Microstructure and Crystallization Kinetics of Silica-Based Ceramic Cores with Enhanced High-Temperature Property
Abstract
1. Introduction
2. Experimental Procedure
2.1. Raw Materials
2.2. Preparation of Ceramic Cores
2.3. Testing and Characterization
3. Results and Discussion
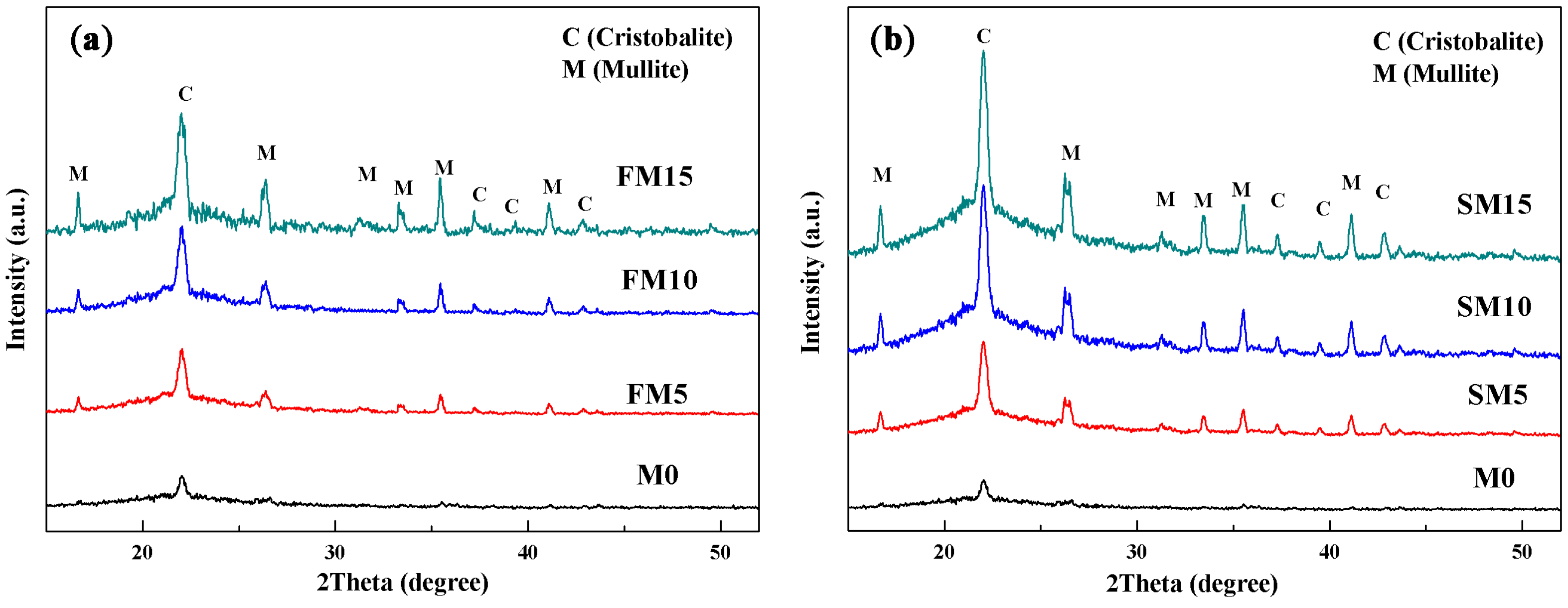
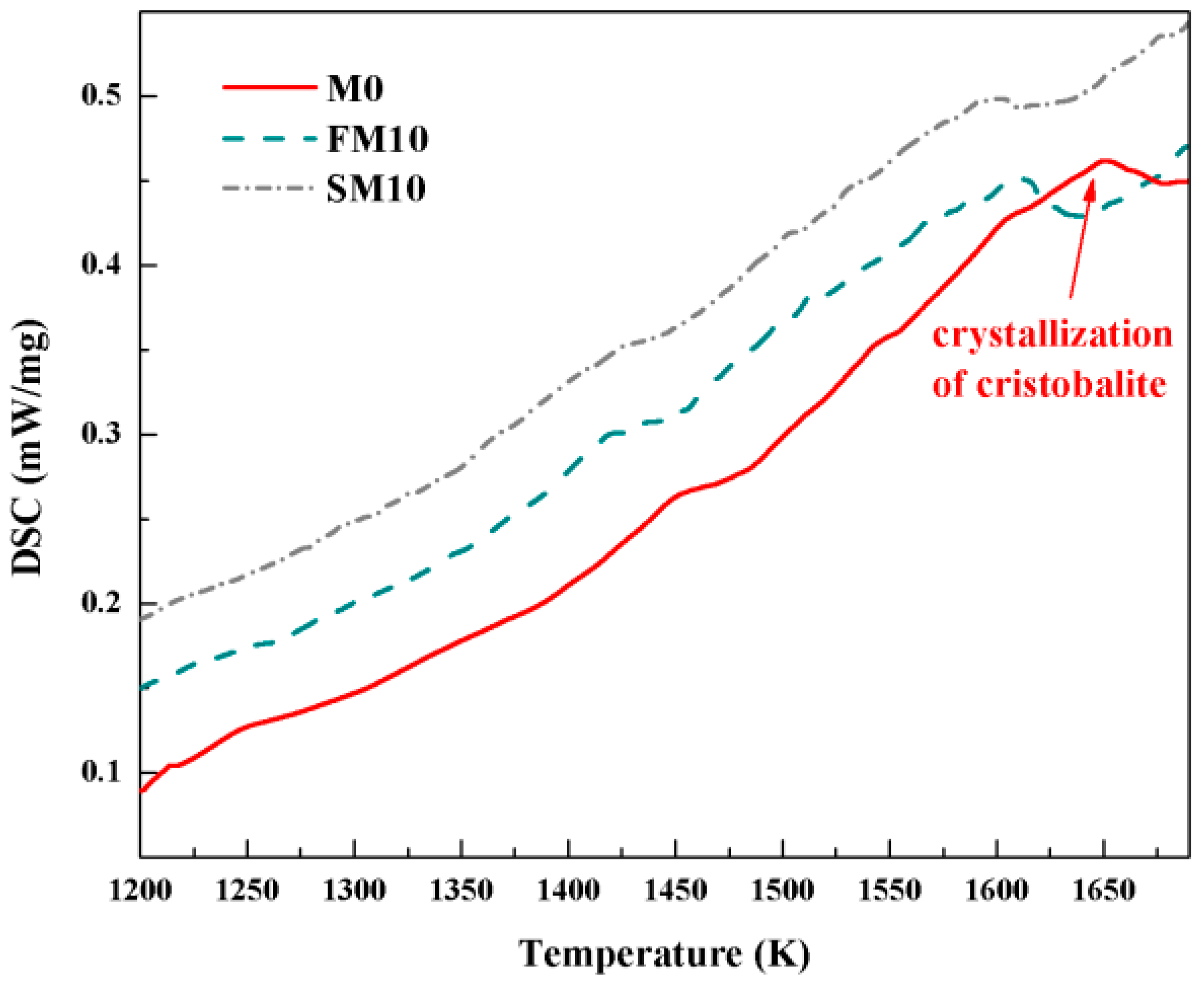
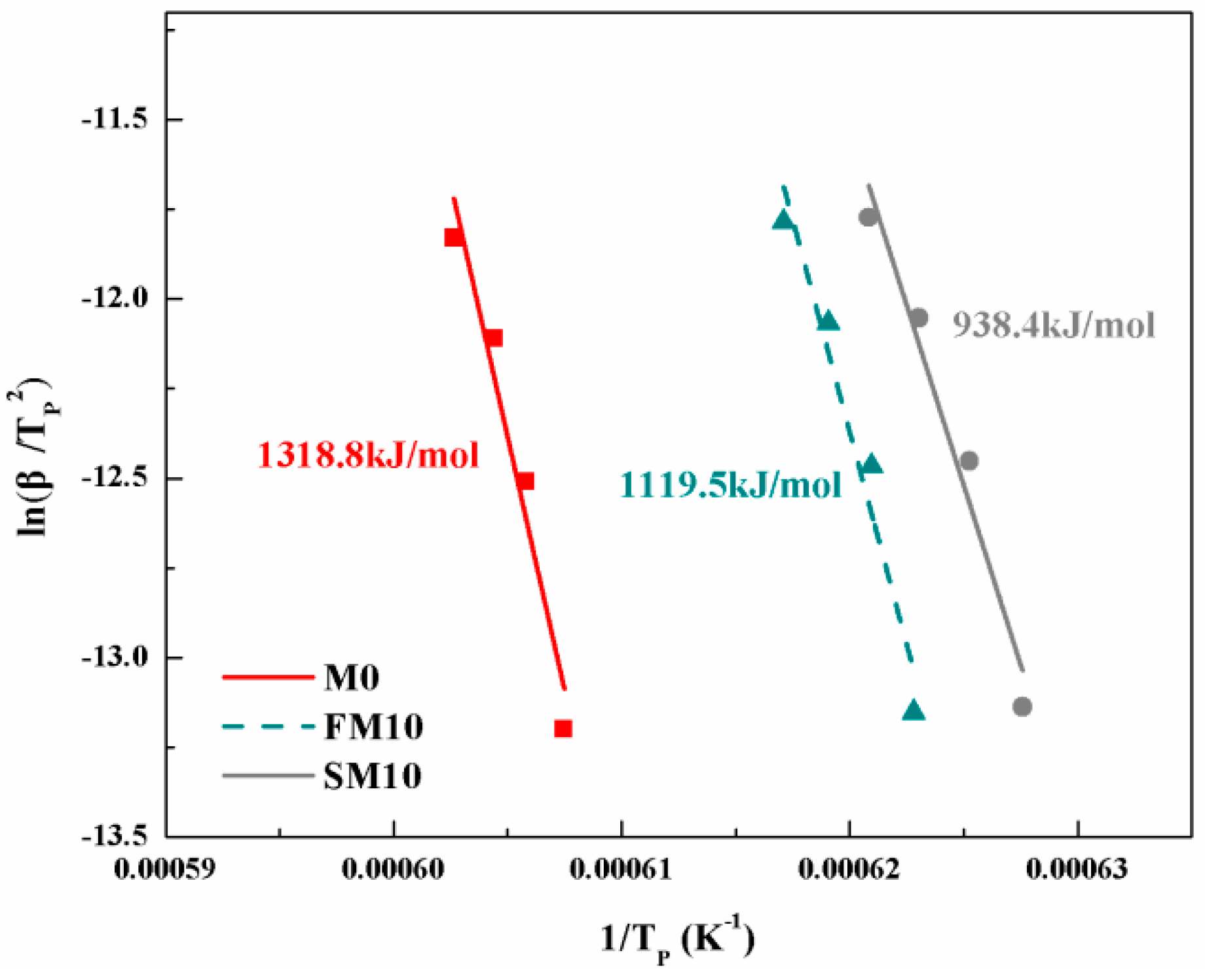
3.1. Phase Composition and Crystallization Kinetics
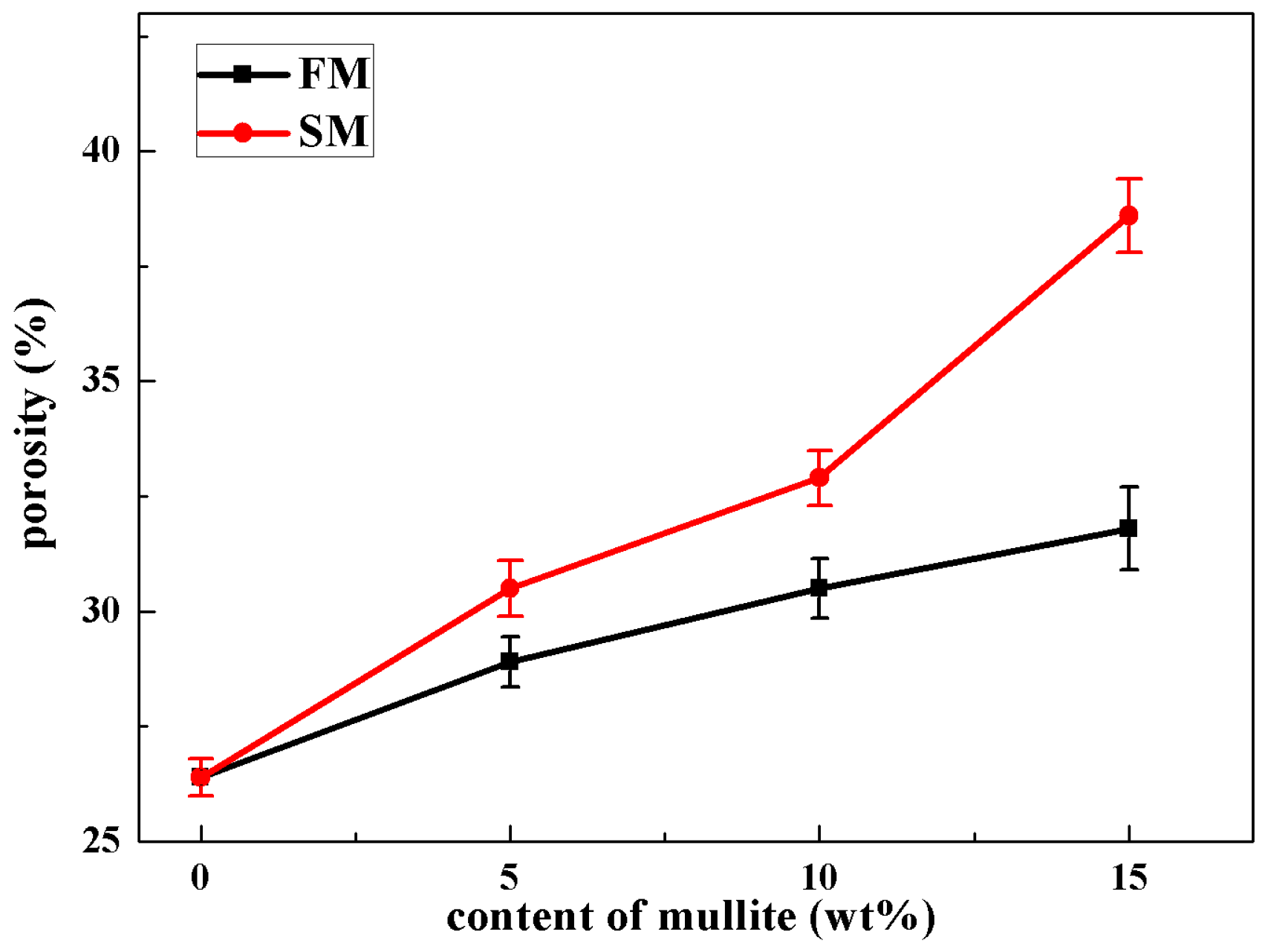
3.2. Microstructures
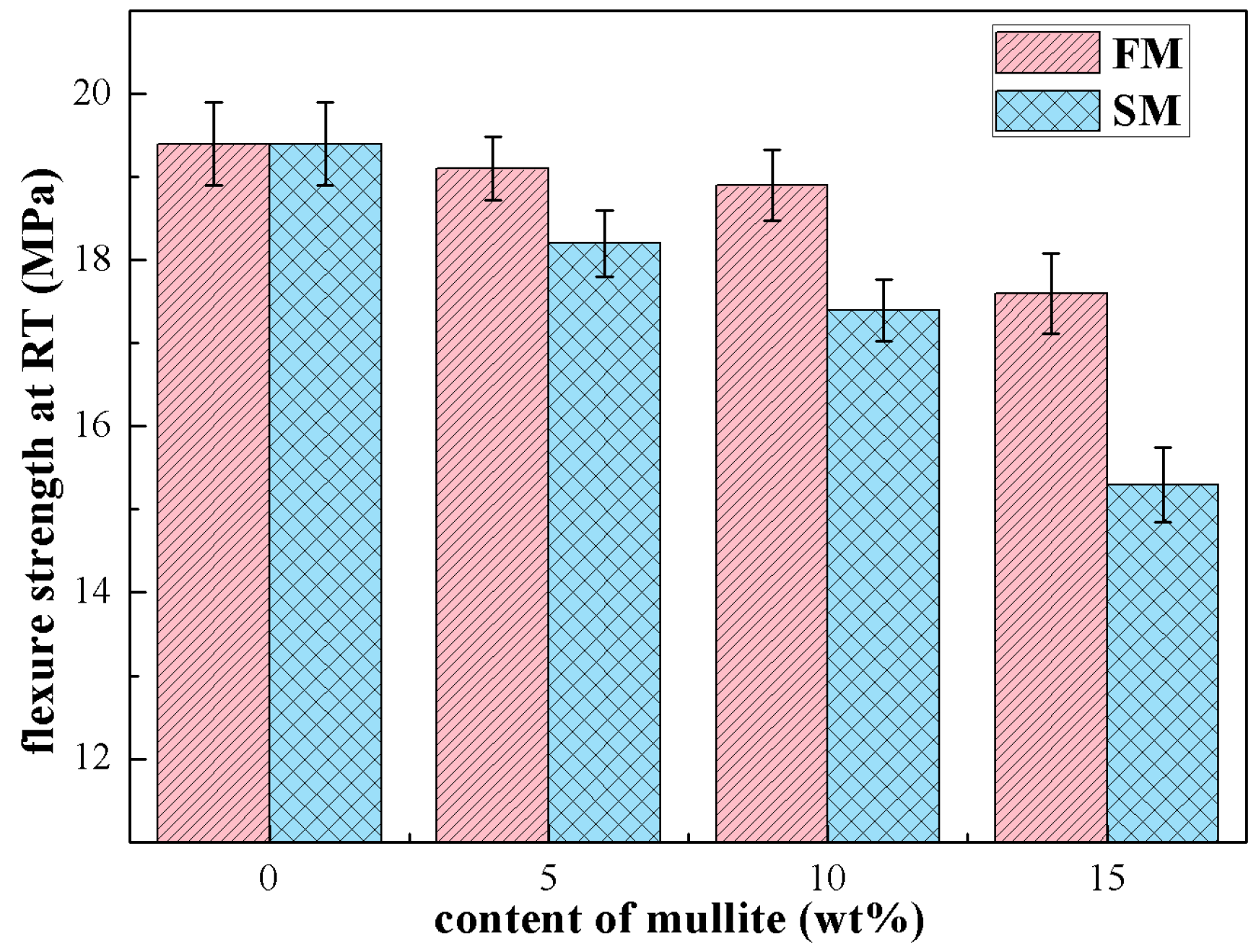
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanyo, J.E.; Schaffoner, S.; Uwanyuze, R.S.; Leary, K.S. An overview of ceramic molds for investment casting of nickel superalloys. J. Eur. Ceram. Soc. 2020, 40, 4955–4973. [Google Scholar] [CrossRef]
- Niu, S.X.; Xu, X.Q.; Li, X.; Chen, X.; Luo, Y.S. Microstructure evolution and properties of silica-based ceramic cores reinforced by mullite fibers. J. Alloys Compd. 2020, 829, 154494. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Yang, Z.G.; Yin, Z.Q.; Chen, B.; Yu, J.B.; Ren, Z.M.; Yu, G.; Zhang, G.L. Investigation of the properties and leaching characteristics of ceramic cores fabricated using BaZrO3 as the raw material. Mater. Chem. Phys. 2021, 272, 124925. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process—A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Gromada, M.; Świeca, A.; Kostecki, M.; Olszyna, A.; Cygan, R. Ceramic cores for turbine blades via injection moulding. J. Mater. Process. Technol. 2015, 220, 107–112. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhao, Z.J.; Yu, J.B.; Ren, Z.M. Preparation of silica ceramic cores by the preceramic pyrolysis technology using silicone resin as precursor and binder. Mater. Chem. Phys. 2019, 223, 676–682. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.S.; Liu, Y.S.; Zeng, Q.F.; Liang, J.J. Silica strengthened alumina ceramic cores prepared by 3D printing. J. Eur. Ceram. Soc. 2021, 41, 2938–2947. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T. Silica and silica-based materials for biotechnology, polymer composites, and environmental protection. Materials 2022, 15, 7703. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, Z.; Zhao, Z.; Yu, J.; Ren, Z.; Zhang, G.; Yu, G. Microstructure and properties of SiO2-based ceramic cores with ball-shaped powders by the preceramic polymer technique in N2 atmosphere. Mater. Chem. Phys. 2020, 243, 122609. [Google Scholar] [CrossRef]
- Bae, J.; Kim, D.; Halloran, J.W. Mechanical and kinetic studies on the refractory fused silica of integrally cored ceramic mold fabricated by additive manufacturing. J. Eur. Ceram. Soc. 2019, 39, 618–623. [Google Scholar] [CrossRef]
- Jin, W.F.; Tao, Y.; Wang, X.; Gao, Z. The effect of carbon nanotubes on the strength of sand seeped by colloidal silica in triaxial testing. Materials 2021, 14, 6119. [Google Scholar] [CrossRef]
- Chao, C.; Lu, H. Optimal composition of zircon-fused silica ceramic cores for casting superalloys. J. Am. Ceram. Soc. 2002, 85, 773–779. [Google Scholar] [CrossRef]
- Wilson, P.J.; Blackburn, S.; Greenwood, R.W.; Prajapti, B.; Smalley, K. The role of zircon particle size distribution, surface area and contamination on the properties of silica-zircon ceramic materials. J. Eur. Ceram. Soc. 2011, 31, 1849–1855. [Google Scholar] [CrossRef]
- Liang, J.J.; Lin, Q.H.; Zhang, X.; Jin, T.; Zhou, Y.Z.; Sun, X.F.; Choi, B.G.; Kim, I.S.; Do, J.H.; Jo, C.Y. Effects of alumina on cristobalite crystallization and properties of silica-based ceramic cores. J. Mater. Sci. Technol. 2017, 33, 204–209. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yeo, J.; Lee, J.S.; Choi, S.C. Influence of silicon carbide as a mineralizer on mechanical and thermal properties of silica-based ceramic cores. Ceram. Int. 2016, 42, 14738–14742. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Zhou, L.; Xu, X.; Niu, S.; Li, X.; Chen, X. Microstructure and properties evolution of silicon-based ceramic cores fabricated by 3D printing with stair-stepping effect control. J. Eur. Ceram. Soc. 2021, 41, 4650–4657. [Google Scholar] [CrossRef]
- Kazemia, A.; Faghihi-Sani, M.A.; Alizadeh, H.R. Investigation on cristobalite crystallization in silica-based ceramic cores for investment casting. J. Eur. Ceram. Soc. 2013, 33, 3397–3402. [Google Scholar] [CrossRef]
- Breneman, R.C.; Halloran, J.W. Effect of cristobalite on the strength of sintered fused silica above and below the cristobalite transformation. J. Am. Ceram. Soc. 2015, 98, 1611–1617. [Google Scholar] [CrossRef]
- Yang, H.L.; Li, Z.S.; Ding, Y.D.; Ge, Q.Q.; Shi, Y.J.; Jiang, L. Effect of silicon source (fly ash, silica dust, gangue) on the preparation of porous mullite ceramics from aluminum dross. Materials 2022, 15, 7212. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, B.; Zhu, Q.; Zhang, X.; Zhang, H.; Yu, J. Preparation and properties of mullite-bonded porous fibrous mullite ceramics by an epoxy resin gel-casting process. Ceram. Int. 2017, 43, 5478–5483. [Google Scholar] [CrossRef]
- Brzeziński, K.; Duda, A.; Styk, A.; Kowaluk, T. Photogrammetry-based volume measurement framework for the particle density estimation of LECA. Materials 2022, 15, 5388. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Zhang, Y.; Chen, J. K value method calculation of crystallization of porous silicon-based ceramic cores and the corresponding performance analysis. Rare. Metal Mat. Eng. 2013, 42, 535–537. [Google Scholar]
- Uhlman, A.R. Crystallization and Melting in Glass Forming Systems; Plenum Press: New York, NY, USA, 1969; pp. 172–197. [Google Scholar]
- Li, X.; Zhang, Z.; Yang, Y.; Fan, J.; Kong, J.; Wang, K. The crystallization mechanism of zr-based bulk metallic glass during electron beam remelting. Materials 2020, 13, 3488. [Google Scholar] [CrossRef] [PubMed]
- Augis, I.A.; Bennett, J.E. Calculation of the Aroma Parameters for Heterogeneous Solid State Reactions Using a Modification of the Kissinger Method. J. Therm. Anal. 1978, 13, 283–292. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, S.H.; Ahn, H.K. Observation of nucleation effect on crystallization in lithium aluminosilicate glass by viscosity measurement. J. Non-Cryst. Solids 2004, 336, 195–201. [Google Scholar] [CrossRef]





| Raw Powders | Chemical Formula | Purity | Average Grain Size | Producer |
|---|---|---|---|---|
| Fused silica | SiO2 | >99.95% | 30.57 μm | Jiangsu |
| Fused mullite | 3Al2O3·2SiO2 | >95% | 18.9 μm | Henan |
| Sintered mullite | 3Al2O3·2SiO2 | >95% | 19.9 μm | Shandong |
| Raw Powders | Compositions and Contents (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | Na2O | K2O | Fe2O3 | CaO | MgO | |
| Fused mullite | 75.88 | 22.94 | 0.24 | 0.17 | 0.11 | 0.16 | <0.1 |
| Sintered mullite | 73.56 | 22.28 | 0.58 | 0.34 | 1.64 | 0.6 | 0.54 |
| Phases | M0 | FM5 | FM10 | FM15 | SM5 | SM10 | SM15 |
|---|---|---|---|---|---|---|---|
| cristobalite | 2.2 | 7.9 | 11.5 | 14.2 | 11.4 | 18.9 | 24.1 |
| mullite | 0 | 5.2 | 10.2 | 15.4 | 5.1 | 10.3 | 15.5 |
| amorphous silica | 97.8 | 86.9 | 78.3 | 70.4 | 83.5 | 70.8 | 60.4 |
| Sample | β = 20 K/min | β = 15 K/min | β = 10 K/min | β = 5 K/min |
|---|---|---|---|---|
| M0 | 1659.4 | 1654.5 | 1650.7 | 1646.4 |
| FM10 | 1620.5 | 1615.4 | 1610.5 | 1605.7 |
| SM10 | 1610.8 | 1605.1 | 1599.4 | 1593.5 |
| Sample | β = 20 K/min | β = 15 K/min | β = 10 K/min | β = 5 K/min | Mean Value of n |
|---|---|---|---|---|---|
| M0 | 1.67 | 1.58 | 1.49 | 1.38 | 1.53 |
| FM10 | 1.90 | 1.80 | 1.71 | 1.56 | 1.74 |
| SM10 | 2.04 | 1.95 | 1.85 | 1.61 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Niu, S.; Wang, D.; Li, J.; Jiao, Q.; Guo, X.; Xu, X. Microstructure and Crystallization Kinetics of Silica-Based Ceramic Cores with Enhanced High-Temperature Property. Materials 2023, 16, 606. https://doi.org/10.3390/ma16020606
Li X, Niu S, Wang D, Li J, Jiao Q, Guo X, Xu X. Microstructure and Crystallization Kinetics of Silica-Based Ceramic Cores with Enhanced High-Temperature Property. Materials. 2023; 16(2):606. https://doi.org/10.3390/ma16020606
Chicago/Turabian StyleLi, Xin, Shuxin Niu, Dongsheng Wang, Jie Li, Qi Jiao, Xinlong Guo, and Xiqing Xu. 2023. "Microstructure and Crystallization Kinetics of Silica-Based Ceramic Cores with Enhanced High-Temperature Property" Materials 16, no. 2: 606. https://doi.org/10.3390/ma16020606
APA StyleLi, X., Niu, S., Wang, D., Li, J., Jiao, Q., Guo, X., & Xu, X. (2023). Microstructure and Crystallization Kinetics of Silica-Based Ceramic Cores with Enhanced High-Temperature Property. Materials, 16(2), 606. https://doi.org/10.3390/ma16020606





