Abstract
The double perovskite halides A2BIBIIIX6 provide flexibility for various formulation adjustments and are of less toxicity in comparison with well-discussed complex lead halide derivatives. Such type of structure can be formed by replacing two Pb2+ ions in the cubic lattice with a pair of non-toxic heterovalent (monovalent and trivalent) metal cations, such as silver and indium. The aim of this work is to briefly characterize the phase equilibria in the ternary system CsBr-AgBr-InBr3 and investigate the thermodynamic availability of synthesis of Cs2AgInBr6 double perovskite phase by solid-state sintering or melt crystallization. The results demonstrate the unfeasibility of the Cs2AgInBr6 phase but high stability of the corresponding binary bromides perspective for optoelectronics.
1. Introduction
New materials for “green” energy technologies, including photovoltaics, have been of great importance over the past decades [1,2]. Metal halide perovskite photovoltaic systems have attracted interest from the scientific community and industry representatives. Interest was initiated by the work of Kojima et al. in 2006 using lead halide perovskite in a photoelectrochemical cell with a structure similar to colored solar cells, and later in 2009, it was published with an efficiency of 3.8% [3,4]. Solid-state perovskite solar cells developed by Kim et al. and Lee et al. in 2012 with efficiencies of 9.7% and 10.9%, respectively, became a breakthrough in the field of perovskite solar cells [5,6]. With each passing year, the efficiency of lead-based perovskite solar cells improved, which made them attractive materials all around the world. Nowadays, the efficiency of perovskite solar cells has exceeded 25% [7]. The rapid increase in efficiency is attributed to the excellent optoelectronic properties of lead halide-based perovskites, originated from a suitable forward bandgap, high absorption coefficient, long-range charge diffusion length, balanced electron and hole mobility, high dielectric constant, excellent carrier mobility and low binding energy of halide excitons. Complex halides with perovskite or perovskite-like structure have found application in optoelectronics, such as emitters in light-emitting diodes (LED) [8,9], lasers [10], materials for photodetectors [11,12].
Despite their impressive characteristics, serious disadvantages of the most efficient light harvesters with perovskite structure are their toxicity [13,14], poor resistance to heat [15,16], light [16,17], oxygen [17], and self-decomposition [18]. The presence of lead in the chemical composition of hybrid organo-inorganic perovskites of lead halides makes them toxic due to the easy dissolution of Pb2+ ions in water, which can cause environmental pollution harmful to humans and the ecosystem [15,17]. Researchers have made significant efforts to find less toxic and more stable alternative perovskites with good photophysical properties [19,20,21]. To reduce toxicity, efforts have been made in the past few years to replace lead with a divalent non-toxic metal cation while maintaining the perovskite structure. Sn and Ge appear to be the most suitable alternatives to Pb in the perovskite structure. It has been observed that non-toxic Sn-based halide perovskites decompose very quickly upon contact with air, mainly due to the Sn2+ oxidation state, which makes them less stable than Pb-based perovskites [22]. The low light absorption, low dielectric constant, and low optical conductivity of Ge-based lead-free perovskites are responsible for their low photovoltaic characteristics [23]. Materials in which other bivalent elements of the periodic table were used have also been tested as a substitute for lead in the perovskite structure. They also exhibit reduced optoelectronic properties due to the large band gap, high effective carrier masses, and low absorption [24,25].
Recent theoretical calculations show that the halide structure of double perovskite, A2BIBIIIX6, which can be formed by replacing two toxic Pb2+ ions in the crystal lattice with a pair of non-toxic heterovalent (monovalent and trivalent) metal cations, is a promising alternative for the implementation of high-performance, lead-free, and stable perovskite solar cells. Consequently, the crystal structure of double perovskite A2BIBIIIX6 deserves in-depth study since it provides the possibility of easier substitution and inclusion of various metal cations with different oxidation states in the B-position, various organic and inorganic ions in the A-position and variations in the halide composition in X-positions [26,27,28]. Halide double perovskites with Ag+ in the BI-position and a heavier halogen in the X-position can give a band gap suitable for photovoltaics [20,29].
Recently, a number of double perovskite compounds were synthesized [20,21,26]. At the same time, much more compounds with a double perovskite structure are predicted experimentally. Some of them would demonstrate a direct bandgap and high charge carrier mobility that is optimal for application in light-emitting diodes or photovoltaics. According to Wang et al. [30], the Cs2AgInBr6 phase has a direct bandgap of ~1.4 eV and excellent mobility of charge carriers that is promising for obtaining high-efficiency solar cells. Elsewhere, the authors found that Cs2AgInBr6 is stable mechanically [31]. According to Xiao et al. [29], in the ternary system CsBr-AgBr-InBr3, the formation of binary bromides CsAgBr2 and Cs3In2Br9 is thermodynamically more favorable. Consequently, here, we present the most recent experimental results on the synthesis of the double perovskite Cs2AgInBr6 and also investigations of phase equilibria for the ternary CsBr-AgBr-InBr3 system using single or binary bromides as precursors.
2. Experimental
2.1. Analysis Methods
X-ray analysis of the samples was carried out using a Rigaku 2500 D-max diffractometer (Rigaku, Tokyo, Japan) with a proportional point detector on CuKα radiation (λ = 1.5418 Å) rotating copper anode in the range 2θ of 10–80° with a step of 0.02°. The obtained data were processed using WinXPow (STOE & Cie, Version 1.07, Darmstadt, Germany) and Jana2006 (ECA-SIG#3/Institute of Physics, Prague, Czech Republic) applications.
To determine the elemental composition of the obtained samples, the materials were examined by X-ray emission microanalysis using a Leo Supra 50 VP instrument (LEO Carl Zeiss SMT Ltd., Oberkochen, Germany) with an X/MAX X-ray energy dispersive detector (EDS) (Oxford Instruments, High Wycombe, UK) at a electron accelerating voltage of 21 kV.
The Raman spectra were studied on an InVia Raman Microscope spectrometer (Renishaw, New Mills, UK) equipped with an argon laser with a wavelength of 532 nm and a helium-neon (He-Ne) laser with a power of 20 mW with a wavelength of 632.8 nm and a spectral filter (5% of the total intensity). All spectra were obtained using a 50× objective by 100-fold signal accumulation, excitation time 1 s. A plate of (100)-oriented single-crystal silicon with a characteristic Raman mode of 521.5 cm−1 served as a standard sample for the preliminary calibration of the device.
Differential scanning calorimetry was carried out on a high-temperature differential scanning calorimeter DSC 404 C Pegasus (NETZSCH, Selb, Germany), at a temperature range of 200–400 °C in an argon atmosphere.
2.2. Synthesis of Samples in Ternary System CsBr-AgBr-InBr3
Solid-phase and heterophase ampoule synthesis was used as the main approach in the synthesis of the compositions of double perovskites, binary bromides, as well as the study of various binary sections in the composition of these ternary systems [2]. Cesium bromide was taken after Sigma-Aldrich (99.999%). Silver bromide was synthesized via an ion-exchange process using silver nitrate (“Reahim”, pure) and potassium bromide (“Rushim”, pure) as precursors. A light-yellow precipitate was washed out with MilliQ distilled water and heated at 60 °C in nitrogen atmosphere. Indium tribromide was synthesized by the solution method using elementary indium (“RedkyMetal.RF”, 99.999%) and hydrobromic acid (“Reahim”, analytical grade) [32,33]. We dissolve metallic indium in a solution of hydrobromic acid. Further, this solution is evaporated at 60 °C.
This approach allows one to study phase equilibria in a closed vessel. Samples of simple bromides were placed in quartz ampoules with an inner diameter of 8 mm, the total weight of the samples was 2 g. The ampoules were sealed after their evacuation (0.072 bar). At the same time, when placing the components of the system in ampoules and choosing the conditions for heat treatment of the ampoules, the differences in the volatility of the components were taken into account.
Several points from the CsBr-AgBr-InBr3 system were selected for analysis of two “double perovskites” predicted theoretically (Figure 1a).
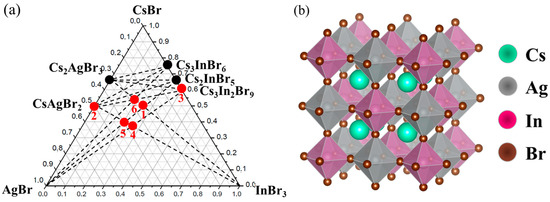
Figure 1.
Phase triangle for the (a) CsBr-AgBr-InBr3 and (b) CsBr-AgBr-InBr3 ternary systems with selected compositions marked as points.
According to the literature [22,29], the above-mentioned ternary systems contain phases with a double perovskite structure. Double perovskites of the composition Cs2AgInBr6 have a face-centered cubic structure with the space group Fm3m and with a lattice constant of about 11 Å. Unlike ABX3 perovskite, Cs2AgInBr6 consists of AgBr6 and InBr6 octahedra alternating along the [100], [010], and [001] directions. The B-site cations Ag+ and In3+ are formed in the form of an ordered structure of rock salt, as shown in Figure 1b.
Sample weights per 1 g of the finished sample are shown in Table 1 for the CsBr-AgBr-InBr3 system.

Table 1.
Weighed weights for samples of the CsBr-AgBr-InBr3 system, calculated per 1 g of the final product.
Samples of theoretically predicted composition Cs2AgInBr6 were synthesized by the solid-phase ampoule method. The conditions for annealing were T = 300–650 °C and then annealed at this temperature for 96 h, according to the conditions of solid-phase synthesis of similar compositions [34].
3. Results and Discussion
Point ‘1’ in CsBr-AgBr-InBr3 Gibbs’s triangle corresponds to a “double perovskite” composition Cs2AgInBr6. The synthesis was performed at different temperatures (300 °C—solid-phase synthesis, 450 °C—partially melted synthesis, 650 °C—crystallization from the melt) to reach the most optimal thermodynamic parameters for the perovskite phase formation. The samples obtained at 300°C and assumed as products of solid-phase synthesis are light grey-colored powders, while the samples annealed at 650 °C correspond to melt crystallization and are “single-piece” and dark-grey colored.
The XRD pattern of the “double perovskite” Cs2AgInBr6 sample, annealed at 300 °C demonstrates some reflections at the same 2Θ value as for double halides, namely, Cs2AgBr3 (PDF#01-072-9840) and Cs3In2Br9 (Springer materials ID: sd_1712349). Similar results were reported recently by Fan et al. [35], who discussed the vapor-deposited Cs2AgBiBr6 thin films.
Three binary systems are parts of the CsBr-AgBr-InBr3 ternary system. In the CsBr–AgBr binary system, there are two phases of cesium bromoargenates(I) CsAgBr2 and Cs2AgBr3 [36], and in the CsBr–InBr3 system, three binary cesium bromoindates(III) bromides, namely, Cs3In2Br9 [37], Cs3InBr6, and Cs2InBr5 [38] are formed. In the ternary diagram, the ‘double perovskite’ phase belongs to two double sections, including AgBr—Cs2InBr5 and InBr3—Cs2AgBr3.
According to the phase XRD analysis performed (Figure 2), the Cs2AgInBr6 phase was not formed, but the sample ‘1’, annealed at 300 °C included two phases of binary bromides Cs3In2Br9, Cs2AgBr3. Samples ‘1’, annealed at 450 and 650 °C include the phases Cs3In2Br9, Cs2AgBr3, and Cs2InBr5. Additionally, some reflections of the AgBr phase would be found, which are not unique only for this phase but overlap with the reflections of the phases of binary bromides, namely, Cs3In2Br9 and Cs2AgBr3.
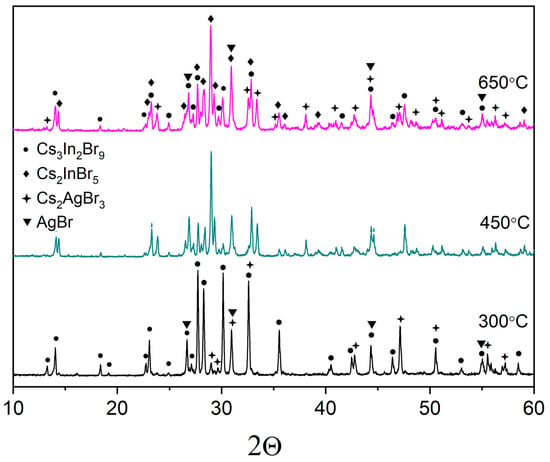
Figure 2.
The diffraction pattern for the theoretical composition Cs2AgInBr6 (•—Cs3In2Br9 [Springer materials ID: sd_1712349]; ♦—Cs2InBr5 [37]; +—Cs2AgBr3 [PDF #01-072-9840]; ▼—AgBr (PDF-2 #79-149).
The reflections of the Cs2InBr5 phase corresponded to the hydrate form Cs2InBr5·H2O [39] which is not shown in the phase diagram reported earlier by Dudareva et al. [38]. This may be due to the fact that X-ray phase analysis occurred in open air and there were water vapors in air. No reflections of the unhydrous Cs2InBr5 phase were found in XRD data for other samples in the ternary system CsBr-AgBr-InBr3. The hydration reaction proceeds at room temperature [39] according to the Equation (1):
Cs3In2Br9 + CsBr + 2H2O = 2Cs2InBr5∙H2O
In order to refine the stoichiometry in the course of ampoule synthesis, some of the samples were additionally studied by the EDX method. Figure 3 and Table 2 show the results of EDX spectroscopy of the samples synthesized by the ampoule method at 300 °C and 650 °C, respectively. It can be assumed that for the samples obtained by the ampoule synthesis, deviations from theoretical values can be associated both with the uneven distribution of indium and silver in an ampoule during the heat treatment and also with various morphology of the crystallites of the resulting phases, i.e. limitations of the EDS method (Figure 3).
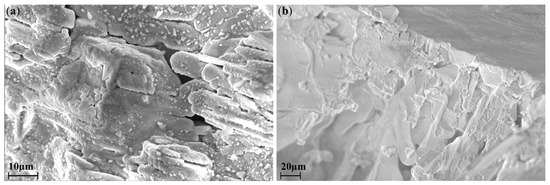
Figure 3.
SEM images of samples obtained by the ampoule method at (a) 300 °C (b) 650 °C.

Table 2.
The atomic percentages of the elemental composition of the samples obtained by the ampoule method at T = 300 °C и 650 °C.
The Raman spectra of the samples synthesized at 300 °C and 650 °C (Figure 4) contain intense vibrational modes corresponding to the Cs3In2Br9 phase: ν (A1g)—165 cm−1, ν (Eg)—216 cm−1 и 111 cm−1 [39]. There are also two “shoulders” at 143 and 210 cm−1, presumably, corresponding to the Raman modes of the Cs2InBr5 phase [40]. It is remarkable that the crystalline phase Cs3In2Br9 is not shown in the binary phase diagram CsBr—reported previously by Dudareva et al. [38]. The Raman spectrum of the composition ‘1’ annealed at 450 °C corresponds well to a superposition of the two spectra discussed above (Figure 4).
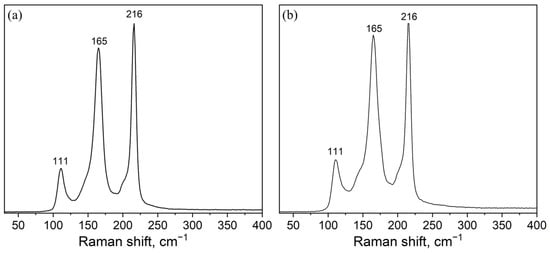
Figure 4.
Raman spectra of samples of the theoretical composition Cs2AgInBr6 obtained by the ampoule method at (a) 300 °C (b) 650 °C. Laser excitation 532 nm.
To study the phase equilibria in the ternary system of bromides, a number of additional compositions were synthesized and studied for its phase analysis.
A Cs3In2Br9 sample ‘3’ obtained at a temperature of 300 °C and an annealing time of 96 h according to the XRD analysis (Figure 5) is a single-phase sample of the desired phase Cs3In2Br9 (Springer materials ID: sd_1712349). The Raman data also correspond well to the spectrum of Cs3In2Br9 phase described by Zhou et al. [39].
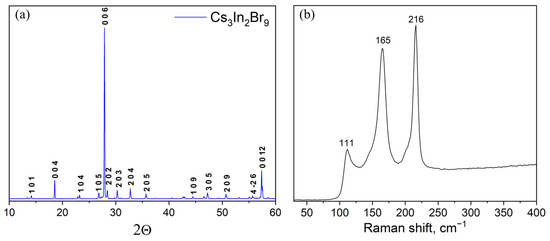
Figure 5.
(a) XRD data and (b) characteristic Raman spectrum of a sample of general composition ‘3’ (Cs3In2Br9 phase).
Samples of the CsAgBr2 binary bromide (point ‘2’) obtained at a temperature of 260 °C and an annealing time of 96 h were also single-phase, according to the corresponding XRD data (Figure 6a). The typical Raman spectrum of caesium dibromoargenate(I) CsAgBr2 is given in Figure 6b. The spectrum includes two maxima, namely, the strongest one at 155 cm−1 and less intensive at 124 cm−1 that, most likely, correspond to vibrational models ν1 and δ of [AgBr4]3− vibrations [41].
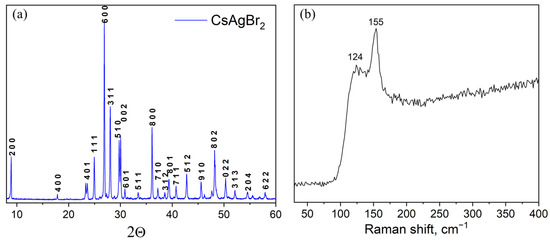
Figure 6.
(a) XRD data and (b) Raman spectrum of a sample ‘2’ (CsAgBr2 phase).
In order to get more information about the named above binary phase equilibria and the CsBr-AgBr-InBr3 ternary system, three additional compositions were synthesized at three intersections, namely, the CsAgBr2-InBr3 and AgBr-Cs3InBr6 sections (point ‘4’), CsAgBr2-InBr3 and AgBr-Cs3In2Br9 sections (point ‘5’), as well as CsAgBr2-Cs3In2Br9 and AgBr-Cs2InBr5 (point ‘6’). The annealing temperature in all cases was 200 °C to avoid evaporation of volatile indium(III) bromide from the reaction zone in an ampoule.
The XRD patterns of samples ‘4’, ‘5’, and ‘6’ are given in Figure 7, Figure 8 and Figure 9. Identification of reflections in diffractograms was performed using the XRD data to the stable and metastable phased known for the binary systems (see Figure 1a). No new phases of ternary bromides were found. The diffraction patterns showed the presence of reflections of binary bromides as CsAgBr2 (PDF-2 (38–850)), Cs3In2Br9 (Springer materials ID: sd_1712349), and a Cs3InBr6 (Materials projects ID:mp-1112651), as well as a precursor AgBr (PDF-2 (79–149)). We found no reflections of the less volatile crystalline CsBr that makes an assumption of having a stable quasi-binary cross-section Cs2AgBr3—Cs3In2Br9.
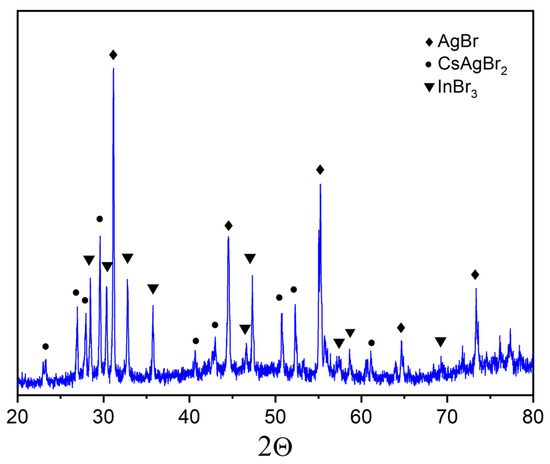
Figure 7.
XRD data for point ‘4’ (intersection of incisions CsAgBr2-InBr3 and AgBr-Cs3In2Br9) (♦—AgBr PDF-2 (79–149); •—CsAgBr2 PDF-2 (38–850); ▼—InBr3 PDF-2 (79–281)).
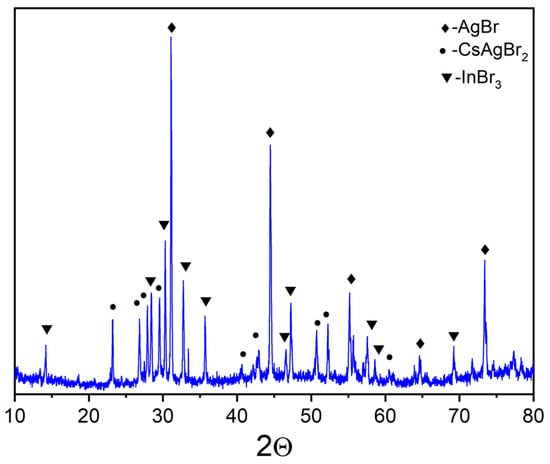
Figure 8.
XRD data for point ‘5’ (intersection of incisions AgBr-Cs2InBr5 and CsAgBr2-InBr3) (♦—AgBr PDF-2 (79–149); •—CsAgBr2 PDF-2 (38–850); ▼—InBr3 PDF-2 (79–281)).
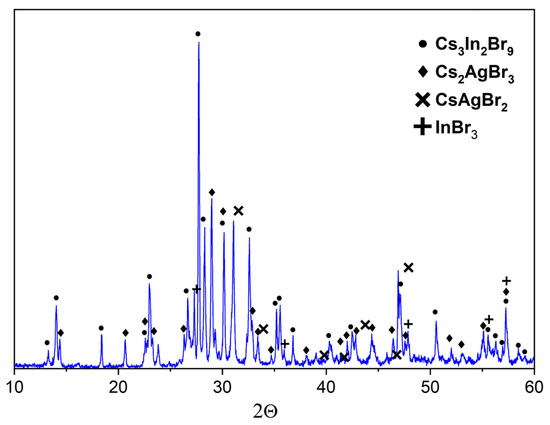
Figure 9.
XRD data for point ‘6’ (intersection of incisions CsAgBr2-Cs3In2Br9 and AgBr-Cs3InBr6) (•—Cs3In2Br9 [Springer materials ID: sd_1712349]; ♦—Cs2AgBr3 (PDF#01-072-9840); ✕—CsAgBr2 PDF-2 (38–850); +—InBr3 PDF-2 (79–281)).
Point ‘4’ is on the intersection of incisions of CsAgBr2-InBr3 and AgBr-Cs3In2Br9. The corresponding XRD data (Figure 7) showed the presence of strong reflections of the AgBr and also sharp and strong reflections of other two binary bromides of cesium bromoargenate(I) CsAgBr2 (PDF-2 (79–149)) and a single volatile bromide InBr3 (PDF-2 (79–281)). No reflections of cesium bromoindates(III) were found. Most likely, this means that the binary system AgBr-Cs3In2Br9 is not stable, while the CsAgBr2-InBr3 section is a thermodynamically stable cross-section.
Point ‘5’ is on the intersection of AgBr-Cs2InBr5 and CsAgBr2-InBr3. The corresponding XRD data (Figure 8) showed the presence of reflections of silver bromide AgBr, caesium dibromoargenate(I) CsAgBr2, and indium bromide InBr3 phases. This means that the binary system AgBr-Cs2InBr5 is metastable while CsAgBr2-InBr3 is a quasi-binary one.
Point ‘6’ is on the intersection of three incisions of CsAgBr2-Cs3In2Br9, AgBr-Cs3InBr6, and Cs2AgBr3-InBr3. The corresponding XRD data (Figure 10) showed the presence of reflections of CsAgBr2 (PDF-2 (79–149)), Cs2AgBr3 (PDF#01-072-9840), Cs3In2Br9 (Springer materials ID: sd_1712349), and InBr3 (PDF-2 (79–281)) phases. This means that the binary system AgBr-Cs3InBr6 is not stable, while CsAgBr2-Cs3In2Br9 and Cs2AgBr3-InBr3 would have thermodynamically stable cross-sections.
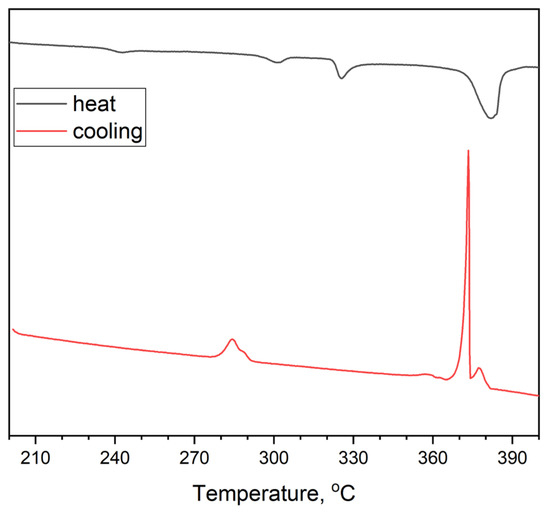
Figure 10.
DSC data for the composition of point 6 at the intersection of the sections Cs3InBr6-AgBr, CsAgBr2-Cs3In2Br9, and Cs2AgBr3-InBr3, obtained by displacement of binary bromides CsAgBr2 and Cs3In2Br9.
Figure 9 shows DSC data for a sample ‘6’ obtained by mixing of CsAgBr2 and Cs3In2Br9 binary bromides in stoichiometric amounts. It is shown that the heating curve has four endothermic minima. The last one at 372 °C (645 K) is the strongest and, most likely, corresponds to a liquidus. At lower temperatures, the minimum at 234 °C (507 K) is close to the eutectic temperature in the AgBr—InBr3 binary system. The nets minimum at 290 °C (563 K) is above the melting temperature of CsAgBr2 (270 °C) [36] and is close to the value of the eutectic temperature for the CsBr—InBr3 binary system. The third endothermic effect at 328 °C (601 K) could be associated with the melting of the Cs3In2Br9 phase, which is not present in the CsBr—InBr3 phase diagram reported elsewhere [42].
The cooling curve differs from the heating process. At higher temperatures, there is a smaller maximum at 380 °C (653 K) and the following narrower and sharper maximum at 372 °C (645 K). Most likely, such exothermic effects correspond to crystallization of Cs3In2Br9 phase and crystallization of indium(III) bromide (420 °C, 693 K). Then, there is a very weak maximum at 356 °C (629 K).
At lower temperatures, two crystallization maxima are observed at 293 °C (566 K) and 287 °C (551 K). Probably, the processes correspond to crystallization of Cs2AgBr3 and CsAgBr2 (270 °C). Moreover, below 250 °C, no crystallization of binary eutectics is found, as observed for the AgBr—InBr3 binary system at the heating process. Thus, it can be assumed that the phase composition of the sample after DSC measurement differs from the initial one, which may be a consequence of the reaction proceeding, for example, according to reaction 1.
Thus, the summary of XRD and DSC results for point ‘7’ indicates the possibility of solid-phase process (2) at 200 °C:
2CsAgBr2 + 2Cs3In2Br9 = 4Cs2AgBr3 + 4InBr3
The possibility of the process (2) contradicts the hypothesis about the quasi-binary characteristics of the section CsAgBr2—Cs3In2Br9 that originated from the instability of the double perovskite phase Cs2AgInBr6. At the same time, process (2) could take place in an open system and be negligible in closed systems, such as in ampoules.
Thus, a brief investigation of phase equilibria in the ternary system CsBr-AgBr-InBr3 gave some controversial results. Nevertheless, the phase analysis of the compositions demonstrated higher thermodynamic stability of three quasi-binary intersections, namely, Cs3In2Br9—Cs2AgBr3, Cs2AgBr3—InBr3, CsAgBr2—InBr3. The stability of the first one led to the full chemical transformation of cesium bromide as a precursor for the “double perovskite” composition in point ‘1’. The last two quasi-binary sections resulted from the high volatility of indium(III) bromide.
4. Conclusions
Investigation of the phase equilibria in the ternary system CsBr-AgBr-InBr3 is a brief one but gives important information on the processes that take part in the system at sub-solidus temperature region and above the melting temperatures. The proposed synthesis approaches for the estimated double perovskite phase by solid-phase synthesis and crystallization from the melt were not enough, and the Cs2AgInBr6 phase is unfeasible. The high stability of the phases of the binary bromides Cs3In2Br9 and Cs2AgBr3 prevents the formation of the double perovskite phase. Analysis of the ternary system CsBr-AgBr-InBr3 demonstrates three quasi-binary sections, namely, Cs3In2Br9—Cs2AgBr3, Cs2AgBr3—InBr3 and CsAgBr2—InBr3, which would be important for the synthesis of binary bromides and investigation of solid solutions in the ternary system or its sections.
Author Contributions
Conceptualization, A.V.G. and R.K.K.; methodology, A.V.G., R.K.K. and J.Z.Y.; formal analysis, R.K.K. ang J.Z.Y.; investigation, R.K.K., J.Z.Y. and A.V.K.; resources, A.V.G.; writing—original draft preparation, R.K.K.; writing—review and editing, A.V.G.; visualization, R.K.K. and J.Z.Y.; supervision, A.V.G. and A.V.K.; project administration, A.V.G.; funding acquisition, A.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (RSF) (grant 22-23-00585).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to for their colleagues Dmitry O. Charkin and Shodruz T. Umedov (Lomonosov Moscow State University) for the manuscript discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sirotkin, O.S.; Sirotkin, R.O.; Shibaev, P.B. Effect of the character of homo- and heteronuclear chemical bond on the intermolecular interaction energy and properties of halogens and hydrogen halides. Russ. J. Inorg. Chem. 2011, 56, 1104–1108. [Google Scholar] [CrossRef]
- Umedov, S.T.; Grigorieva, A.V.; Lepnev, S.L.; Knotko, A.V.; Koji, N.; Shinichi, O.; Shevelkov, A.V. Indium Doping of Lead-Free Perovskite Cs2SnI6. Front. Chem. 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Miyasaka, T.; Shirai, Y. Novel photoelectrochemical cell with mesoscopic electrodes sensitized by lead-halide compounds (2). In Proceedings of the 210th ECS Meeting, Cancun, Mexico, 29 October–3 November 2006. Abstract #397. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with effciency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratory. Best Research Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev220630.pdf (accessed on 30 June 2022).
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Cho, H.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Chu, L.; Hu, R.; Liu, W.; Ma, Y.; Zhang, R.; Yang, J.; Li, X. Screen printing large-area organometal halide perovskite thin films for efficient photodetectors. Mater. Res. Bull. 2018, 98, 322. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, B.; Zheng, K.; Yang, S.; Li, Y.; Deng, W.; He, R. Formamidinium lead bromide (FAPbBr3) perovskite microcrystals for sensitive and fast photodetectors. Nano-Micro Lett. 2018, 10, 43. [Google Scholar] [CrossRef]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 75, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Babayigit, A.; Thanh, D.D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar]
- Conings, B.; Drijkoningen, J.; Gauguelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef]
- Wilks, R.G.; Bar, M. Perovskite solar cells: Danger from within. Nat. Energy 2017, 2, 16204. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Yang, J.-H.; Fu, Y.; Yang, D.; Xu, Q.; Yu, L.; Wei, S.-H.; Zhang, L. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 2017, 139, 2630–2638. [Google Scholar] [CrossRef]
- Filip, M.R.; Hillman, S.; Haghighirad, A.A.; Snaith, H.J.; Giustino, F. Band gaps of the lead-free halide double perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from theory and experiment. J. Phys. Chem. Lett. 2016, 7, 2579–2585. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Ostrikov, K.K.; Wasalathilake, K.C.; Yan, C.; Wang, H.; Tesfamichael, T. Insight into lead-free organic-inorganic hybrid perovskites for photovoltaics and optoelectronics: A first-principles study. Org. Electron. 2018, 59, 99–106. [Google Scholar] [CrossRef]
- Filip, M.R.; Giustino, F. Computational screening of homovalent lead substitution in organic-inorganic halide perovskites. J. Phys. Chem. C 2015, 120, 166–173. [Google Scholar] [CrossRef]
- Körbel, S.; Marques, M.A.; Botti, S. Stability and electronic properties of new inorganic perovskites from high-throughput ab initio calculations. J. Mater. Chem. C 2016, 4, 3157–3167. [Google Scholar] [CrossRef]
- Volonakis, G.; Haghighirad, A.A.; Milot, R.L.; Sio, W.H.; Filip, M.R.; Wenger, B.; Johnston, M.B.; Herz, L.M.; Snaith, H.J.; Giustino, F. Cs2InAgCl6: A New Lead-Free Halide Double Perovskite with Direct Band Gap. J. Phys. Chem. Lett. 2017, 8, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wu, T.; Li, Y.; Jiang, L.; Deng, W.; Han, K. Combining theory and experiment in the design of a lead-free ((CH3NH3)2AgBiI6) double perovskite. New J. Chem. 2017, 41, 9598–9601. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Li, S.; Liu, J.; Guo, Y.; Niu, G.; Yao, L.; Fu, Y.; Gao, L.; Dong, Q.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Du, K.; Meng, W.; Mitzi, D.; Yan, Y. Chemical origin of the stability difference between Cu(I)- and Ag(I)-based halide double perovskites. Angew. Chem. Int. Ed. 2017, 56, 12107–12111. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, Y.; Zhang, M.; Shi, J.; Cai, W. Promising Lead-Free Double-Perovskite Photovoltaic Materials Cs2MM′Br6 (M = Cu, Ag, and Au; M′ = Ga, In, Sb, and Bi) with an Ideal Band Gap and High Power Conversion Efficiency. J. Phys. Chem. C 2021, 125, 21160–21168. [Google Scholar]
- Menedjhi, A.; Bouarissa, N.; Saib, S.; Bauamama, K. Halide double perovskite Cs2AgInBr6 for photovoltaic’s applications: Optical properties and stability. Optik 2021, 243, 167198. [Google Scholar] [CrossRef]
- Ensslin, F.; Dreyer, H.Z. Über einige Salze des Indiums. I. Anorg. Allg. Chem. 1942, 249, 119–132. [Google Scholar] [CrossRef]
- Ensslin, F.; Ziemeck, B.; DeSchaepdryver, L.Z. Zur Chemie des Indiums. VI. Die Löslichkeit von Indium(III)-chlorid, Indium(III)-bromid und Indium(III)-jodid im Wasser bei verschiedenen Temperaturen. Anorg. Chem. 1947, 254, 293–296. [Google Scholar] [CrossRef]
- Du, K.; Meng, W.; Wang, X.; Yan, Y.; Mitzi, D. Bandgap Engineering of Lead-Free Double Perovskite Cs2AgBiBr6 through Trivalent Metal Alloying. Angew. Chem. Int. Ed. 2017, 56, 8158–8162. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Peng, H.; Zheng, Z.; Chen, Z.; Tan, S.; Chen, X.; Luo, Y.; Su, Z.; Luo, J.; Liang, G. Single-Source Vapor-Deposited Cs2AgBiBr6 Thin Films for Lead-Free Perovskite Solar Cells. Nanomaterials 2019, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Lyalikov, K.S. Doklady Akad. Nauk USSR. Dokl. Chem. 1949, 65, 17. [Google Scholar]
- Meyer, G.Z. Zur Kenntnis der Chloro- und Bromo-Indate (III). A3In2Cl9 (A = Cs, Rb, In, Tl) und Cs3In2Br9−xClx (x = 0, 3, 6, 7, 8). Anorg. Allg. Chem. 1978, 445, 140–146. [Google Scholar] [CrossRef]
- Dudareva, A.G.; Bogatov, Y.E.; Ivanov-Emin, B.N.; Fedorov, P.I. Study of the interaction of indium tribromide with cesium bromide. J. Inorg. Chem. 1971, XVI, 2586. [Google Scholar]
- Zhou, L.; Liao, J.-F.; Huang, Z.-G.; Wei, J.-H.; Wang, X.-D.; Li, W.-G.; Su, C.-Y. Highly Red Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. IR and Raman Spectra of Inorganic and Coordination Compounds; Mir: Moscow, Russia, 1991; p. 537. [Google Scholar]
- Waters, D.N.; Basak, B. Vibrational spectra of dihalogeno-anions of copper(I) and silver(I) in solution in tri-n-butyl phosphate. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 2733–2735. [Google Scholar] [CrossRef]
- Dudareva, A.G.; Bogatov, Y.E.; Konstandia, A.; Ivanov-Emin, B.N.; Fedorov, P.I. Physicochemical study of the interaction of indium tribromide with silver, zinc and lead(II) bromides in a melt. Chem. Chem. Technol. 1971, XIV, 1299. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).