Abstract
To endow synergistically epoxy resin (EP) with excellent fire resistance and high optical transparency, a nitrogen-rich DOPO-based derivate (named as FATP) was synthesized and incorporated into EP. It showed that the incorporation of the FATP reduced the fire hazard of the EP, as demonstrated by the fact that the EP/4% FATP blends gained a UL-94 V-0 rating and an LOI value of 35%, with the lowest values of the THR (86.7 MJ/m2), the PHRR (1059.3 kW/m2), and the TSP (89.6 MJ/m2). The presence of the FATP also reduced the thermal stability and the crosslinking density whilst improving the curing reaction and the storage modulus of the EP/FATP blends. The TG-FTIR spectra showed that •HPO/•PO free radicals and some nonflammable gases (HN3 and NH3) were produced during the pyrolysis, and the characterization (SEM, Raman spectroscopy, and XPS) of char residues confirmed that the FATP facilitated the formation of continuous and compact carbon layers of greater graphitization degree. It was thus concluded that the FATP played the flame-retardant roles in both the gas and condensed phases. Furthermore, the FREPs kept almost identical transparency as the pristine EP, and mechanical properties were also slightly enhanced. The FREPs presented in this work show promising applications in the fields of advanced optical technology.
1. Introduction
Epoxy resin (EP), one of the most used thermosets, has been an indispensable basic material for high-end manufacturing industries such as 5G communications and rail transit, thanks to its excellent comprehensive performances including thermal stability, mechanic strength, chemical resistance, and transparency. However, like many other petroleum-based polymer matrices, the EP also suffers from intrinsic flammability with serious melt-dripping because of the organic characteristics, which restricts the broad application in the fields requiring harsh fire safety [1,2]. It is well-known that fires as one of common accidents threaten seriously human security and the environment and bring about annual costs of tens of billions of dollars globally [3]. Thus, endowing EP with better fire resistance becomes urgent from both the perspectives of expanding its application and sociological pressure. In the past decades, halogenated flame retardants (FRs) have been used extensively to enhance the fire resistance of EP due to their inexpensiveness and better flame-retardant efficiency. Unfortunately, halogenated FRs release some toxic gases such as halogenated dioxins and dibenzofurans during the combustion [4,5], and thus, a majority of halogenated flame retardants are forbidden in order to abide by RoHS. Therefore, it is an inexorable trend to develop green FRs to meet with the regulations about environmental protection and human health [6,7].
Heretofore, many efforts have been made to prepare halogen-free FRs which could enhance dramatically the fire resistance of the polymer matrix. A lot of works have demonstrated that the incorporation of phosphorus- [8,9], nitrogen- [10,11], silicon- [12,13], and sulfur-containing [14,15] compounds into EP is an effective method to increase the flame retardancy. Among these compounds, phosphorus-containing compounds have been mostly used for their low toxicity and high flame-retardant efficiency [16]. Lately, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) has become a common phosphorus-containing flame retardant for EP due to its better stability and inherent fire retardancy [17]. Nevertheless, it suffers limitations that UL-94 V-1 rating is achieved only at its high loading level when used alone [18]. Luckily, there is a reactive P−H bond in the DOPO, other flame-retardant elements (silicon [19,20], sulfur [21,22], nitrogen [23], and boron [24,25]) or groups (imidazole [26,27], triazole [28,29], and triazine [30,31]) can be integrated into the DOPO through the reaction between the P−H bond and unsaturated bonds including double bonds and epoxy groups, and many DOPO-based derivatives with high flame-retardant efficiency are thus prepared. It is worthwhile pointing out, however, that the introduction of additive FRs inevitably deteriorate mechanical performances or transparency [32]. Especially, the optical transmittance is a vital parameter for EP using in the fields such as LED and transparent coatings; thus, a lot of works have been conducted to prepare flame-retardant EP resins (FREPs) with better transparency [33,34,35,36]. Wang et al. [37] reported that the addition of a 10 wt.% phosphorus/nitrogen-containing derivative (DPAP) endows EP with a V-0 rating and an LOI of 35.2%, but its transmittance decreases obviously from 87.8% of the pure EP to 72%. Despite some achievements were obtained, it is still a research hotspot to simultaneously endow EP with outstanding flame retardancy and high transparency.
As we all know, nitrogen-containing heterocycles, such as tetrazole, have the nature of thermal stability and flame retardancy [38]. Particularly, 5-amino-1H-tetrazole (ATZ) is a stable nitrogen-rich heterocycle with unique four nitrogen atoms and thus is considered as a greatest potential material to fabricate a variety of high-efficient flame retardants [39]. Additionally, 2-furaldehyde is a bio-based compound and can promote powerfully char-forming capacity [40,41]. Thus, in this work, a nitrogen-rich DOPO-based compound (FATP) was synthesized using 2-furaldehyde, 5-amino-1H-tetrazole, and DOPO as raw materials. Subsequently, FREPs with different FATP loading levels were prepared using the 4,4-Diaminodiphenylmethane (DDM) as a curing agent. The effect of the FATP on the curing behavior, flame retardancy, thermal stability, combustion behavior, mechanical properties, and transparency of FREPs was investigated in detail. Finally, the flame-retardant mechanism was proposed through the analysis of pyrolysis gaseous products and char residues.
2. Experimental
2.1. Synthesis of the FATP
The synthesis route of the FATP by the one-pot method is illustrated in Scheme 1. The detail experimental process is described as follows. ATZ (3.403 g, 0.04 mol) and 250 mL absolute alcohol were feed into a 500 mL round three-necked flask connected with a magnetic stirrer, a reflux condenser, and a constant pressure dropping funnel. After the complete dissolution of ATZ at 50 °C, 2-furaldehyde (3.843 g, 0.04 mol) in absolute alcohol (20 mL) was added dropwise through the constant pressure dropping funnel. Subsequently, the mixture was stirred for 12 h at 75 °C, and then, DOPO (8.64 g, 0.04 mol) was added into the above mixture, followed by stirring for another 12 h. Finally, the solution was cooled to room temperature, and the solid precipitate was then filtered, washed with absolute alcohol three times and dried in a vacuum oven at 80 °C overnight to give 10.76 g of FATP as white powder (71% yield).
Scheme 1.
Synthesis route of the FATP.
2.2. Preparation of Flame-Retardant Epoxy Thermosets (FREPs)
Firstly, the predetermined FATP was added into the preheated EP at 160 °C and was magnetically stirred to obtain a homogeneous and transparent solution. Then, after the above solution was cooled to 90 °C, the DDM was added and stirred to dissolve completely. Finally, the mixture was injected rapidly into a preheated silicone rubber mold, and the curing process was performed in an oven at 100 °C for 2 h and 150 °C for 3 h. The specific formulas of the pure EP and the FREPs are listed in Table 1.

Table 1.
Formulas of the pure EP and the FREPs.
3. Results and Discussion
3.1. Characterization of the FATP
The spectra of FTIR, 1H NMR, and 31P NMR are shown in Figures S1 and S2. In the FTIR spectrum of the FATP, some characteristic peaks appeared at 1640 cm−1 (υC=N), 1244 cm−1 (υP=O), 1215 cm−1 (υP−O−Car), and 750 cm−1 (υP−C). It was noteworthy that triple absorption peaks at 3150–3465 cm−1 ascribed to υ-NH2 of ATZ were merged into a single peak at 3432 cm−1 (σNH) for the FATP. The disappearance of the stretching vibration peaks at 2435 (σC=N, DOPO) and 1691 cm−1 (σCHO, FA) revealed the consumption of DOPO and FA. As seen in Figure S2a, the signals at 6.35–6.45 ppm were assigned to the proton of −NH−, the multiple signals at 7.00–7.86 and 8.15–8.37 ppm corresponded to the protons of aromatic hydrogens and heterocycle, respectively, and the ones at 5.36–5.45 ppm are due to the resonance of the aliphatic C−H bond. Furthermore, two chemical shifts appeared at 27.01 ppm and 27.75 ppm in the 31P NMR spectrum of the FATP due to the existence of chiral carbon, which was connected with the phosphorus. It can be inferenced that the target product FATP was synthesized successfully according to the above test results.
3.2. Curing Behaviors of FREPs
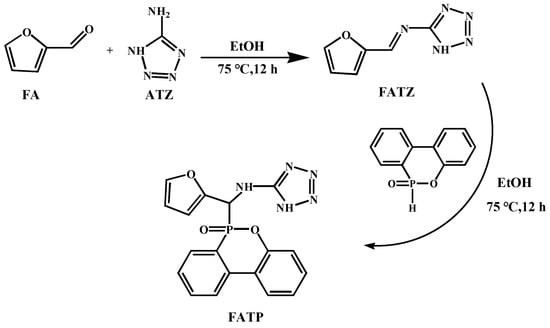
Figure 1 presents the non-isothermal DSC curves as well as the linear fitting curves of the ln(β/Tp2) and ln(β) against 1/Tp following the Kissinger (Equation (1)) and the Ozawa (Equation (2)) methods [42,43]. The reaction activation energy (Ea) was obtained from the slope of the fitted curves, as listed in Table 2.
where in β, Tp, A, and R are the heating rate, the exothermic peak temperature, the pre-exponential factor, and the ideal gas constant (8.314 J/K·mol), respectively. As shown in Figure 1, all specimens displayed a single exothermic peak, and the Tp values moved towards a great temperature with the increasing β. It might be due to that the a lower β offers more time to proceed the curing process. Furthermore, the Tp and Ea values reduced with the increasing FATP content in case of the same β, indicating that the FATP improved the reactivity of the EP. The improved ring-opening reactivity contributed to both the polarization of epoxy ring, which originated from the hydrogen bond between −NH in the FATP and the epoxy group in the EP and the catalysis of the –N=N group from the tetra-azole of the FATP [44].
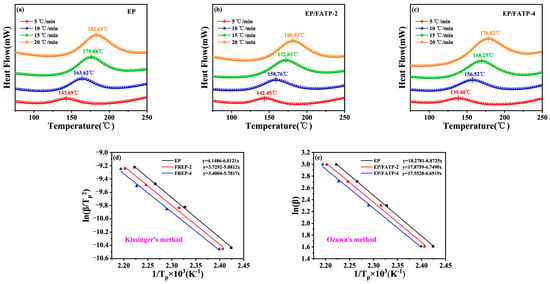
Figure 1.
Non-isothermal DSC curves of the EP (a), FREP-2 (b), and FREP-4 (c) at different β and fitting curves of ln (β/Tp2) (d) and ln β (e) versus 1/Tp × 103.

Table 2.
Ea values calculated according to the Kissinger and the Ozawa methods.
3.3. Flame Retardancy of the FREPs
The UL-94 rating and the LOI values were measured, and the corresponding data are summarized in Table 3. Obviously, the pristine EP was readily flammable and could not be self-extinguished once ignited, giving an LOI value of 25% and no UL-94 rating. It is satisfactory that the flame retardancy of FREPs was improved significantly with the loading of the FATP. In the case of the 2% FATP content, FREP-2 presented an LOI value of 32.5% and passed the UL-94 V-1 testing. With the FATP loading level up to 4% FATP (phosphorus content of 0.33%), the FRP-4 sample achieved an LOI value of 35% and a UL-94 V-0 rating. It demonstrated that the FATP had a better flame-retardant efficiency on the EP matrix.

Table 3.
UL-94 rating and LOI values of the FREPs.
3.4. Thermal Stability of the FREPs
Thermogravimetric analysis (TGA) was measured under N2 to assess the effect of the FATP on the thermal decomposition behavior of the FREPs. Figure S3 and Table 4 show the resultant curves and data, respectively. As seen, the FATP presented a two-stage decomposition process. The first-stage occurred at 240–300 °C due to the thermal cracking of the tetrazole to release NH3 and trinitrides, and the second-stage ranging from 400 °C to 500 °C was attributed to the thermal cracking of phosphine group. Although the FATP gave a lower T5% of 243.5 °C, its char residue at 700 °C was up to 33.2%, revealing that the FATP had a superior charring capacity. As seen, the pure EP and the FREPs displayed similar single-stage thermal decomposition processes in the range of 350–550 °C. It was also found that the T5% values of EP/FATP-2 and EP/FATP-4 were reduced by 19.5 °C and 29.4 °C, respectively, as comparison with the pristine EP. It reflected that the FATP with a lower T5% value accelerated the early degradation of the EP matrix. Noteworthy, the increasing FATP contents resulted in a lower Rmax and enhanced the CR700 values of the FREPs. It might be owing to the fact that the FATP decomposed early at low temperature and produced some nonflammable gases, which restrained the further burning of the EP. Additionally, the char-forming capacity was improved by the reaction between the Furan ring in the FATP and the degradation products of the EP.

Table 4.
Data from TGA and DTG curves measured under nitrogen atmosphere.
3.5. Analysis of Combustion Behaviors
The cone calorimeter test (CCT) is generally used to simulate the burning behaviors in a real fire, since it provides some characteristic parameters during the combustion [45,46]. Figure 2 illustrates the variations of the heat release rate (HRR), the total heat release (THR), the smoke release rate (SPR), and the total smoke release (TSP) with the time, and some crucial data are listed in Table 5.
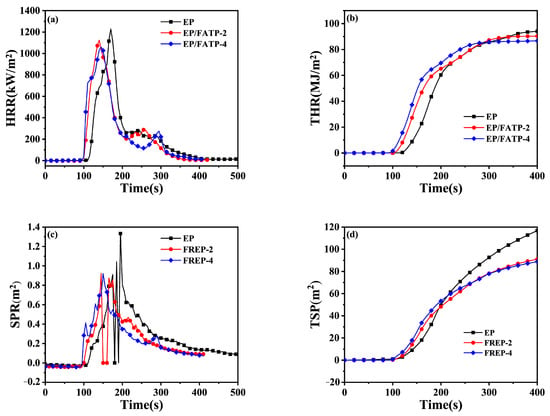
Figure 2.
The variations of the HRR (a), the THR (b), the SPR (c), and the TSP (d) with the time.

Table 5.
Some crucial data from the CCTs for the pure EP and the FREPs.
As compared to the pristine EP, the FREPs presented lower values of time to ignition (TTI), e.g., 111 s for EP > 96 s for EP/FATP-2 > 85 s for EP/FATP-4. It is probably due to the fact that FATP with lower T5% facilitated the early decomposition of the EP matrix.
The THR and the peak of the HRR (the PHRR) are important parameters to characterize the combustion intensity. The pure EP burned intensely and gave the highest values of the THR (95.7 MJ/m2) and the PHRR (1229.3 kW/m2). Clearly, the values of both the THR and the PHRR tended to decrease with the incorporation of the FATP. For example, the FREP-4 sample achieved the lowest values of the THR (86.7 MJ/m2) and the PHRR (1059.3 kW/m2), which were decreased by 13.8% and 9.4% by contrast with the pristine EP, respectively. It confirmed that the incorporation of the FATP suppresses effectively the heat release during burning.
Since the excess toxic smoke causes a mortal threat to victims in a fire, the TSP is a crucial parameter to assess fire safety of polymeric substances [47]. The pure EP displayed a higher TSP value of 139.7 MJ/m2. By contrast, the corresponding value of EP/FATP-4 was reduced by 35.9%, indicating that the FATP had better smoke suppression.
The average effective heat combustion (av-EHC) is defined as the ratio of the HRR to the mass loss rate (MLR) and often used to assess the combustion degree of volatiles in the gas phase [48]. The av-EHC values of the pure EP, FREP-2, and FREP-4 were achieved to be 24.47, 23.96, and 23.56 MJ/kg, respectively. It suggests that the incomplete combustion of volatiles occurs with the incorporation of the FATP. In contrast to the neat EP, the greater av-COY/av-CO2Y values of the FREPs further demonstrated the occurrence of the incomplete combustion. The above-mentioned phenomena are probably attributed to the fact that the phosphorus-containing species, which are produced from the degradation of the FATP, could quench combustible and during combustion, and the combustion chain reaction is thus interrupted [49,50]. What is more, the char residues of FREP-2 (15.5%) and FREP-4 (17.3%) were far more than that of the neat EP (9.4%). It indicates that the FATP promotes the formation of char residues which can isolate the transition of oxygen and flammable gases; it hence plays a flame-retardant effect in the condensed phase.
3.6. Characterization of Char Residues
Figure 3 shows the digital graphs and SEM micrographs of the char residues after CCTs for all samples. As seen in Figure 3a1,a2, the neat EP had fewer char residues with an expansion height of 1.6 cm. With the incorporation of the FATP, much more and intact char residues were left after CCTs, with the expansion heights of 2.5 cm for FREP-2 and 2.8 cm for FREP-4, respectively. It is mainly due to the fact that the dehydration and carbonization of EP is facilitated by phosphoric acid which are produced from the decomposition of FATP, and the char residue thus forms readily [51]. Furthermore, the char residues of the neat EP showed many holes and some cracks (Figure 3a3), which were conductive to the transmission of the heat and flammable gases from the inner EP matrix to the exterior environment. By contrast, a few voids were found in the char residues of FREP-2 (Figure 3b3). With the increasing FATP content, the char residues of FREP-4 became continuous and compact (Figure 3c3), which is in favor to suppress effectively the transmission of the heat and combustible volatiles.
Figure 3.
Digital photos and SEM micrographs of char residues after CCTs: (a1–a3) pure EP; (b1–b3) FREP-2; and (c1–c3) FREP-4.
It is well accepted that a Raman spectrometer was applied to evaluate the graphitization degree of carbon materials. As illustrated in the Raman spectra (Figure S4), two strong signals appeared at 1351 cm−1 and 1582 cm−1, which contributed to the D band representing disordered carbon and the G band representing graphitized carbon, respectively. The area ratio (ID/IG) of the D band to the G band is usually adapted to characterize the graphitization degree of carbon materials, and a lower ID/IG value means a higher graphitization degree [52]. The calculated ID/IG values showed a downtrend with the increasing FATP contents, that is, 2.64 for the pure EP > 2.62 for the EP/FATP-2 > 2.47 for the EP/FATP-4. The result confirmed that the incorporation of the FATP results in an enhancement of the graphitization degree, thus constituting readily a stable and compact carbon layer.
XPS was further applied to analyze the specific elemental composition and the valence state. Figure S5 depicts the XPS spectra of the pure EP and FREP-4, and the specific elemental compositions are listed in Table 6. In addition to C, N, and O presenting in the char residues of the pure EP, an extra P element appeared in the char residues of FREP-4. As calculated, the C/O value of FREP-4 (6.53) was far greater than that of the neat EP (1.81). It indicated that the FATP had the char-forming capacity of the EP matrix and enhanced the resistance of the char to thermal oxidization, thus having a flame-retardant effect in the condensed phase.

Table 6.
Element compositions of the char residues.
Figure S6 shows the C1s, O1s, N1s, and P2p spectra of the FAEP-4 specimen. As seen in the C1s spectrum, the appearance of three signals means that the carbon in char residues had three valence states. The peak at 284.7 eV was assigned to the C−C and C−H bonds in aliphatic and aromatic fragments, the one at 286.2 eV was attributed to the C−O−C and P−O−C bonds, and the one at 288.2 eV belonged to the C=O bond. The above bonds might be contributed to the carbonization and dehydration of the EP matrix during combustion. With respective to the O1s spectrum, the peak at 531.3 eV belonged to the C=O and P=O bonds, and the one at 533.1 eV was ascribed to P−O−, C−O−C, or −O− in the P−O−P bond. As for the N1s spectrum, the signals at 398.5 eV and 400.4 eV were attributed to the C−N bond and the N=N bond in the tetra-azole [53,54]. The P2p spectrum presented two signals at 133.6 eV and 134.7 eV, which were ascribed to the P−O−C and P=O bonds, respectively. The results revealed that the FATP was decomposed to produce phosphorous oxides during combustion, which can catalyze the carbonization and dehydration of the EP matrix, and thus plays flame retardant roles in the condensed phase.
3.7. Gaseous Products Analysis
To better analyze the impact of the FATP in the gas phase, the gaseous volatiles released during the thermal decomposition of the pure EP and FREP-4 were detected via TG-FTIR. Figure 4 depicts the TG-FTIR spectra of the gases at different temperatures and the overall 3D TG-FTIR spectra. The main pyrolysis gases during the thermal decomposition of the pure EP and EP/FATP-4 were similar, including H2O (3622–3681 cm−1), aliphatic compounds (2750–3142 cm−1), aromatic compounds (3032 cm−1, 1600 cm−1, 1512 cm−1, and 827 cm−1), bisphenol A (1259 cm−1, 1337 cm−1, and 1175 cm−1), and carbonyl compounds (1732 cm−1). By contrast, in the spectra of FREP-4, some new characteristic peaks appeared at 1604 cm−1, 1255 cm−1, and 1050 cm−1, which contributed to the stretching vibration of P−O−Ph, P−O, and P−O−C, respectively. Therefore, it can be speculated that the decomposition of FATP produced ·HPO and ·PO radicals, which subsequently captured the reactive radicals such as H∙ and OH∙ to break off the combustion chain reaction. Two other peaks at 2309 cm−1 (HN3) and 919 cm−1 (NH3) were also found. Additionally, it was observed that the incorporation of FATP decreased both the initial temperature of the gas release (385 °C for the pristine EP and 356 °C for FREP-4) and the peak intensity. Figure S7 further shows the variations of the peak intensities of some representative flammable volatiles with the time. As seen, EP/FATP-4 gave a lower intensity than the pure EP, suggesting that the FATP plays flame-retardant roles in the gas phase through the suppression effect on the release of flammable volatiles.
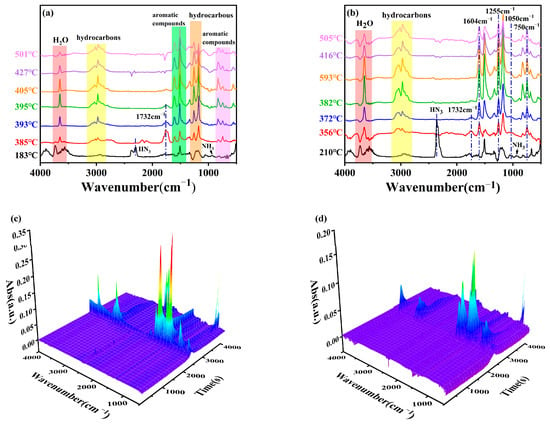
Figure 4.
FTIR spectra of gaseous pyrolysis products of EP (a) and FREP-4 (b) at different temperatures; 3D TG-IR diagrams of EP (c) and FREP-4 (d) gaseous pyrolysis products.
3.8. Possible Flame-Retardant Mechanism
Considering the above analysis of the combustion behavior, gaseous pyrolysis products, and the char residues, a possible flame-retardant mechanism was proposed and presented in Figure 5. In the gas phase, •HPO and •PO free radicals, which result from the decomposition of the phosphorous-containing fragment in the FATP, act as quenchers to capture the reactive groups such as •OH and •H. The chain reaction during the combustion is thus interrupted. Meanwhile, some nonflammable gases including HN3 and NH3 are also released and can dilute flammable volatiles and take a portion of heat away. In the condensed phase, the phosphorous-containing group in the FATP is decomposed into phosphoric acid, which promotes the catalytic carbonization and dehydration of the EP matrix. It, thereof, promotes the formation of a dense and expendable carbon layer in the surface of the EP matrix, which can hinder the transmission of the flammable gases and heat between the exterior environment and inner EP matrix. It is a synergistic effect among phosphaphenanthrene, furan, and tetra-azole, which means a better diphasic flame-retardant mechanism occurs; superior fire safety is thus achieved for FREPs.
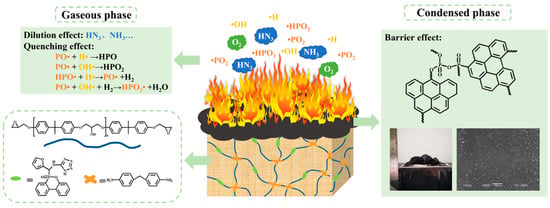
Figure 5.
A possible flame-retardant mechanism for FREPs.
3.9. Thermal and Mechanical Properties
The glass transition temperatures () of the neat EP and FREPs were tested via DSC. The corresponding DSC curves are illustrated in Figure S8, and the Tg values and the specific heat capacity values (ΔCp) are summarized in Table 7. As seen, the Tg values of FREPs were lower than that of the pristine EP and showed a downward trend with the increasing FATP content. This phenomenon might contribute to the reduction of the crosslinking density of FREPs upon the loading of the FATP. Conversely, the ΔCp values showed an upward trend with the increasing FATP contents. It can be deduced that much more hydrogen bonds were constructed between −NH in the FATP and the epoxy group in the EP.

Table 7.
Tg and ΔCp values obtained from DSC curves.
The dynamical mechanical performances were tested according to DMA; the variations of the storage modulus () and the loss tangent () with the temperature are illustrated in Figure S9, in which the temperature at the peak of is defined as . The crosslinking density () can be achieved according to the rubber elasticity theory with the equation [55], where and R donate the storage modulus at temperature of and the gas constant (8.314 J/kmol), respectively. The values of , at 50 °C, at , and are presented in Table 8. It was found that the FREPs gave lower values than that of the pristine EP and the of the FREPs was reduced accordingly [56]. The result is in agreement with that of the DSC. What we especially pointed out is that the value depends on both the temperature and the FATP contents. In the case of the temperature below , for instance 50 °C, the values of the FREPs were higher than that of the pristine EP and displayed an upward trend with the increasing FATP content. It contributed to the construction of much more hydrogen bonds and the introduction of the rigid phosphaphenanthrene, furan, and tetra-azole in the FATP. At the temperature of , a contrary tendency of the against the FATP contents was observed. Specifically, the value was decreased from 42.4 MPa for the pure EP to 37.7 MPa for FREP-2 and 33.8 MPa for FREP-4. This phenomenon contributed to the fact that the negative effect of the reduced crosslinking density on the stiffness predominates over the positive effect of hydrogen bonds and rigid groups when polymer materials are in the rubbery state.

Table 8.
DMA data of the pure EP and the FREPs.
The effects of the FATP content on the mechanical properties of the FREPs were further evaluated through tensile and flexural testing, with results listed in Table 9. The pristine EP presented a tensile strength of 72.2 MPa and a flexural strength of 126.1 MPa. In the case of the 4 wt.% FATP loading, the corresponding values of EP/FATP-4 were increased to 74.8 MPa and 135.5 MPa, with an increase of 3.6% and 7.5% by comparison with the neat EP, respectively. Additionally, the incorporation of the FATP with rigid chain structure resulted in enhancements of the elastic and flexural moduli, which were similar with results in the temperature below .

Table 9.
Tensile and flexural properties of the pure EP and the FREPs.
3.10. Transparency Analysis
EP thermosets with better transparency are generally used as optical materials such as LED and transparent coatings. It is well-known that the improvement of the flame retardancy is obtained at the expenses of the transparency of the EP, which is critical to the expended application of Ethe P. Therefore, the effect of the FATP on the optical transmittance of FREPs was explored. Figure 6 presents the curves of the transmittance against the wavelength in the range of 200–800 nm and optical images. Obviously, the transmittance (800 nm) of the EP was 89.3%, and the corresponding values of FREPs were almost the same, namely, 89.1% for EP/FATP-2 and 88.3% for FREP-4. Its better transparency allowed the emblems of Changzhou University to be clearly visible under all samples. The results demonstrated that the incorporation of the FATP had no negative roles on the transparency of EP thermosets. The maintenance of the better transparency contributed to the fact that the above-mentioned hydrogen bonds enhanced the compatibility between the FATP and the EP, thus leading to a uniform dispersion of the former within the later matrix. Moreover, UV-B and even UV-A were absorbed completely upon the introduction of FATP, indicating that the FREPs possessed better ultraviolet (UV) shielding capability. This specificity of FREPs is due to the fact that both the aromatic ring and the phosphorous-containing groups had the UV absorbability.
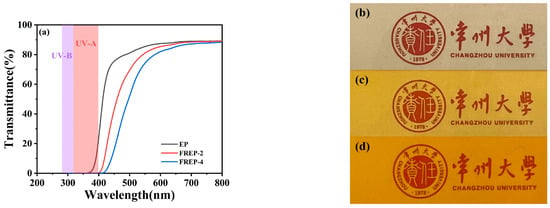
Figure 6.
(a) Transmittance spectra of the pure EP and the FREPs in the range of 200–800 nm; optical images of the pure EP (b), FREP-2 (c), and FREP-4 (d).
4. Conclusions
A nitrogen-rich DOPO-based derivate (FATP) was successfully synthesized by the one-pot method using 2-furaldehyde, 5-amino-1H-tetrazole, and DOPO as raw materials. FREPs were further prepared by curing the mixture of the EP and the FATP in the presence of the DDM as a curing agent. The analysis of the curing behavior showed that the FATP improved the reactivity of the EP and promoted the curing reaction. The TGA results confirmed that the incorporation of the FATP decreased T5% and Tmax as well as the maximum decomposition rate. When 4% FATP (0.33 wt.% phosphorus content) was introduced, the FREP-4 specimen achieved a UL-94 V-0 rating and an LOI value of 35%. Meanwhile, the EP/FATP-4 gave the lowest values of the THR (86.7 MJ/m2), the PHRR (1059.3 kW/m2), and the TSP (139.7 MJ/m2), with the reductions of 13.8%, 9.4%, and 35.9% as compared with the neat EP, respectively. The analysis of the pyrolysis gases revealed that the FATP played flame-retardant roles in the gas phase through the release of •HPO/•PO free radicals quenching reactive groups such as •OH and •H and some nonflammable gases (HN3 and NH3) diluting combustible volatiles. The subsequent characterization (SEM, Raman spectroscopy, and XPS) of char residues confirmed that the FATP promoted the formation of continuous and compact carbon layers with a greater graphitization degree, which acted as a physical barrier to inhibit the transmission of flammable gases and the heat between the exterior environment and the inner EP matrix, thus playing a flame-retardant effect in the condensed phase. The DSC and DMA results revealed that the storage modulus at 50 °C was increased although the Tg and Ve declined with the incorporation of the FATP. Compared with the pristine EP, FREP-4 kept almost the same transmittance while slightly enhancing mechanical properties. In summary, the FREPs not only possessed better flame retardancy, fire safety, and extra UV shielding capabilities, but also kept high transparency and mechanical strength, thus having promising applications in the fields of advanced optical technology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16020519/s1, Figure S1. FTIR spectra of FA, ATZ, DOPO and FATP; Figure S2. 1H NMR spectrum of FATP (a), 31P NMR spectra of DOPO (b) and FATP(c); Figure S3. TGA and DTG curves under nitrogen atmosphere; Figure S4. Raman spectra of the char residue for pure EP and FREPs; Figure S5. XPS spectra of the char residue; Figure S6. C1s(a), N1s(b), O1s (c), and P2p (d) spectra of char residues of FREP-4; Figure S7. Variation of peak intensity of some typical flammable volatiles with time; Figure S8. DSC curves of pure EP and FREPs; Figure S9. DMA curves of pure EP and FREPs.
Author Contributions
Formal analysis, B.C.; Investigation, J.L. and W.X.; Resources, L.W.; Writing—original draft, J.L.; Writing—review & editing, Z.L.; Methodology, B.W.; Supervision, B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.; Li, S.; Zhu, Z.; Yin, X.; Wang, L.; Weng, Y.; Wang, X. A novel DOPO-based flame retardant containing benzimidazolone structure with high charring ability towards low flammability and smoke epoxy resins. Polym. Degrad. Stab. 2020, 183, 109426. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; Zhang, Y.; Hu, L.; Wang, T.; Han, R.; He, J.-L.; Luo, W.; Liu, Z.-G.; Deng, J.-N.; et al. Imide-DOPO derivative endows epoxy resin with excellent flame retardancy and fluorescence without losing glass transition temperature. Composites B Eng. 2021, 230, 109533. [Google Scholar] [CrossRef]
- Price, D.; Anthony, G.; Carty, P. Fire Retardant Materials; Elsevier: Cambridge, UK, 2001. [Google Scholar]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Zaikov, G.E.; Lomakin, S.M. Ecological issue of polymer flame retardancy. J. Appl. Polym. Sci. 2002, 86, 2449–2462. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V.; Plasseraud, L.; Boni, G. New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins. ACS Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Song, L.; Hu, Y. Intrinsically flame-retardant bio-based epoxy thermosets: A review. Composites B Eng. 2019, 179, 107487.1–107487.13. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Huang, S.; Tian, X.; Song, L.; Yu, Q.; Wang, Z. Performance comparison of flame retardant epoxy resins modified by DPO-PHE and DOPO-PHE. Polym. Degrad. Stab. 2018, 156, 89–99. [Google Scholar] [CrossRef]
- Cheng, J.; Duan, H.; Yang, S.; Wang, J.; Zhang, Q.; Ding, G.; Hu, Y.; Huo, S. A P/N-containing flame retardant constructed by phosphaphenanthrene, phosphonate, and triazole and its flame retardant mechanism in reducing fire hazards of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49090. [Google Scholar] [CrossRef]
- Niu, H.; Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Phosphorus-Free Vanillin-Derived Intrinsically Flame-Retardant Epoxy Thermoset with Extremely Low Heat Release Rate and Smoke Emission. ACS Sustain. Chem. Eng. 2021, 9, 5268–5277. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, P.; Wang, H.; Wang, L.; Yu, B.; Yang, F. A facile one-step synthesis of highly efficient melamine salt reactive flame retardant for epoxy resin. J. Mater. Sci. 2020, 55, 12836–12847. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Zhang, W.; Yang, R.; Li, J. Fabrication of eco-friendly and multifunctional sodium-containing polyhedral oligomeric silsesquioxane and its flame retardancy on epoxy resin. Composites B Eng. 2020, 191, 107961. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, W.; Yang, R.; He, J.; Li, J.; Zhao, F. Facile synthesis of lithium containing polyhedral oligomeric phenyl silsesquioxane and its superior performance in transparency, smoke suppression and flame retardancy of epoxy resin. Comp. Sci. Technol. 2020, 189, 108004. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Sulfur’s role in the flame retardancy of thio-ether-linked hyperbranched polyphosphoesters in epoxy resins. Eur. Polym. J. 2020, 122, 109390. [Google Scholar] [CrossRef]
- Perez, R.; Sandler, J.; Altstädt, V.; Hoffmann, T.; Pospiech, D.; Ciesielski, M.; Döring, M.; Braun, U.; Balabanovich, A.; Schartel, B. Novel phosphorus-modified polysulfone as a combined flame retardant and toughness modifer for epoxy resins. Polymer 2007, 48, 778. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Yu, B.; Wang, J.; Wang, C.; Zeng, W.; Hu, Y. Economical and environment-friendly synthesis of a novel hyperbranched poly(aminomethylphosphine oxide-amine) as co-curing agent for simultaneous improvement of fire safety, glass transition temperature and toughness of epoxy resins. Chem. Eng. J. 2017, 322, 618–631. [Google Scholar] [CrossRef]
- Chen, R.; Hu, K.; Tang, H.; Wang, J.; Zhu, F.; Zhou, H. A novel flame retardant derived from DOPO and piperazine and its application in epoxy resin: Flame retardance, thermal stability and pyrolysis behavior. Polym. Degrad. Stab. 2019, 166, 334. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Q.; Luo, Z.; Wang, B. Facile synthesis of a reactive P/N/S-containing compound toward highly effective flame retardancy of epoxy resin with high transparency and improved mechanical strength. Fire Saf. J. 2021, 126, 103472. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, W.; He, X.; Yang, R. High-efficiency flame retardency of epoxy resin composites with perfect T-8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs). Comp. Sci. Technol. 2016, 127, 8. [Google Scholar] [CrossRef]
- Wang, J. Mechanistic study of the flame retardancy of epoxy resin with a novel phosphorus and silicon-containing flame retardant. J. Macromol. Sci. 2020, 59, 479. [Google Scholar] [CrossRef]
- Jiang, G.; Xiao, Y.; Qian, Z. A novel phosphorus-, nitrogen- and sulfur-containing macromolecule flame retardant for constructing high-performance epoxy resin composites. Chem. Eng. J. 2023, 451, 137823. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Nitrogen/sulfur-containing DOPO based oligomer for highly efficient flame-retardant epoxy resin. Polym. Degrad. Stab. 2019, 171, 109023. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Xu, B.; Qu, L.; Fang, D. Cross-linkable phosphorus/nitrogen-containing aromatic ethylenediamine endowing epoxy resin with excellent flame retardancy and mechanical properties. Compostites A Appl. S. 2022, 162, 107145. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, H.; Ji, S.; Ma, H. Novel phosphorus/nitrogen/boron-containing carboxylic acid as co-curing agent for fire safety of epoxy resin with enhanced mechanical properties. J. Hazard. Mater. 2021, 402, 123769. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, H. A novel nitrogen, phosphorus, and boron ionic pair compound toward fire safety and mechanical enhancement effect for epoxy resin. Polym. Adv. Technol. 2019, 31, 885. [Google Scholar] [CrossRef]
- Huo, S.; Yang, S.; Wang, J.; Cheng, J.; Zhang, Q.; Hu, Y.; Ding, G.; Zhang, Q.; Song, P.; Wang, H. A liquid phosphaphenanthrene-derived imidazole for improved flame retardancy and smoke suppression of epoxy resin. ACS Appl. Polym. Mater. 2020, 2, 3566. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Li, C.; Wang, X.; Cai, H. Synthesis of a DOPO-containing imidazole curing agent and its application in reactive flame retarded epoxy resin. Polym. Degrad. Stab. 2018, 159, 78. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Li, L.; Zhang, X. Synthesis of a phosphaphenanthrene/triazole oligomer for simultaneous improvement of flame retardancy and smoke suppression of epoxy resins. Comp. Commun. 2021, 28, 100965. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.; Liu, X.; Dong, Y. Gas-phase flame-retardant effects of a bi-group compound based on phosphaphenanthrene and triazine-trione groups in epoxy resin. Polym. Degrad. Stab. 2016, 133, 350. [Google Scholar] [CrossRef]
- Wirasaputra, A.; Yao, X.; Zhu, Y.; Liu, S.; Yuan, Y.; Zhao, J.; Fu, Y. Flame-retarded epoxy resins with a curing agent of DOPO-triazine based anhydride. Macromol. Mater. Eng. 2016, 301, 982. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Pang, F.-Q.; Xu, Y.-L.; Jian, R.-K. Multifunctional phosphorus-containing triazolyl amine toward self-intumescent flame-retardant and mechanically strong epoxy resin with high transparency. Ind. Eng. Chem. Res. 2020, 59, 11918. [Google Scholar] [CrossRef]
- Pang, F.Q.; Liu, X.D.; Zheng, X.T.; Lin, Y.C.; Jian, R.K. An intrinsic flame retardant epoxy resin with high transparency and strengthened mechanical property. J. Appl. Polym. Sci. 2021, 138, e51230. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Wang, J.; Pan, Z.; Zhou, H. Epoxy resin modified with chitosan derivatives and DOPO: Improved flame retardancy, mechanical properties and transparency. Polym. Degrad. Stab. 2022, 199, 109931. [Google Scholar] [CrossRef]
- Qiao, H.; Su, L.; Liu, C.; Zhang, H.; Chen, M. From laboratory to industrialization: Eco-friendly flame retardant endowing epoxy resin with excellent flame retardancy, transparency, and mechanical properties. Polym. Adv. Technol. 2022, 33, 1695. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Tang, Q.; Zhang, K.; Deng, W.; Zhang, L.; Wang, R.; Chen, J.; Deng, J.; Liao, W.; et al. Highly efficient flame-retardant and transparent epoxy resin. Polym. Adv. Technol. 2021, 32, 2940. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wang, J.; Liu, J.; Long, S.; Wang, D. Synthesis of multifunctional flame retardant with toughening and transparency and its application in epoxy resin. React. Funct. Polym. 2022, 176, 105289. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N.S.S.; Shafiei, N. Use of tetrazoles in catalysis and energetic applications: Recent developments. Mol. Catal. 2021, 513, 111788. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrolysis 2019, 139, 104. [Google Scholar] [CrossRef]
- Meng, J.; Zeng, Y.; Chen, P.; Zhang, J.; Yao, C.; Fang, Z.; Ouyang, P.; Guo, K. Flame retardancy and mechanical properties of bio-based furan epoxy resins with high crosslink density. Macromol. Mater. Eng. 2020, 305, 19000587. [Google Scholar] [CrossRef]
- Xie, W.; Huang, S.; Tang, D.; Liu, S.; Zhao, J. Synthesis of a furfural-based DOPO-containing co-curing agent for fire-safe epoxy resins. RSC Adv. 2020, 10, 104. [Google Scholar]
- He, K. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1417. [Google Scholar]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. 1970, 2, 301. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Xia, L.; Zheng, X. Effect of a novel P/N/S-containing reactive flame retardant on curing behavior, thermal and flame-retardant properties of epoxy resin. J. Anal. Appl. Pyrolysis 2017, 127, 360–368. [Google Scholar] [CrossRef]
- Huo, S.; Zhou, Z.; Jiang, J.; Sai, T.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Flame-retardant, transparent, mechanically-strong and tough epoxy resin enabled by high-efficiency multifunctional boron-based polyphosphonamide. Chem. Eng. J. 2022, 427, 131578. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Yu, R.; Yang, K.; Guo, L.; Yan, H. Phosphorus-free hyperbranched polyborate flame retardant: Ultra-high strength and toughness, reduced fire hazards and unexpected transparency for epoxy resin. Composites B Eng. 2022, 242, 110101. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, H.; Wan, C.; Liu, C.; Zhao, H.; Ma, H. Simultaneously improving the thermal stability, mechanical properties and flame retardancy of epoxy resin by a phosphorus/nitrogen/sulfur-containing reactive flame retardant. Mater. Today Commun. 2021, 30, 103108. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yang, S.; Cheng, J.; Ding, G.; Huo, S. Facile construction of one-component intrinsic flame-retardant epoxy resin system with fast curing ability using imidazole-blocked bismaleimide. Composites B Eng. 2019, 177, 107380. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Wang, J.; Xi, W. High-performance flame retardancy by char-cage hindering and free radical quenching effects in epoxy thermosets. Polymer 2015, 68, 262. [Google Scholar] [CrossRef]
- Qian, L.; Ye, L.; Qiu, Y.; Qu, S. Thermal degradation behavior of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymer 2011, 52, 5486. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.-C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.H.; Song, P.; Wang, H. Engineering MXene surface with POSS for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2020, 401, 123342. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-Q.; Fu, T.; Xu, Y.-J.; Li, D.-F.; Wang, X.-L.; Wang, Y.-Z. Novel phosphorus-containing halogen-free ionic liquid toward fire safety epoxy resin with well-balanced comprehensive performance. Chem. Eng. J. 2018, 354, 208. [Google Scholar] [CrossRef]
- Buten, C.; Lamping, S.; Körsgen, M.; Arlinghaus, H.F.; Jamieson, C.; Ravoo, B.J. Surface functionalization with carboxylic acids by photochemical microcontact printing and tetrazole chemistry. Langmuir 2018, 34, 2132. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular theory of rubber elasticity. Polymer 1979, 20, 1317. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, D. Novel cyclolinear cyclotriphosphazene-linked epoxy resin for halogen-free fire resistance: Synthesis, characterization, and flammability characteristics. Ind. Eng. Chem. Res. 2012, 51, 15064. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).