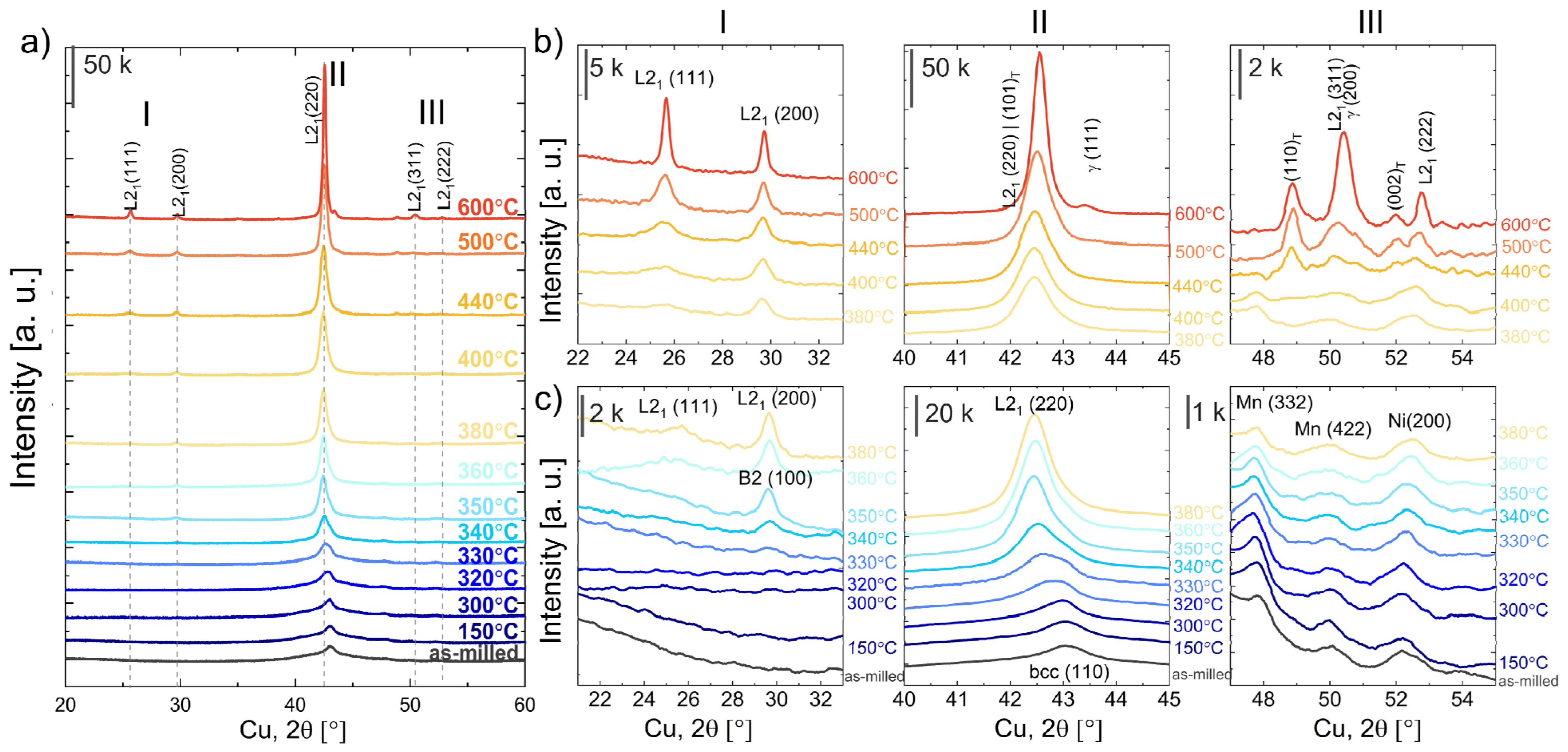
3.3. Effect of Powders’ Annealing on the Phase Transformations
3.4. XRD Phase Analysis of Annealed Powders
4. Summary and Conclusions
- (1)
- Further ordering of L21 phase and its growth (the fully ordered L21 Heusler structure was observed for powder annealed at 600 °C);
- (2)
- Dissolution of the residual Ni and Mn;
- (3)
- MnNi (P4/mmm) phase formation;
- (4)
- γ (fcc) phases precipitation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karaca, H.E.; Karaman, I.; Basaran, B.; Ren, Y.; Chumlyakov, Y.I.; Maier, H.J. Magnetic Field-Induced Phase Transformation in NiMnCoIn Magnetic Shape-Memory Alloys-A New Actuation Mechanism with Large Work Output. Adv. Funct. Mater. 2009, 19, 983–998. [Google Scholar] [CrossRef]
- Bruno, N.M.; Wang, S.; Karaman, I.; Chumlyakov, Y.I. Reversible martensitic transformation under low magnetic fields in magnetic shape memory alloys. Sci. Rep. 2017, 7, 40434. [Google Scholar] [CrossRef]
- Liu, J.; Gottschall, T.; Skokov, K.P.; Moore, J.D.; Gutfleisch, O. Giant magnetocaloric effect driven by structural transitions. Nat. Mater. 2012, 11, 620–626. [Google Scholar] [CrossRef]
- Bruno, N.M.; Salas, D.; Wang, S.; Roshchin, I.V.; Santamarta, R.; Arroyave, R.; Duong, T.; Chumlyakov, Y.I.; Karaman, I. Acta Materialia on the microstructural origins of martensitic transformation arrest in a NiCoMnIn magnetic shape memory alloy. Acta Mater. 2018, 142, 95–106. [Google Scholar] [CrossRef]
- Kainuma, R.; Imano, Y.; Ito, W.; Sutou, Y.; Morito, H.; Okamoto, S.; Kitakami, O.; Oikawa, K.; Fujita, A.; Kanomata, T.; et al. Magnetic-field-induced shape recovery by reverse phase transformation. Nature 2006, 439, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Peruman, K.V.; Chokkalingam, R.; Mahendran, M. Annealing effect on phase transformation in nano structured Ni-Mn-Ga ferromagnetic shape memory alloy. Phase Transit. 2010, 83, 509–517. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ren, Y.; Nie, Z.; Liu, D.M.; Zuo, L.; Choo, H.; Li, H.; Liaw, P.K.; Yan, J.Q.; McQueeney, R.; et al. Structural transition of ferromagnetic nanoparticles. J. Appl. Phys. 2007, 101, 063530. [Google Scholar] [CrossRef]
- Tian, B.; Chen, F.; Liu, Y.; Zheng, Y.F. Structural transition and atomic ordering of Ni49.8Mn28.5Ga21.7 ferromagnetic shape memory alloy powders prepared by ball milling. Mater. Lett. 2008, 62, 2851–2854. [Google Scholar] [CrossRef]
- Santos, I.A.; Coelho, A.A.; Gama, S. Structural, microstructural and magnetocaloric investigations in high-energy ball. Solid State Commun. 2008, 148, 289–292. [Google Scholar] [CrossRef]
- Mostafaei, A.; Vecchis, P.R.D.; Stevens, E.L.; Chmielus, M. Acta Materialia Sintering regimes and resulting microstructure and properties of binder jet 3D printed Ni-Mn-Ga magnetic shape memory alloys. Acta Mater. 2018, 154, 355–364. [Google Scholar] [CrossRef]
- Tian, F.; Cao, K.; Zhang, Y.; Zeng, Y.; Zhang, R.; Chang, T.; Zhou, C.; Xu, M.; Song, X.; Yang, S. Giant spontaneous exchange bias triggered by crossover of superspin glass in Sb-doped Ni50Mn38Ga12 Heusler alloys. Sci. Rep. 2016, 6, 30801. [Google Scholar] [CrossRef]
- Hosoda, H.; Takeuchi, S.; Inamura, T.; Wakashima, K.; Hosoda, H.; Takeuchi, S.; Inamura, T.; Wakashima, K. Material design and shape memory properties of smart composites composed of polymer and ferromagnetic shape memory alloy particles Material design and shape memory properties of smart composites composed of polymer and ferromagnetic shape memory alloy particles. Sci. Technol. Adv. Mater. 2004, 5, 503–509. [Google Scholar] [CrossRef]
- Tian, B.; Chen, F.; Tong, Y.X.; Li, L.; Zheng, Y.F. Bending properties of epoxy resin matrix composites fi lled with Ni-Mn-Ga ferromagnetic shape memory alloy powders. Mater. Lett. 2009, 63, 1729–1732. [Google Scholar] [CrossRef]
- Stevens, E.; Kimes, K.; Salazar, D.; Mostafaei, A.; Rodriguez, R.; Chernenko, V.; Chmielus, M.; Acierno, A.; Patricia, L. Mastering a 1.2 K hysteresis for martensitic para-ferromagnetic partial transformation in Ni-Mn (Cu)-Ga magnetocaloric material via binder jet 3D printing. Addit. Manuf. 2020, 37, 101560. [Google Scholar] [CrossRef]
- Scheerbaum, N.; Hinz, D.; Gutfleisch, O.; Müller, K.-H.; Schultz, L. Textured polymer bonded composites with Ni-Mn-Ga magnetic shape memory particles. Acta Mater. 2007, 55, 2707–2713. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Wang, Z.H.; Guo, E.J.; Tan, C.L.; Tian, X.H. Crystallization kinetics of Ni 51 Mn 36 Sn 13 free-standing alloy thin films. Vaccum 2016, 130, 124–129. [Google Scholar] [CrossRef]
- Huang, M. Kinetic study on the phase transformations in sputter deposited NiMn thin films. J. Appl. Phys. 2005, 97, 064906. [Google Scholar] [CrossRef]
- Ladwig, P.F.; Yang, Y.; Ding, L.; Tsu, I.F.; Chang, Y.A. Paramagnetic-to-antiferromagnetic phase transformation in sputter-deposited Ni-Mn thin films. J. Electron. Mater. 2003, 32, 1155–1159. [Google Scholar] [CrossRef]
- Huang, M.; Ladwig, P.F.; Chang, Y.A. Phase transformations in sputter deposited NiMn thin films. Thin Solid Films 2005, 478, 137–140. [Google Scholar] [CrossRef]
- Tian, B.; Chen, F.; Tong, Y.X.; Li, L.; Zheng, Y.F.; Liu, Y.; Li, Q.Z. Phase transition of Ni-Mn-Ga alloy powders prepared by vibration ball milling. J. Alloys Compd. 2011, 509, 4563–4568. [Google Scholar] [CrossRef]
- Sánchez-Alarcos, V.; Recarte, V.; Pérez-Landazábal, J.I.; Larumbe, S.; Caballero-Flores, R.; Unzueta, I.; García, J.A.; Plazaola, F.; Rodríguez-Velamazán, J.A. Mechanically induced disorder and crystallization process in Ni-Mn-In ball-milled alloys. J. Alloys Compd. 2016, 689, 983–991. [Google Scholar] [CrossRef]
- Liu, D.M.; Nie, Z.H.; Ren, Y.; Wang, Y.D.; Pearson, J.; Liaw, P.K.; Brown, D.E. Structural transitions and magnetic properties of Ni50Mn 36.7In13.3 particles with amorphous-like phase. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 3062–3070. [Google Scholar] [CrossRef]
- Matyja, E.; Prusik, K.; Zubko, M.; Dercz, G.; Glowka, K. Structure of the Ni-Co-Mn-In alloy obtained by mechanical alloying and sintering. J. Alloys Compd. 2019, 801, 529–535. [Google Scholar] [CrossRef]
- Recarte, V.; Unzueta, I.; García, J.A.; Plazaola, F.; Roca, P. La Effect of high-energy ball-milling on the magnetostructural properties of a Ni45Co5Mn35Sn15 alloy. J. Alloys Compd. 2021, 858, 158350. [Google Scholar] [CrossRef]
- Peterlechner, M.; Bokeloh, J.; Wilde, G.; Waitz, T. Study of relaxation and crystallization kinetics of NiTi made amorphous by repeated cold rolling. Acta Mater. 2010, 58, 6637–6648. [Google Scholar] [CrossRef]
- Belyaev, S.; Sergey, M.; Frolova, N.; Pilyugin, V.; Resnina, N.; Slesarenko, V.; Zeldovich, V. Structure and Properties of TiNi Alloy Subjected to Severe Plastic Deformation and Subsequent Annealing. Mater. Sci. Forum 2013, 738–739, 518–524. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature with Heating Rate in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Lelito, J. Crystallization Kinetics Analysis of the Amorphouse Mg 72 Zn 24 Ca 4 Alloy at the Isothermal Annealing. Materials 2020, 13, 2815. [Google Scholar] [CrossRef]
- Chen, L.C.; Spaepen, F. Analysis of calorimetric measurements of grain growth. J. Appl. Phys. 1991, 69, 679–688. [Google Scholar] [CrossRef]
- Jin, J.; Li, F.; Yin, G.; Wang, X.; Gong, P. Thermochimica Acta Influence of substitution of Cu by Ni on the crystallization kinetics of TiZrHfBeCu high entropy bulk metallic glass. Thermochim. Acta 2020, 690, 178650. [Google Scholar] [CrossRef]


| β [°C/min] | 70MA TP1 [°C] | 70MA TP2 [°C] | 100MA TP1 [°C] | 100MA TP2 [°C] |
|---|---|---|---|---|
| 10 | 363.2 | 439.3 | 358.0 | 441.3 |
| 20 | 374.2 | 451.2 | 368.9 | 453.2 |
| 30 | 380.7 | 459.2 | 375.9 | 461.2 |
| 40 | 387.6 | 468.3 | 381.7 | 466.3 |
| 50 | 389.9 | 471.5 | 386.6 | 471.0 |
| Sample | Ea [kJ/mol] P1 | Ea [kJ/mol] P2 |
|---|---|---|
| 70MA | 195 (8) | 202 (12) |
| 100MA | 185 (6) | 228 (3) |
| Alloy Composition, State (Form) | Manufacturing Method | Activation Energy Ea | Process | Ref. |
|---|---|---|---|---|
| Ni45.5Co4.5Mn36.6In13.4 powder | Mechanical alloying (100MA) | 185(6) kJ/mol 228(3) kJ/mol | 300 °C to 340 °C amorphous phase → bcc 340 °C: bcc → B2 360 °C: (P1) B2 → L21 (P1) 440 °C: (P2) Mn (α-Mn) → MnNi (P4/mmm) | This work |
| Ni50Mn34In16 powder | high-energy ball milling | 164.0 kJ/mol (1) 173.7 kJ/mol (2) | two-step (1) (2) transformation amorphous-like phase → B2-ordered structure | [22] |
| Ni49.8Mn28.5Ga21.7 powders | high-energy ball milling | 209(8) kJ/mol | fcc → bcc | [21] |
| Ni50Mn36.7In13.3 powder | high-energy ball milling | No data | 523 K (250 °C) amorphous like phase → B2 annealing at 684 K (411 °C) 30 min B2 → L21 with tetragonal distortion | [23] |
| Ni49.8Mn28.5Ga21.7 powders | high-energy ball milling | No data | (305 °C) fct → bcc (fct—face-centered tetragonal) (360 °C) L21 nucleation and growth with increased annealing temperature (sample annealed at 800 °C—full Heusler structure) | [8] |
| NiMn films 1 | DC magnetron sputtering | 139 kJ/mol | disordered fcc → L10 (L10—tetragonal) | [19] |
| NiMn films 1 | DC magnetron sputtering | 121 kJ/mol 140 kJ/mol | (1) Amorphous → fcc (2) fcc → L10 | [18] |
| NiMn films 1 | DC magnetron sputtering | No data | (1) 160 °C MnO formation (2) 320 °C—amorphous like phase → fcc (3) 360 °C fcc → L10 (4) 440 °C grain growth | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matyja, E.; Prusik, K.; Zubko, M.; Świec, P.; Dercz, G.; Loskot, J. Crystallization Kinetics and Structure Evolution during Annealing of Ni-Co-Mn-In Powders Obtained by Mechanical Alloying. Materials 2023, 16, 645. https://doi.org/10.3390/ma16020645
Matyja E, Prusik K, Zubko M, Świec P, Dercz G, Loskot J. Crystallization Kinetics and Structure Evolution during Annealing of Ni-Co-Mn-In Powders Obtained by Mechanical Alloying. Materials. 2023; 16(2):645. https://doi.org/10.3390/ma16020645
Chicago/Turabian StyleMatyja, Edyta, Krystian Prusik, Maciej Zubko, Paweł Świec, Grzegorz Dercz, and Jan Loskot. 2023. "Crystallization Kinetics and Structure Evolution during Annealing of Ni-Co-Mn-In Powders Obtained by Mechanical Alloying" Materials 16, no. 2: 645. https://doi.org/10.3390/ma16020645
APA StyleMatyja, E., Prusik, K., Zubko, M., Świec, P., Dercz, G., & Loskot, J. (2023). Crystallization Kinetics and Structure Evolution during Annealing of Ni-Co-Mn-In Powders Obtained by Mechanical Alloying. Materials, 16(2), 645. https://doi.org/10.3390/ma16020645








