Influence of Catalytic Infrared Radiation on the Protective Properties of Industrial Epoxy Primers
Abstract
:1. Introduction
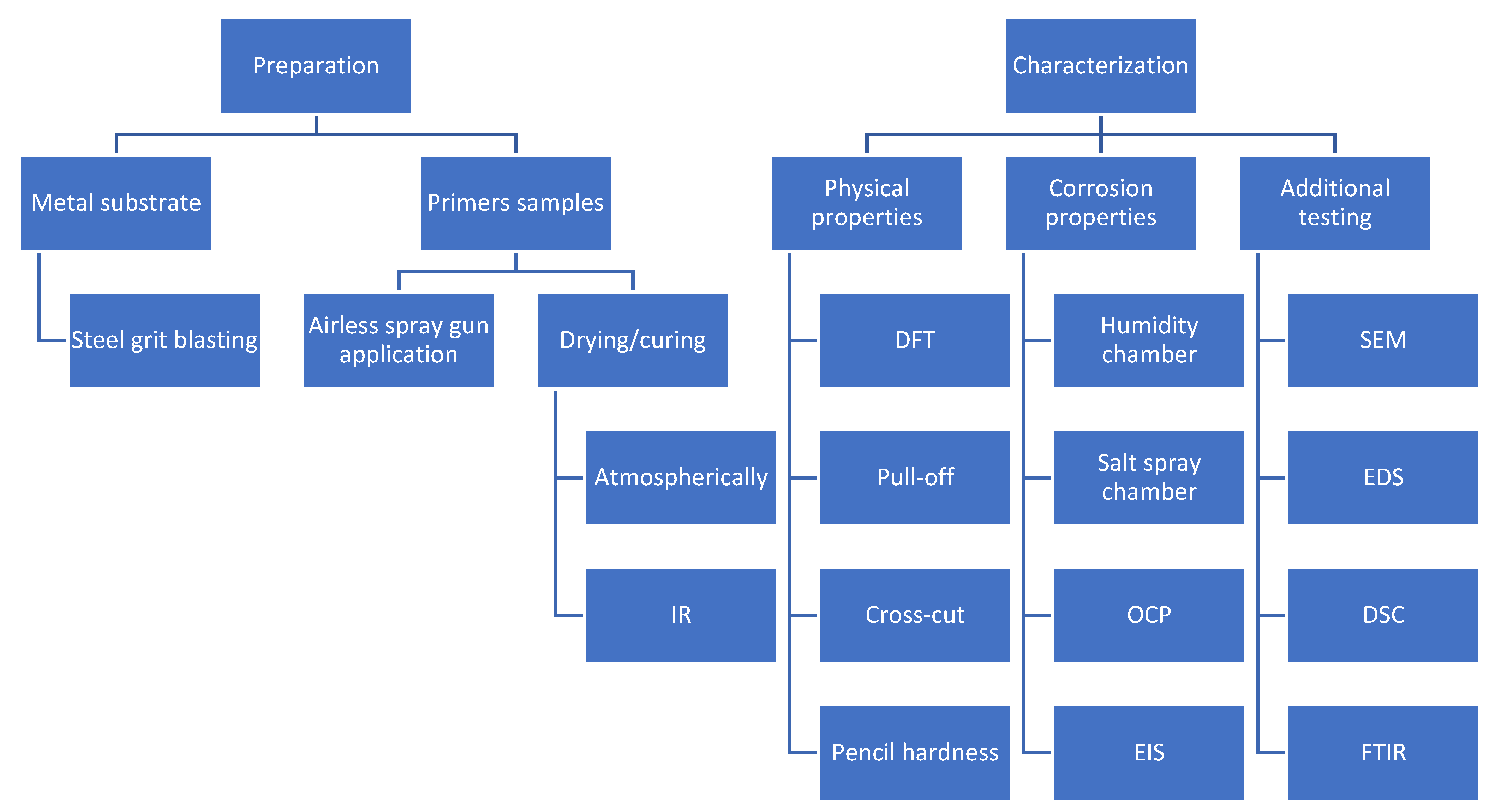
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Pull-off and cross-cut tests revealed great adhesion of both primers. The adhesion was even better when catalytic infrared radiation was applied.
- Catalytic infrared radiation did not affect the primers’ hardness or their results from the corrosion acceleration chambers, in terms of cracking, flaking, and blistering.
- Corrosion at the scribe from the experiments in a salt spray chamber revealed higher cohesive strength of primers cured with catalytic infrared radiation.
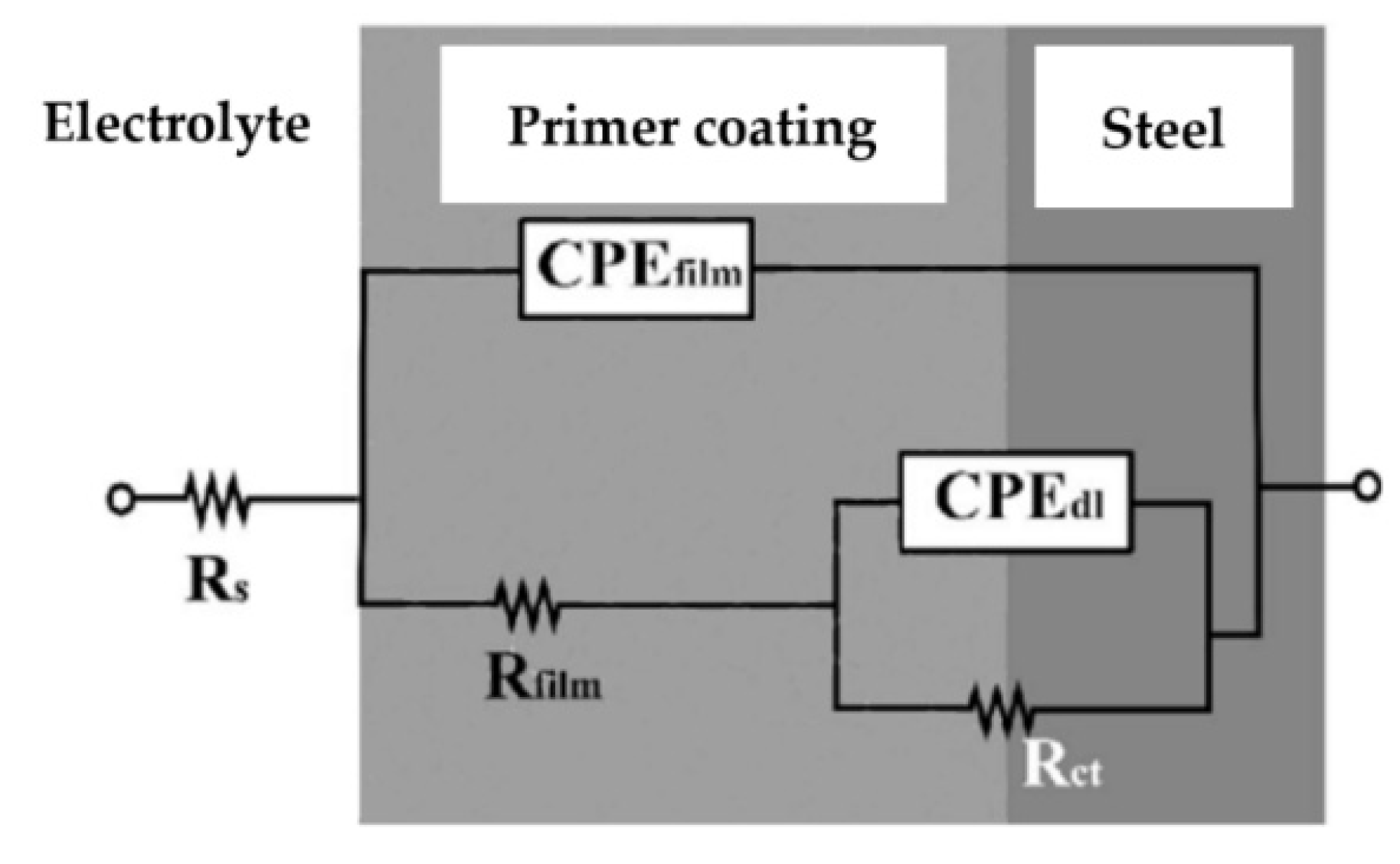
- OCP and EIS results indicate a higher corrosion resistance of IR-cured primers. IR-cured coating protection efficiencies are significant and amount to around 90%.
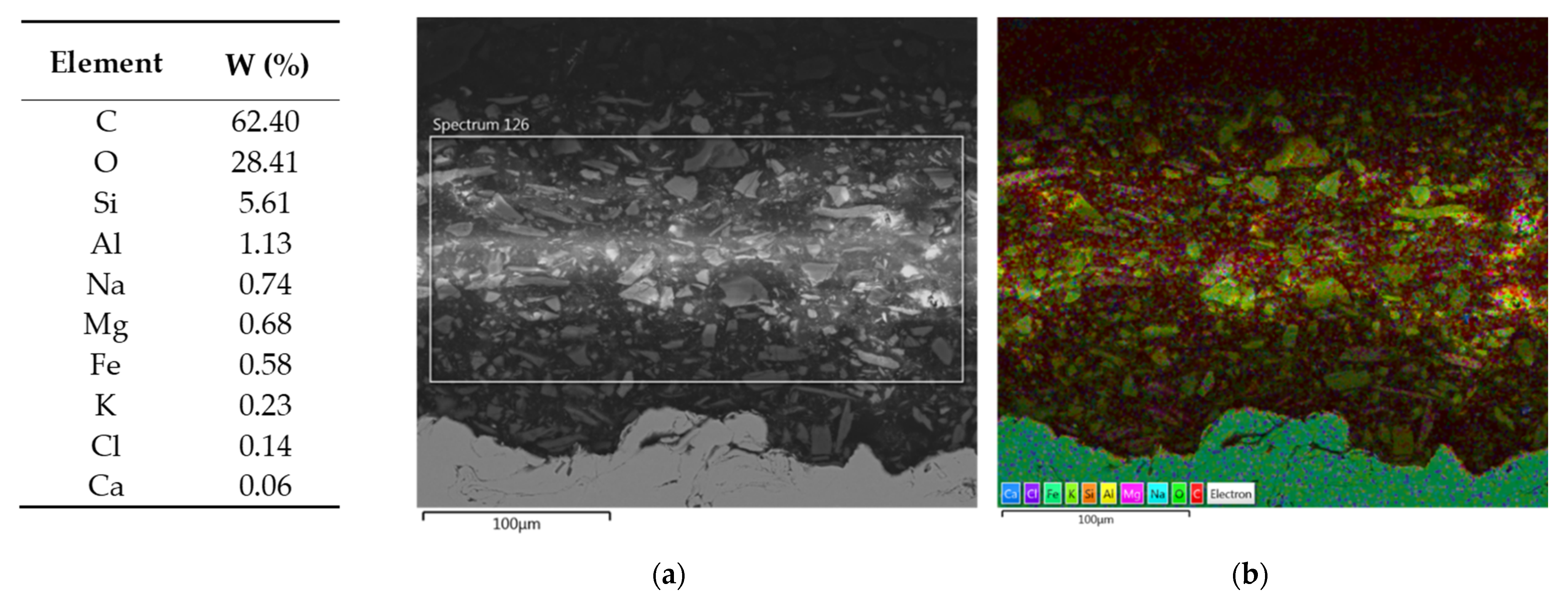
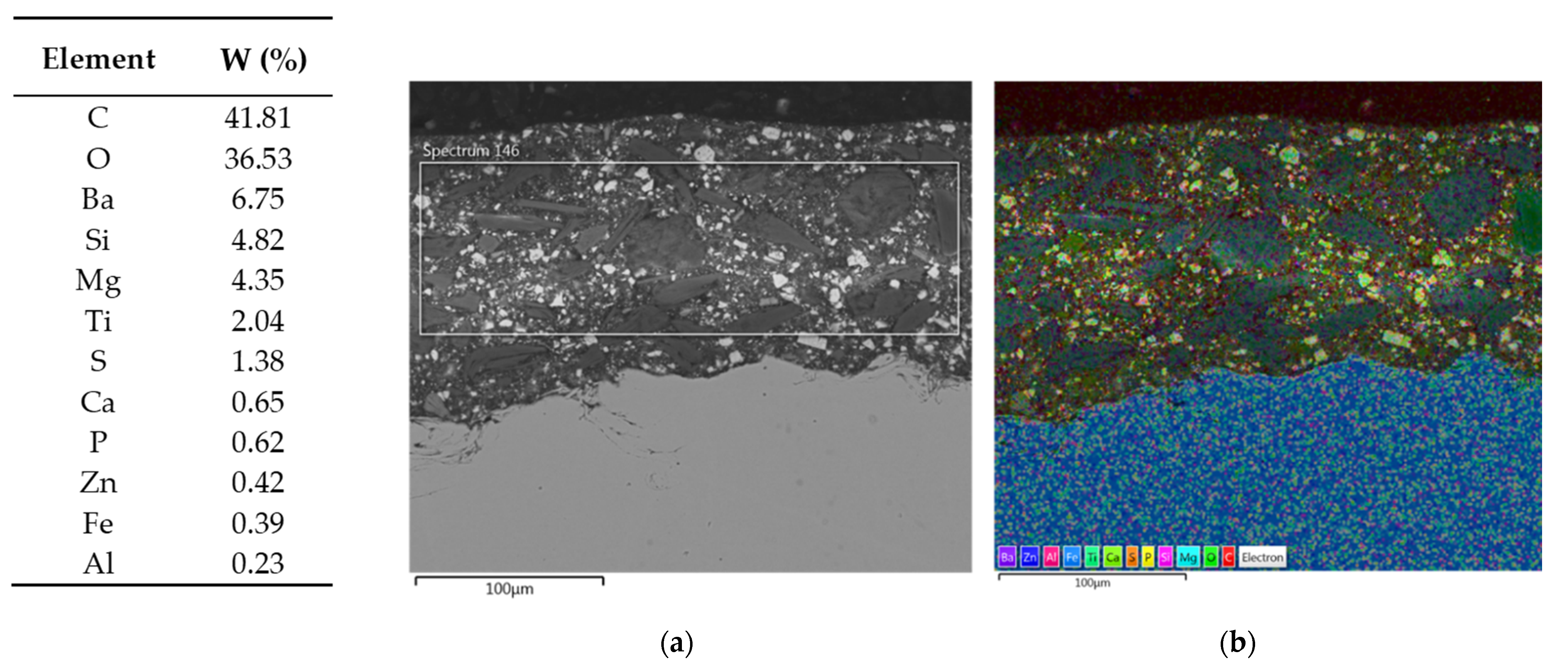
- SEM/EDS helped to better understand specific properties of individual primers.
- DSC analysis confirmed a higher curing degree of IR-cured primers, while the results of FTIR analysis revealed no significant differences between differently dried primers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Stojanović, I.; Cindrić, I.; Janković, L.; Šimunović, V.; Franjić, H. Performance Assessment of Differently Dried Coating Systems for Potential Application in the Power Transformer Industry. Coatings 2022, 12, 331. [Google Scholar] [CrossRef]
- De la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Popoola, A.P.I.; Loto, C.A.; Osifuye, C.O.; Aigbodion, V.S.; Popoola, O.M. Corrosion and wear properties of Ni-Sn-P ternary deposits on mild steel via electroless method. Alex. Eng. J. 2016, 55, 2091–2908. [Google Scholar] [CrossRef]
- Bhuvaneswari, T.K.; Jeyaprabha, C.; Arulmathi, P. Corrosion inhibition of mild steel in hydrochloric acid by leaves extract of Tephrosia purpurea. J. Adhes. Sci. Technol. 2022, 34, 2424–2447. [Google Scholar] [CrossRef]
- Židov, B.; Lin, Z.; Stojanović, I.; Xu, L. Impact of inhibitor loaded mesoporous silica nanoparticles on waterborne coating performance in various corrosive environments. J. Appl. Polym. Sci. 2021, 158, 49614. [Google Scholar] [CrossRef]
- Song, G.-L.; Feng, Z. Modification, Degradation and Evaluation of a Few Organic Coatings for Some Marine Applications. Corros. Mater. Degrad. 2020, 1, 408–442. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, Y.; Takada, R.; Eguchi, Y.; Takahashi, H. Formation of Self-Healing Organic Coatings for Corrosion Protection of Al Alloys by Dispersion of Spherical and Fibrous Capsules. Materials 2023, 16, 3018. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Sugimoto, T.; Yamaguchi, K.; Higashiyama, Y. Detection of Paint Curing by Non-contact Surface Resistivity Measurement. In Proceedings of the Joint Electrostatics Conference, Cambridge, ON, Canada, 12–14 June 2012. [Google Scholar]
- Fink-Jensen, P. Hardness testing of organic coatings. J. Pure Appl. Chem. 1965, 10, 239–292. [Google Scholar] [CrossRef]
- Stojanović, I.; Alar, V.; Mikšić, B.A.; Boršić, I.R. The effect of VpCI chemical pre-treatment on adhesion of organic coatings. In Proceedings of the Eurocorr, Seville, Spain, 9–13 September 2019. [Google Scholar]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Bierwagen, G.P. Reflections on corrosion control by organic coatings. Prog. Org. Coat. 1996, 28, 43–48. [Google Scholar] [CrossRef]
- Ouarga, A.; Noukrati, H.; Iraola-Arregui, I.; Elaissari, A.; Barroug, A.; Youcef, H.B. Development of anti-corrosion coating based on phosphorylated ethyl cellulose microcapsules. Prog. Org. Coat. 2020, 148, 105885. [Google Scholar] [CrossRef]
- De Meijer, M. Review on the durability of exterior wood coatings with reduced VOC-content. Prog. Org. Coat. 2001, 43, 217–225. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Zin, I.M.; Howard, R.L.; Badger, S.J.; Scantlebury, J.D.; Lyon, S.B. The mode of action of chromate inhibitor in epoxy primer on galvanized steel. Prog. Org. Coat. 1998, 33, 203–210. [Google Scholar] [CrossRef]
- Mohseni, M.; Mirabedini, M.; Hasemi, M.; Thompson, G.E. Adhesion performance of an epoxy clear coat on aluminum alloy in the presence of vinyl and amino-silane primers. Prog. Org. Coat. 2006, 57, 307–313. [Google Scholar] [CrossRef]
- Poelman, M.; Olivier, M.-G.; Gayarre, N.; Petitjean, J.-P. Electrochemical study of different ageing tests for the evaluation of a cataphoretic epoxy primer on aluminium. Prog. Org. Coat. 2005, 54, 55–62. [Google Scholar] [CrossRef]
- Bagale, U.D.; Desale, R.; Sonawane, S.H.; Kulkarni, R.D. An Active Corrosion Inhibition Coating of Two Pack Epoxy Polyamide System using Halloysite Nanocontainer. Prot. Met. Phys. Chem. Surf. 2018, 54, 230–239. [Google Scholar] [CrossRef]
- Khodaei, P.; Shabani-Nooshabadi, M.; Behpour, M. Epoxy-Based nanocomposite coating reinforced by a zeolite complex: Its anticorrosion properties on mild steel in 3.5 wt% NaCl media. Prog. Org. Coat. 2019, 136, 105254. [Google Scholar] [CrossRef]
- Roose, P.; Fallais, I.; Vandermiers, C.; Olivier, M.-G.; Poelman, M. Radiation curing technology: An attractive technology for metal coating. Prog. Org. Coat. 2009, 64, 163–170. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, S.; Wang, T.; Xia, L.; Liu, Y.; Wang, X.; Li, L.; Wnag, T. Experimental and Numerical Investigations on Curing a Polyester-Based Powder Coating by Catalytic Infrared Radiation. Appl. Sci. 2023, 13, 2187. [Google Scholar] [CrossRef]
- Schmitz, C.; Strehmel, B. NIR LEDs and NIR lasers as feasible alternatives to replace oven processes for treatment of thermal-responsive coatings. J. Coat. Technol. Res. 2019, 16, 1527–1541. [Google Scholar] [CrossRef]
- ISO 8501-1(en); Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness—Part 1: Rust Grades and Preparation Grades of Uncoated Steel Substrates and of Steel Substrates after Overall Removal of Previous Coatings. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8503-1(en); Preparation of Steel Substrates before Application of Paints and Related Products—Surface Roughness Characteristics of Blast-Cleaned Steel Substrates—Part 1: Specifications and Definitions for ISO Surface Profile Comparators for the Assessment of Abrasive Blast-Cleaned Surfaces. International Organization for Standardization: Geneva, Switzerland, 2012.
- Hempel. “Hempaprime Multi 500”, Product Data Sheet. Date of Publication: August 2023.
- Ching. “ESD 182 K-DB”, Product Data Sheet. Date of Publication: February 2020.
- Khamis, M.; Subramanyam, B.; Dogan, H.; Gwirtz, J.A. Flameless catalytic infrared radiation used for grain disinfestation does not affect hard red winter wheat quality. J. Stored Prod. Res. 2011, 47, 204–209. [Google Scholar] [CrossRef]
- Al-Dabbas, M.A. Heating by Catalytic Gas Infrared Rays. Energy Eng. 2011, 108, 26–45. [Google Scholar] [CrossRef]
- ISO 2808; Paints and Varnishes—Determination of Film Thickness. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 4624; Paints and Varnishes—Pull-off Test for Adhesion. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 2409; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 15184; Paints and Varnishes—Determination of Film Hardness by Pencil Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 6270-1; Paints and Varnishes—Determination of Resistance to Humidity—Part 1: Condensation (Single-Sided Exposure). International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 9227; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4628; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and Intensity of Uniform Changes in Appearance. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 12944-6; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 6: Laboratory Performance Test Methods. International Organization for Standardization: Geneva, Switzerland, 2018.
- Jianguo, L.; Gaoping, G.; Chuanwei, Y. EIS study of corrosion behaviour of organic coating/Dacromet composite systems. Electrochim. Acta 2005, 50, 3320–3332. [Google Scholar] [CrossRef]
- Wright, J.M.; Colling, A. Seawater: Its Composition, Properties and Behaviour, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1995; p. 5. [Google Scholar] [CrossRef]
- Aleksandrov Fabijanić, T.; Šnajdar, M.; Kurtela, M.; Šimunović, V.; Marciuš, M.; Klaić, M. Corrosion Resistance of Nanostructured Cemented Carbides with Alternative FeNi and FeNiCo Binders. Nanomaterials 2023, 13, 1407. [Google Scholar] [CrossRef]
- Samardžija, M.; Alar, V.; Špada, V.; Stojanović, I. Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles. Coatings 2022, 12, 1500. [Google Scholar] [CrossRef]
- Stojanović, I.; Cindrić, I.; Turkalj, L.; Kurtela, M.; Rakela-Ristevski, D. Durability and Corrosion Properties of Waterborne Coating Systems on Mild Steel Dried under Atmospheric Conditions and by Infrared Radiation. Materials 2022, 15, 8001. [Google Scholar] [CrossRef]
- Stojanović, I.; Logar, M.; Fatović, I.; Alar, V.; Rakela-Ristevski, D. Experimental Study of Atmospherically and Infrared-Dried Industrial Topcoats. Coatings 2023, 13, 1343. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Šoić, I.; Martinez, S.; Dubravić, M. Gel-Electrolyte EIS setup used for probing of IR Dried/Cured industrial coatings. Prog. Org. Coat. 2019, 137, 105331. [Google Scholar] [CrossRef]
- Uday, M.; Kiran Kumar, P. Heat transfer studies for infrared radiation assisted curing in polymer composites. J. Phys. Conf. Ser. 2020, 1473, 12027. [Google Scholar] [CrossRef]
- Kralj, M.; Pavković, K.; Stojanović, I.; Anđal, J. Adhesion and anticorrosive properties of DTM coating as related to primer coating. Građevinar 2019, 71, 401–408. [Google Scholar] [CrossRef]
- Wei, H.; Xia, J.; Zhou, W.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Adhesion and cohesion of epoxy-based industrial composite coatings. Compos. Part B Eng. 2022, 193, 108035. [Google Scholar] [CrossRef]
- Selemix. A Guide to the Updated ISO 12944 Norm. Available online: https://en.selemix.com/en/technical-support/expert-know-how/a-guide-to-the-updated-iso-12944-norm/# (accessed on 5 July 2023).
- Kano, K.; Hagiwara, S.; Igarashi, T.; Otani, M. Study on the free corrosion potential at an interface between an Al electrode and an acidic aqueous NaCl solution through density functional theory combined with the reference interaction site model. Electrochim. Acta 2021, 377, 138121. [Google Scholar] [CrossRef]
- Hiromoto, S. Corrosion of metallic biomaterials. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing: Sawston, UK, 2010; Volume 1, pp. 99–121. [Google Scholar] [CrossRef]
- Kalendova, A.; Veselý, D.; Kalenda, P. A study of the effects of pigments and fillers on the properties of anticorrosive paints. Pigment. Resin Technol. 2006, 35, 83–94. [Google Scholar] [CrossRef]
- Vallejo Vitaller, A.; Angst, U.M.; Elsener, B. Laboratory tests simulating corrosion in geothermal power plants: Infuence of service conditions. Geotherm. Energy 2020, 8, 9. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Alam, M.A.; Al-Zahrani, S.M. Fabrication of different protective coatings and studying their mechanical properties and corrosion behavior in sodium chloride solutions. Int. J. Electrochem. Sci. 2015, 10, 373–387. [Google Scholar] [CrossRef]
- Yang, X.; Zou, Y.; Huang, Q.; Su, Z.; Chang, X.; Zhang, M.; Xiao, Y. Improved oxidation resistance of chemical vapor reaction SiC coating modified with silica for carbon/carbon composites. J. Cent. South Univ. Technol. 2010, 17, 1–6. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Luo, P.; Xue, S.; Li, Z.; Cheng, X.; Zhong, J.; Yan, L.; Fan, Y. Synthesis of ZrB2 strengthened Ni–W composite coating and study of its mechanical characters and anti-corrosion performance. Surf. Coat. Technol. 2022, 441, 128553. [Google Scholar] [CrossRef]
- Veselý, D.; Kalendová, A.; Němec, P. Properties of organic coatings depending on chemical composition and structure of pigment particles. Surf. Coat. Technol. 2010, 204, 2032–2037. [Google Scholar] [CrossRef]
- Michel, M.; Ferrier, E. Effect of curing temperature conditions on glass transition temperature values of epoxy polymer used for wet lay-up applications. Constr. Build. Mater. 2020, 231, 117206. [Google Scholar] [CrossRef]
- Acik, G.; Karabulut, H.R.F.; Altinkok, C.; Karatavuk, A.O. Synthesis and characterization of biodegradable polyurethanes made from cholic acid and L-lysine diisocyanate ethyl ester. Polym. Degrad. Stab. 2019, 165, 42–48. [Google Scholar] [CrossRef]
- Sabu, M.; Bementa, E.; Jaya Vins Ruban, Y.; Ginil Mon, S. A novel analysis of the dielectric properties of hybrid epoxy composites. Adv. Compos. Hybrid Mater. 2022, 3, 325–335. [Google Scholar] [CrossRef]
- Colombani, J.; Chauvet, E.; Amat, S.; Dupuy, N.; Gigmes, D. A FTIR/chemometrics approach to characterize the gamma radiation effects on iodine/epoxy-paint interactions in Nuclear Power Plants. Anal. Chim. Acta 2017, 960, 53–62. [Google Scholar] [CrossRef]
- Ramírez-Herrera, C.A.; Cruz-Cruz, I.; Jiménez-Cedeño, I.H.; Martínez-Romero, O.; Elías-Zúñiga, A. Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites. Polymers 2021, 13, 1273. [Google Scholar] [CrossRef]
- González González, M.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy; Theophanides, T., Ed.; IntechOpen: Rijeka, Croatia, 2012; Volume 1, pp. 261–284. [Google Scholar] [CrossRef]
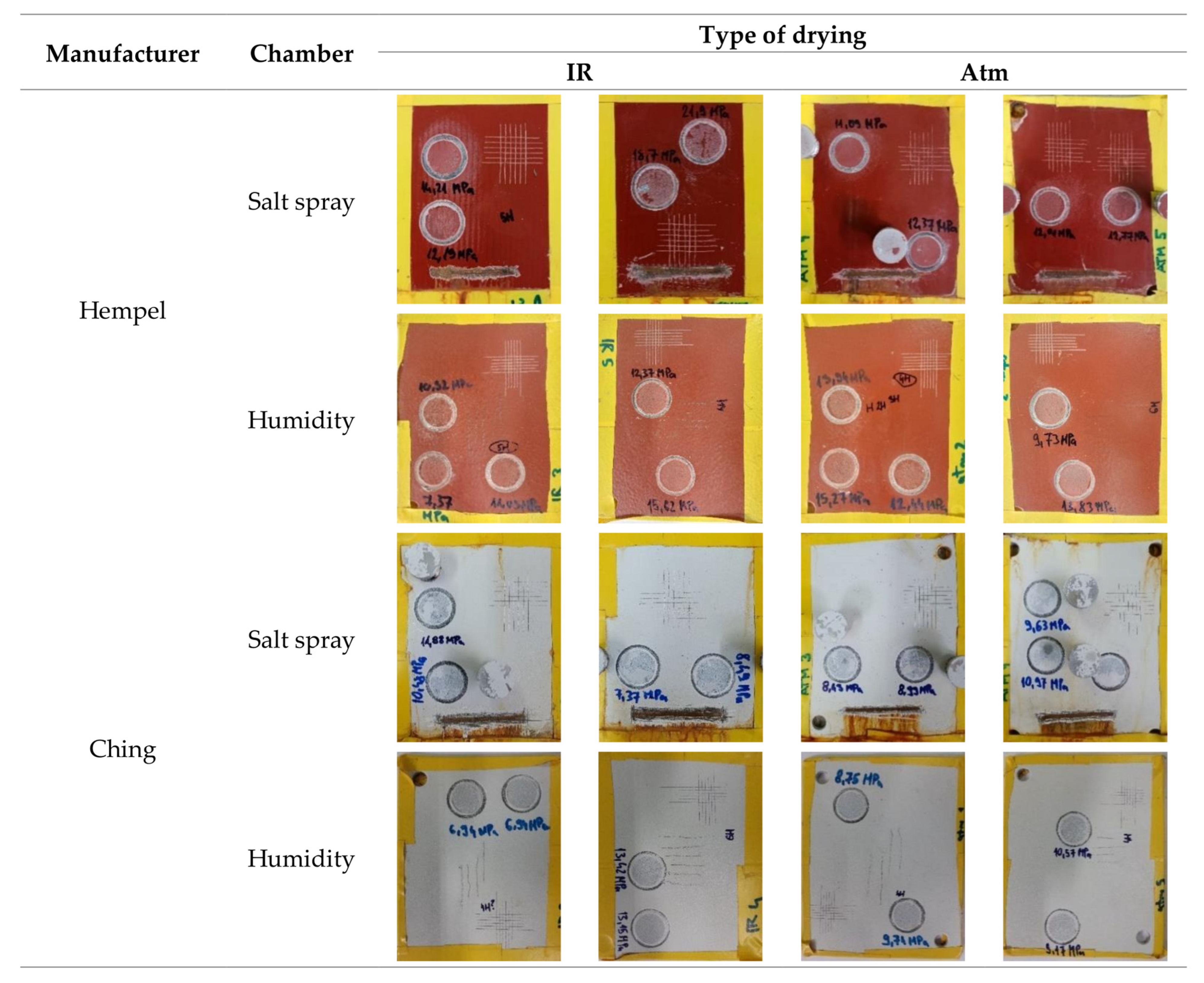





| Manufacturer | Hempel | Ching |
|---|---|---|
| Gloss | Semi-gloss | Matte |
| Solids by volume (Vol. %) | 85 ± 2 | 59 |
| Temperature resistance (°C) | 120 | 130 |
| Surface dry time (h) | 3 | 3 |
| Recommended thickness (µm) | 150 | 60–100 |
| Acceptable thickness (µm) | 100–225 | 60–200 |
| Manufacturer | Type of Drying | DFT (μm) | Pull-Off (MPa) | Cross-Cut | Pencil Hardness |
|---|---|---|---|---|---|
| Hempel | IR | 165 | 21.25 | 0 | H |
| Atm | 153 | 16.64 | 0 | H | |
| Ching | IR | 164 | 14.88 | 0 | H |
| Atm | 155 | 13.47 | 0 | H |
| Chamber | Type of Drying | DFTmean (µm) | Pull-Off (MPa) ISO 4624 | Cross-Cut ISO 2409 | Pencil Hardness ISO 15184 | M (mm) ISO 12944-6 |
|---|---|---|---|---|---|---|
| Salt spray | IR | 168 (±2.5) | 13.2 (±0.4) | 1 | HB | 0.01 |
| 162 (±2.5) | 20.3 (±0.4) | 1 | F | 0.11 | ||
| Atm | 173 (±2.5) | 12.39 (±0.4) | 1 | F | 0.2 | |
| 170 (±2.5) | 11.73 (±0.4) | 1 | H | 0.23 | ||
| Humidity | IR | 135 (±2.5) | 9.57 (±0.4) | 1 | HB | / |
| 141 (±2.5) | 14 (±0.4) | 1 | HB | / | ||
| Atm | 141 (±2.5) | 13.88 (±0.4) | 1 | HB | / | |
| 149 (±2.5) | 11.78 (±0.4) | 1 | F | / |
| Chamber | Type of Drying | DFTmean (µm) | Pull-Off (MPa) ISO 4624 | Cross-Cut ISO 2409 | Pencil Hardness ISO 15184 | M (mm) ISO 12944-6 |
|---|---|---|---|---|---|---|
| Salt spray | IR | 133 (±2.5) | 11.18 (±0.4) | 1 | H | 0.02 |
| 151 (±2.5) | 7.93 (±0.4) | 1 | F | 0.04 | ||
| Atm | 142 (±2.5) | 8.71 (±0.4) | 1 | F | 0.01 | |
| 131 (±2.5) | 10.3 (±0.4) | 1 | H | 0.26 | ||
| Humidity | IR | 123 (±2.5) | 6.84 (±0.4) | 1 | HB | / |
| 102 (±2.5) | 13.29 (±0.4) | 1 | F | / | ||
| Atm | 128 (±2.5) | 9.25 (±0.4) | 1 | HB | / | |
| 129 (±2.5) | 9.87 (±0.4) | 1 | HB | / |
| Chamber | Type of Drying | Rusting ISO 4628-3 | Cracking ISO 4628-4 | Flaking ISO 4628-5 | Blistering ISO 4628-2 |
|---|---|---|---|---|---|
| Salt spray | IR | Ri 1 | 0(S0) | 0(S0) | 0(S0) |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) | ||
| Atm | Ri 1 | 0(S0) | 0(S0) | 0(S0) | |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) | ||
| Humidity | IR | Ri 1 | 0(S0) | 0(S0) | 0(S0) |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) | ||
| Atm | Ri 1 | 0(S0) | 0(S0) | 0(S0) | |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) |
| Chamber | Type of Drying | Rusting ISO 4628-3 | Cracking ISO 4628-4 | Flaking ISO 4628-5 | Blistering ISO 4628-2 |
|---|---|---|---|---|---|
| Salt spray | IR | Ri 1 | 0(S0) | 0(S0) | 0(S0) |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) | ||
| Atm | Ri 1 | 0(S0) | 0(S0) | 0(S0) | |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) | ||
| Humidity | IR | Ri 1 | 0(S0) | 0(S0) | 0(S0) |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) | ||
| Atm | Ri 1 | 0(S0) | 0(S0) | 0(S0) | |
| Ri 1 | 0(S0) | 0(S0) | 0(S0) |
| Day | Ecorr vs. SCE (mV) | |||
|---|---|---|---|---|
| Hempel | Ching | |||
| IR | Atm | IR | Atm | |
| 1 | −135.9 | −419.9 | −163.2 | −261.9 |
| 4 | −226.3 | −506.1 | −147.2 | −476.0 |
| 7 | −224.8 | −488.3 | −148.1 | −380.6 |
| 10 | −221.4 | −523.9 | −145.4 | −362.5 |
| 15 | −209.8 | −516.8 | −136.8 | −331.8 |
| 21 | −258.6 | −522.6 | −135.3 | −231.9 |
| Manufacturer | Type of Drying | Tg (°C) |
|---|---|---|
| Hempel | IR | 38.8 |
| Atm | 36.4 | |
| Ching | IR | 56.9 |
| Atm | 50.5 |
| Wavenumber (cm−1) | Assignment | |||
|---|---|---|---|---|
| Hempel | Ching | |||
| IR | Atm | IR | Atm | |
| 3676.89 | 3676.86 | 3676.58 | 3676.67 | O-H stretching |
| 3305.69 | 3368.37 | 3346.83 | N-H stretching | |
| 2924.63 | 2925.18 | 2925.21 | 2925.19 | C-H stretching of CH2 |
| 2853.78 | 2852.87 | 2854.77 | 2855.27 | CH2 symmetrical and asymmetrical stretching |
| 1607.22 | 1607.47 | 1606.62 | 1606.85 | C=C aromatic cycle stretching, N-H bending of primary amine |
| 1581.12 | 1581.61 | 1581.39 | 1581.91 | C=C aromatic cycle stretching, N-H bending of primary amine |
| 1508.71 | 1508.80 | 1507.71 | 1508.18 | C-C stretching of aromatic cycle |
| 1456.10 | 1460.63 | 1413.19 | 1412.01 | C–H bending of CH2 |
| 1294.63 | 1294.79 | 1297.39 | 1296.65 | C-O symmetric stretching of phenolic ether |
| 1241.27 | 1243.25 | 1243.16 | 1241.35 | C-O symmetric stretching of phenolic ether |
| 1180.68 | 1181.17 | 1181.05 | 1181.13 | C-O symmetric stretching of phenolic ether |
| 1011.91 | 1010.74 | 1011.29 | 1012.78 | C-O symmetric stretching of aliphatic ether |
| 873.07 | 872.42 | C=C bending | ||
| 827.86 | 827.62 | 827.48 | 827.46 | C–H out-of-plane bending of aromatic cycle |
| 762.67 | 761.10 | C–H out-of-plane bending of aromatic cycle | ||
| 724.75 | 723.25 | 711.46 | 711.25 | C–H out-of-plane bending of aromatic cycle |
| 671.37 | 670.49 | 669.75 | 670.42 | C–H out-of-plane bending of aromatic cycle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, I.; Logar, M.; Turkalj, L.; Cindrić, I.; Kurtela, M.; Franjić, H. Influence of Catalytic Infrared Radiation on the Protective Properties of Industrial Epoxy Primers. Materials 2023, 16, 6551. https://doi.org/10.3390/ma16196551
Stojanović I, Logar M, Turkalj L, Cindrić I, Kurtela M, Franjić H. Influence of Catalytic Infrared Radiation on the Protective Properties of Industrial Epoxy Primers. Materials. 2023; 16(19):6551. https://doi.org/10.3390/ma16196551
Chicago/Turabian StyleStojanović, Ivan, Mirta Logar, Lovro Turkalj, Ivan Cindrić, Marin Kurtela, and Hrvoje Franjić. 2023. "Influence of Catalytic Infrared Radiation on the Protective Properties of Industrial Epoxy Primers" Materials 16, no. 19: 6551. https://doi.org/10.3390/ma16196551
APA StyleStojanović, I., Logar, M., Turkalj, L., Cindrić, I., Kurtela, M., & Franjić, H. (2023). Influence of Catalytic Infrared Radiation on the Protective Properties of Industrial Epoxy Primers. Materials, 16(19), 6551. https://doi.org/10.3390/ma16196551








