Strength Degradation of Foamed Lightweight Soil Due to Chemical Erosion and Wet-Dry Cycle and Its Empirical Model
Abstract
:1. Introduction
2. Materials and Scheme of Test
2.1. Test Materials and Specimen Preparation
2.2. Test Plan and Procedures
3. Test Results and Analysis
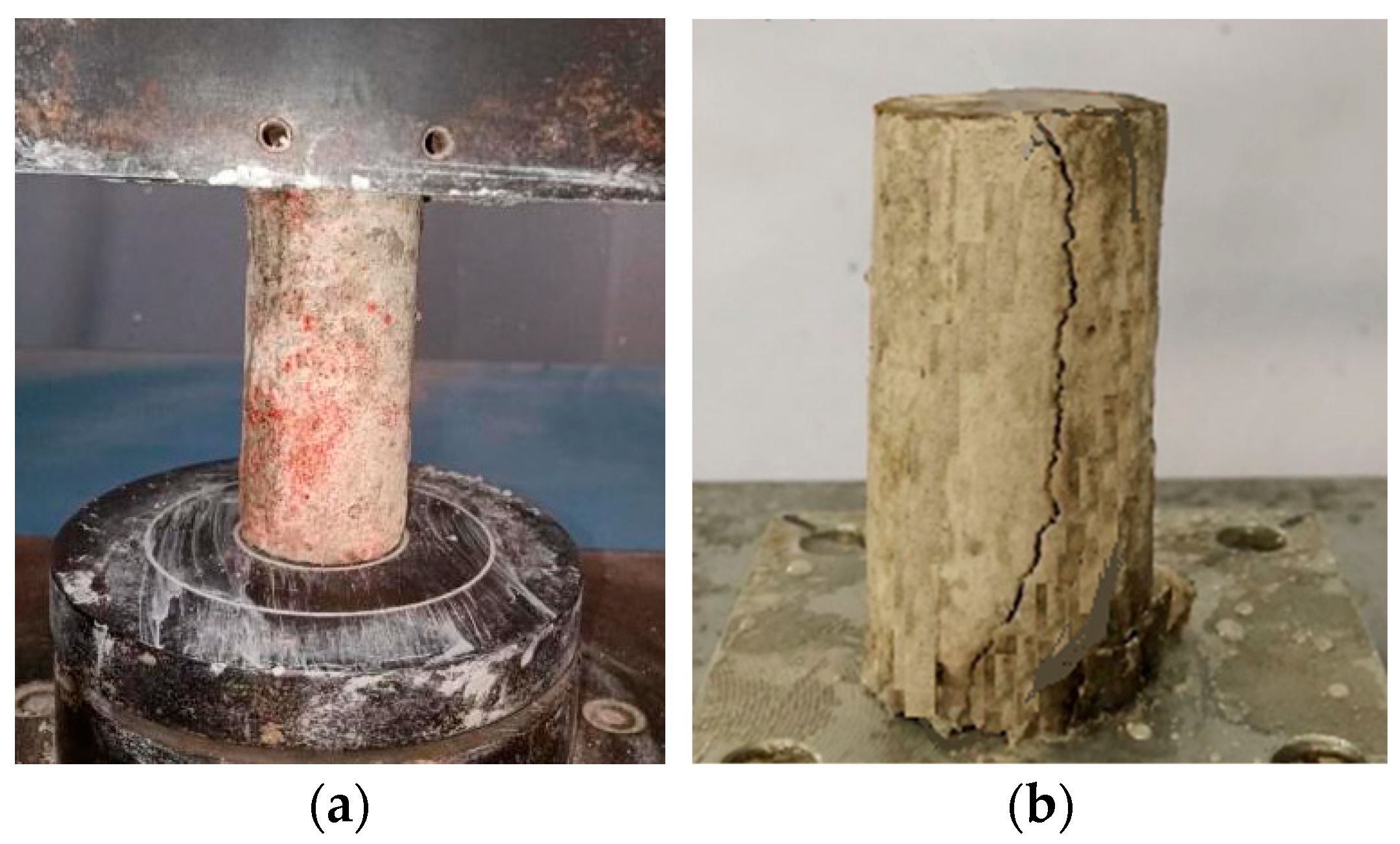
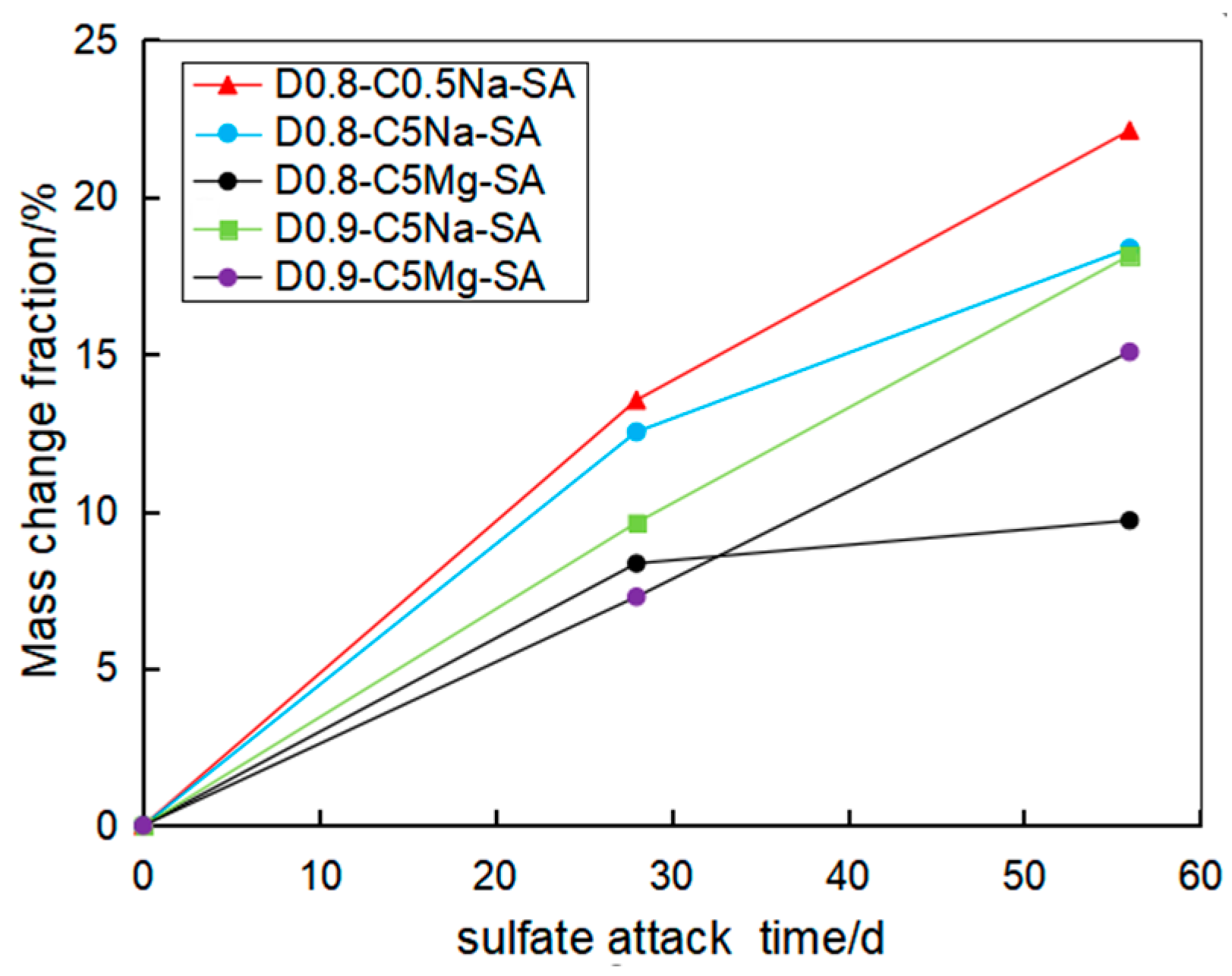
3.1. Appearances and Mass Change
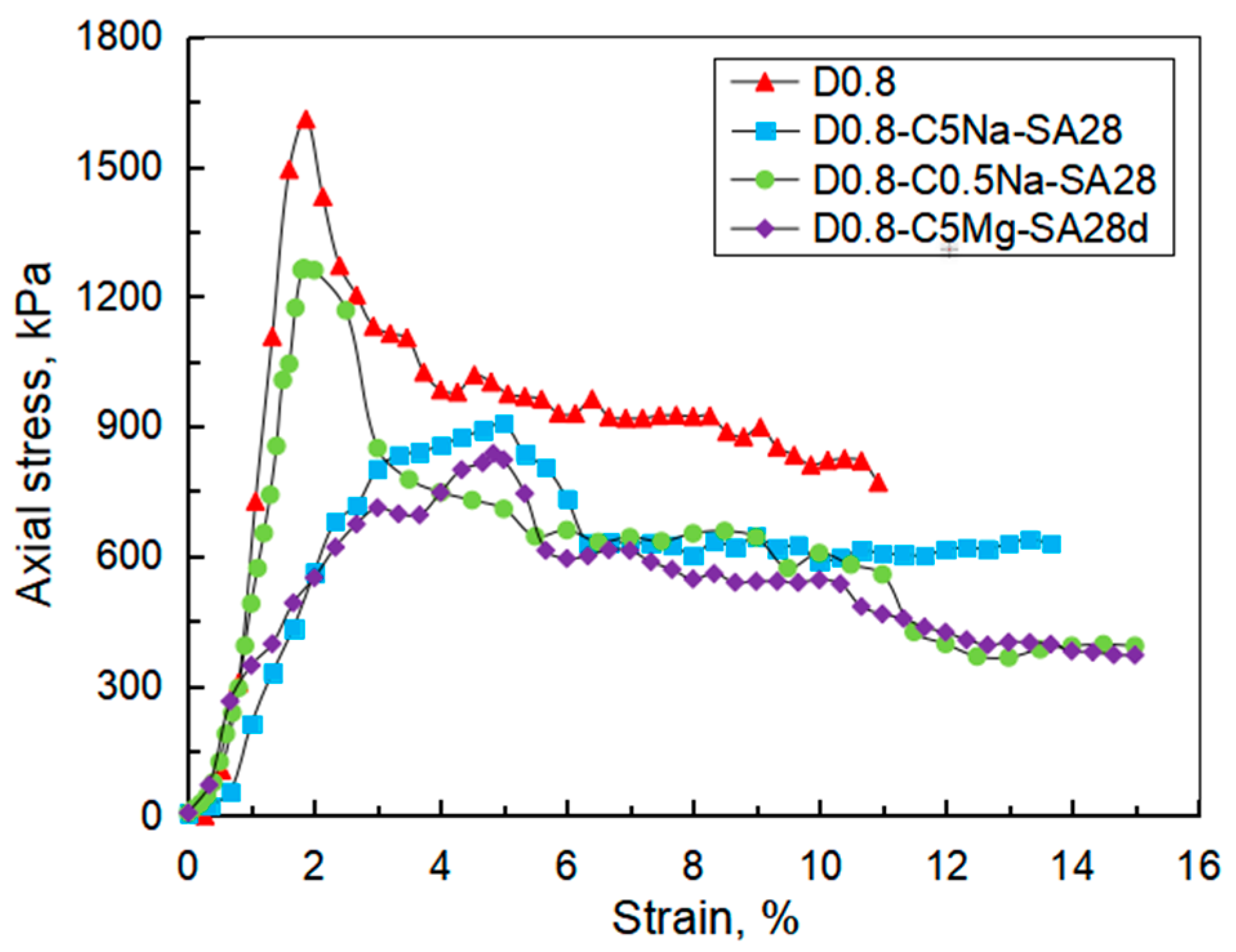
3.2. Stress vs. Strain Curve
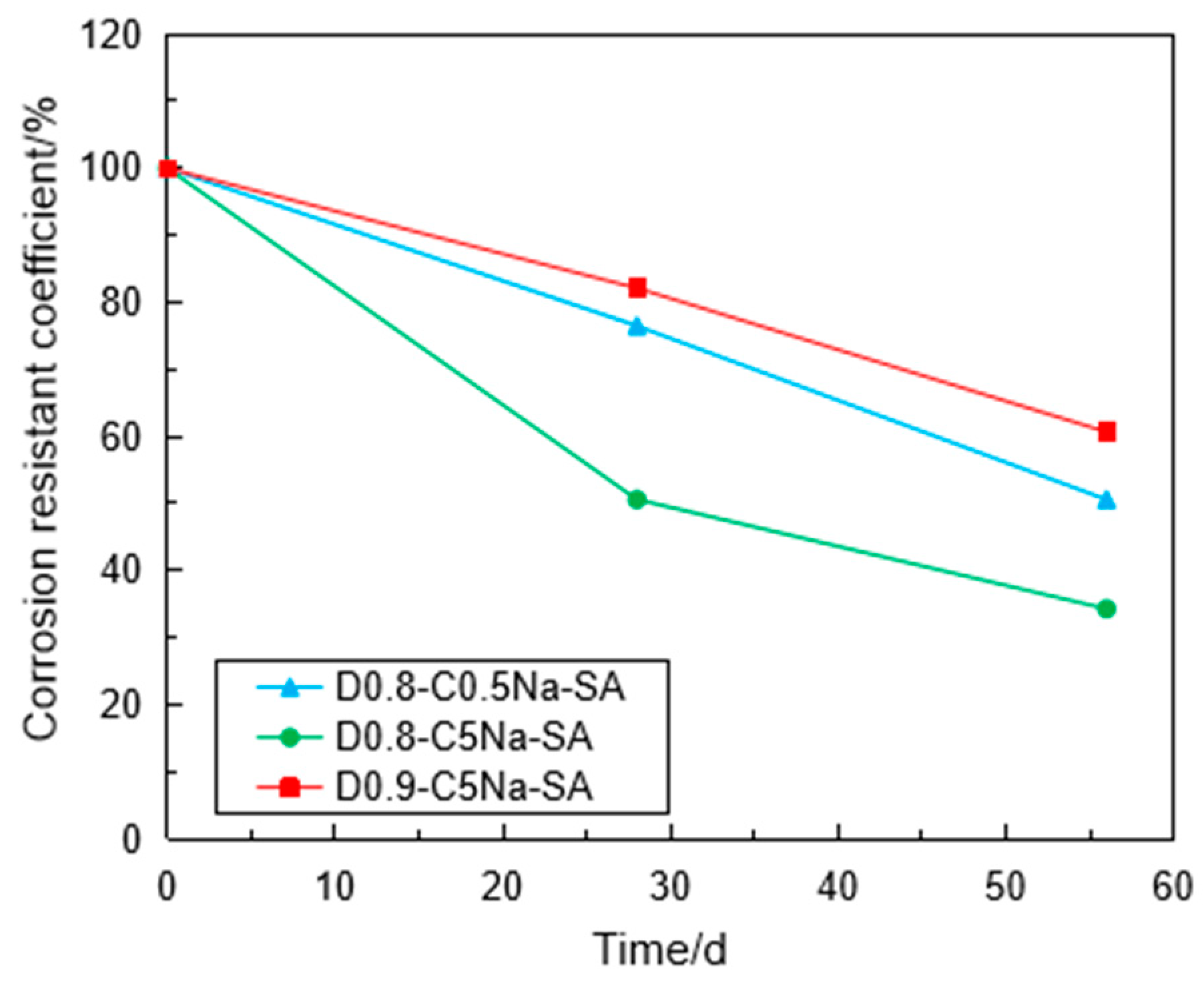
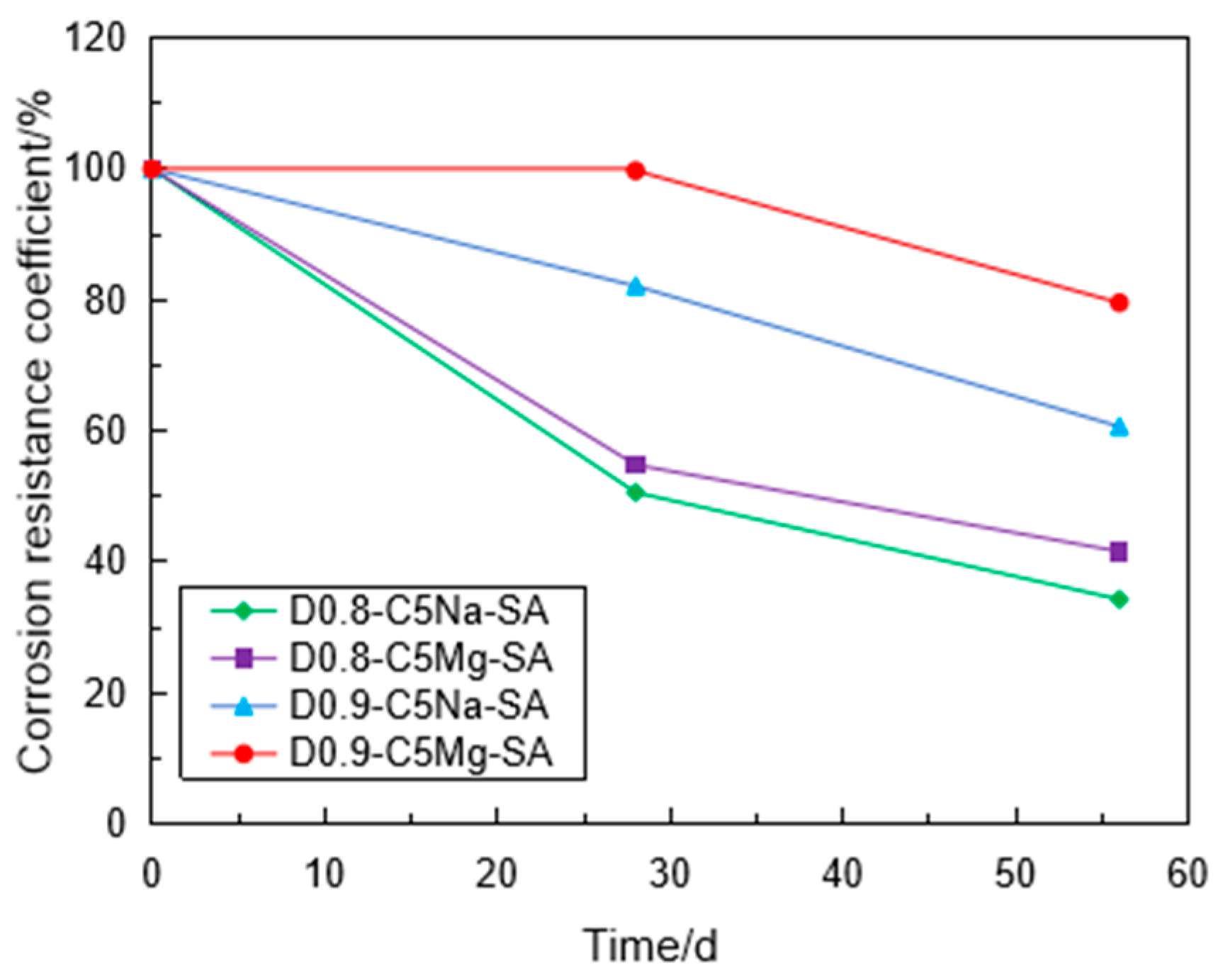
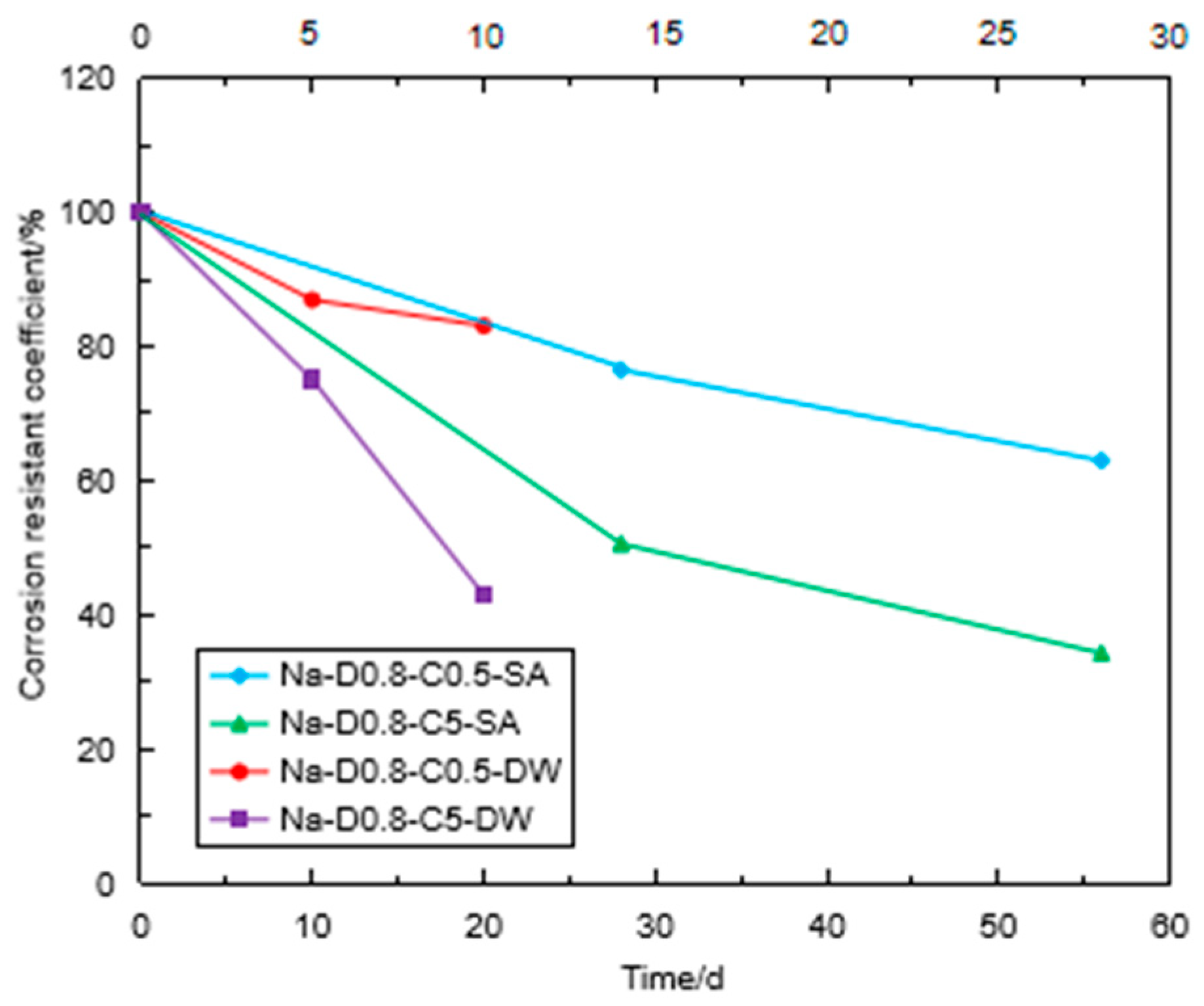
3.3. Corrosion Resistance of the Specimen under Sulfate Attack
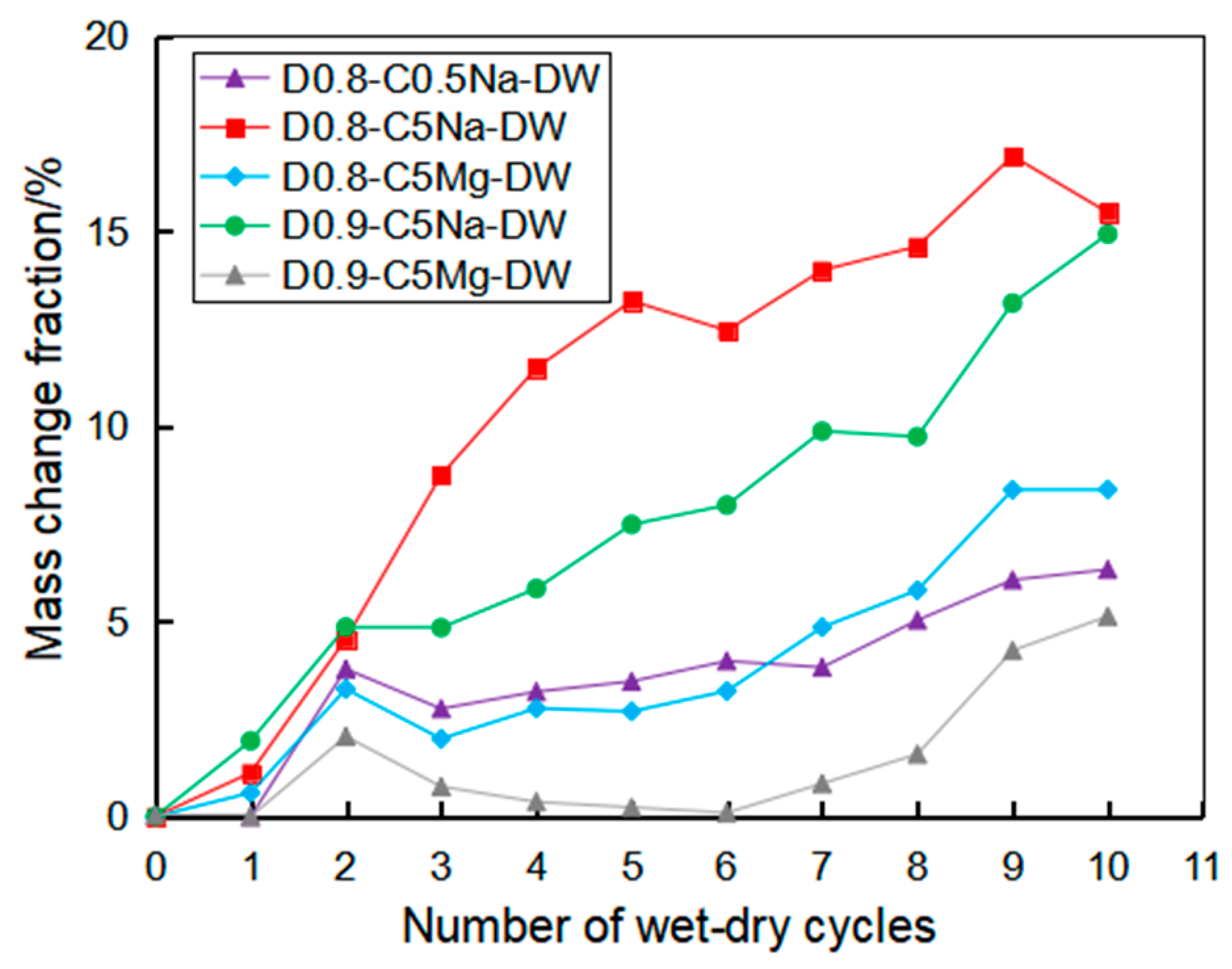
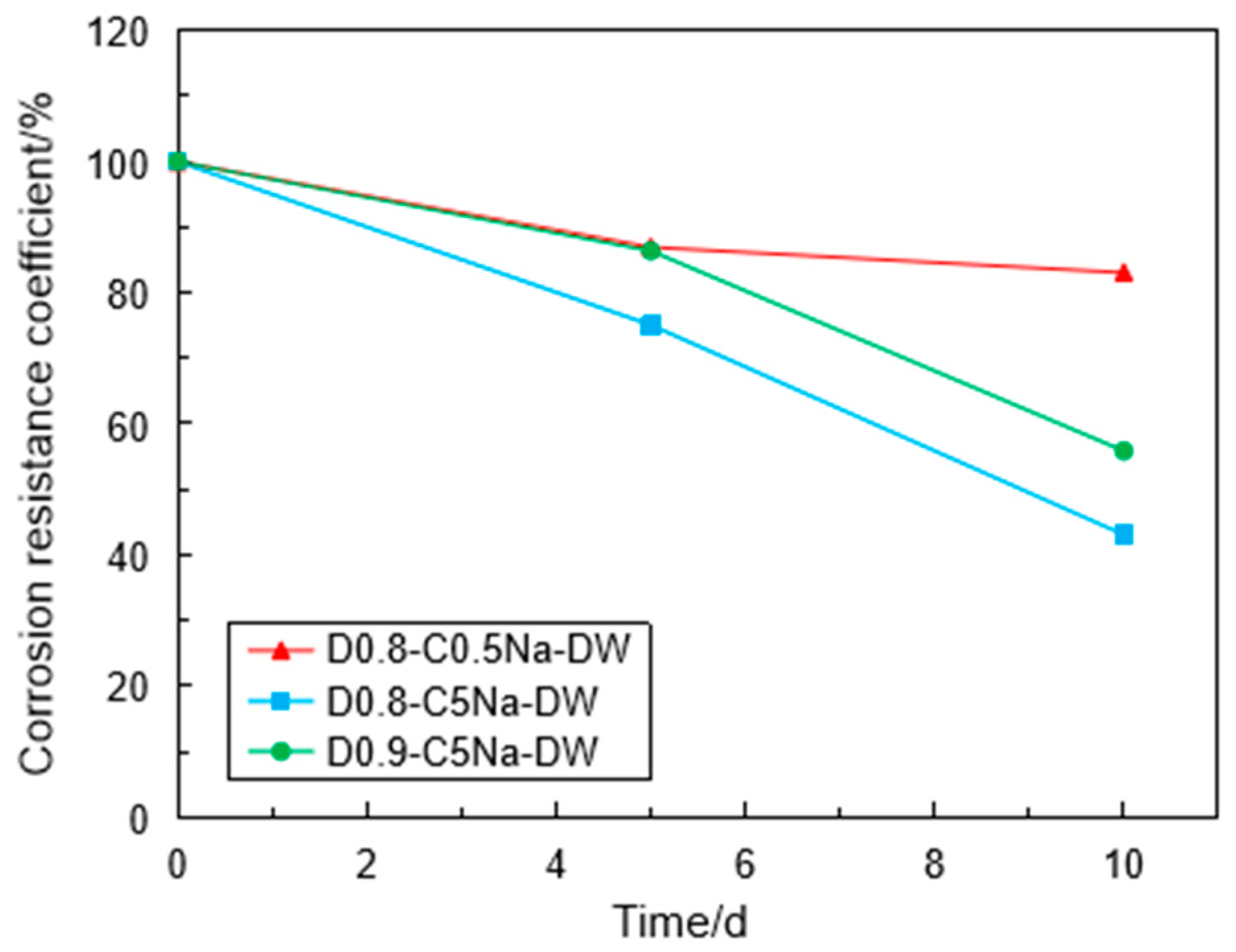
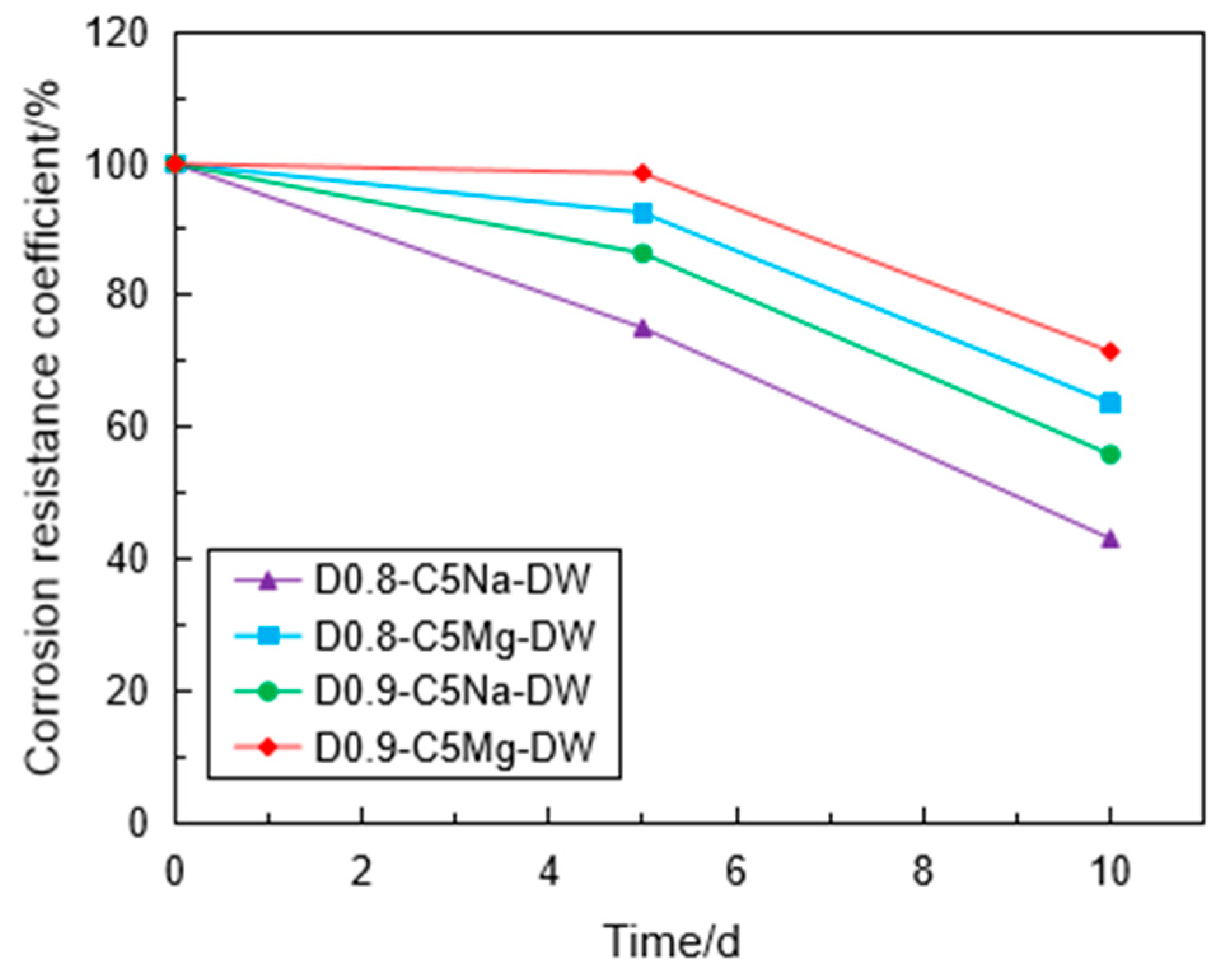
3.4. Corrosion Resistance of the Specimen under Coupling Effect
3.5. Comparison of Sulfate Immersion and Coupling Effect
4. Empirical Method for Predicting the CRC of FLS
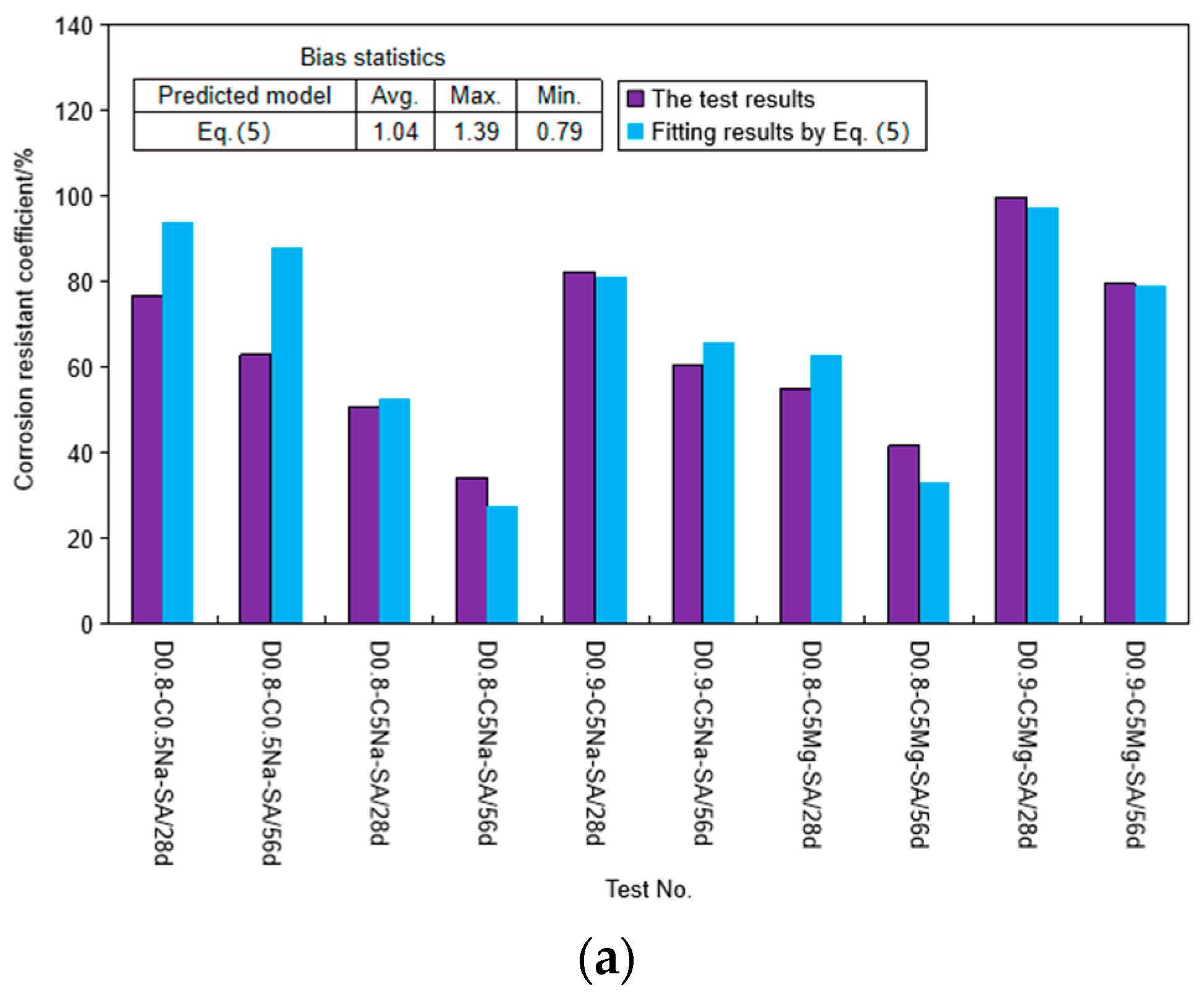
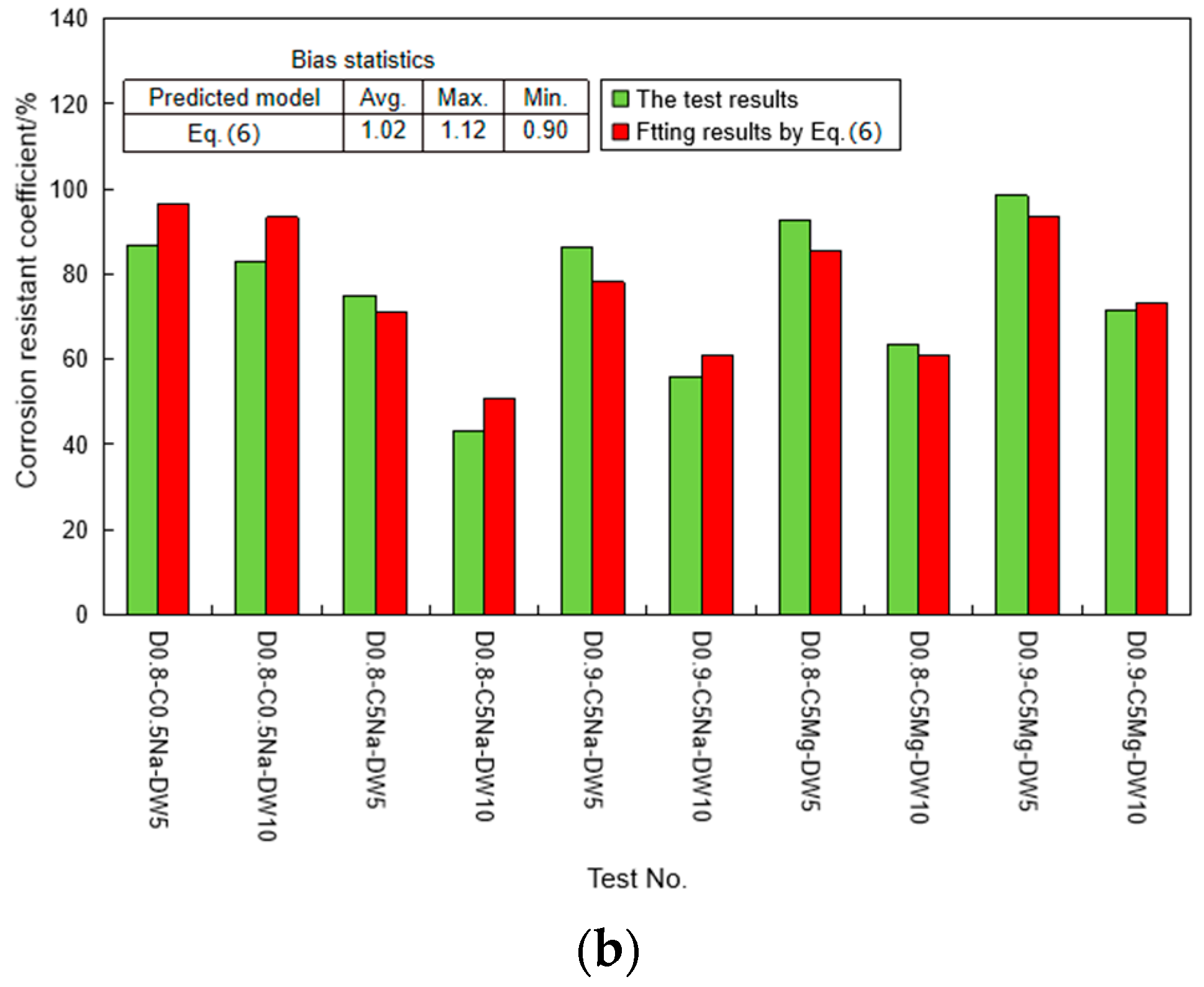
4.1. Strength Degradation Model
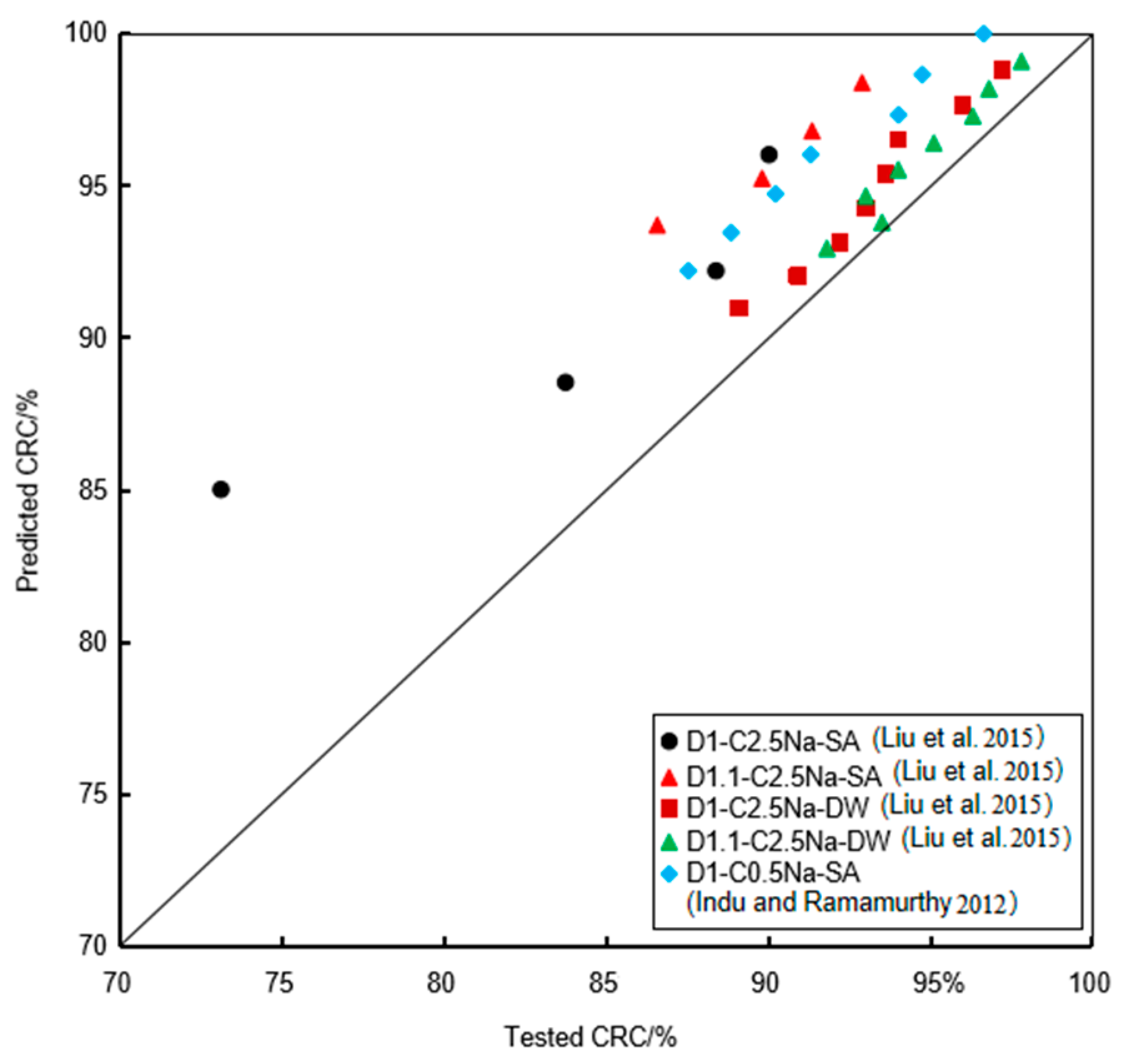
4.2. Determination and Verification of the Proposed Model
4.3. Sensitivity Analysis of Influencing Factors
4.4. Discussion and Limitation
5. Conclusions
- (1)
- The mass of the FLS increased with the sulfate attack time caused by the reaction of sulfate with Calcium aluminates and Calcium hydroxide to generate ettringite and gypsum. Under the erosion of the same sulfate concentration, the increase in mass caused by the MgSO4 solution was less than that by the Na2SO4 solution with the same SO42− concentration. The specimen with 0.9 g/cm3 had relatively smaller mass growth than the specimen with 0.8 g/cm3 under the same condition.
- (2)
- Using the UCT, it can be found that the sulfate attack had a greater impact on the reduction in the specimen strength. For chemical attack, the FLS specimen underwent more serious erosion in the high-concentration sulfate solution, and the high-density FLS specimen had strong erosion resistance. The Na2SO4 solution provided a more severe erosion effect compared to the MgSO4 solution when other conditions were kept constant. However, the erosion of the FLS specimen under the coupling effect of sulfate attack and dry-wet cycling was more significant than that only under chemical soaking.
- (3)
- An empirical model was developed based on the test results to predict the strength degradation of FLS under sulfate immersion and under the coupling effect of chemical attack and wet-dry cycle, and it was further verified using a comparison with the reported studies. The sensitivity analysis indicated that the strength degradation was sensitive to the FLS density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.Z.; Su, R.K.L. A review on durability of foam concrete. Buildings 2023, 13, 1880. [Google Scholar] [CrossRef]
- Raj, A.; Sathyan, D.; Mini, K.M. Physical and functional characteristics of foam concrete: A review. Constr. Build. Mater. 2019, 221, 787–799. [Google Scholar] [CrossRef]
- Shi, X.; Huang, J.; Su, Q. Experimental and numerical analyses of lightweight foamed concrete as filler for widening embankment. Constr. Build. Mater. 2020, 250, 118897. [Google Scholar] [CrossRef]
- Zhang, Z.; Rao, F.; Ye, G.; Liu, J. Mechanical performance and void structure change of foamed cement paste subjected to static and cyclic loading under plane strain conditions. Materials 2022, 15, 1711. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, Y.; Wang, Y.; Yan, C.; Zhang, L.; Yin, L.; Yang, X.; Qiu, Y.; Lan, H. Triaxial axis shear mechanical properties of fiber-reinforced foamed lightweight soil. J. Mater. Civ. Eng. 2021, 33. [Google Scholar] [CrossRef]
- Do, J. Optimization and design guideline of lightweight foamed soil reinforced with waste fishing net. Geomech. Eng. 2022, 29, 657–666. [Google Scholar]
- Yu, X.G.; Huang, H.K.; Lin, L.; Xu, H.C. Study of the density control of the low density sulphate aluminium cement (SAC) foam concrete. Adv. Mater. Res. 2013, 608–609, 1786–1789. [Google Scholar]
- Tian, W.; Han, N. Experiment analysis of concrete’s mechanical property deterioration suffered sulphate attack and drying-wetting cycles. Adv. Mater. Sci. Eng. 2017, 2017, 5673985. [Google Scholar] [CrossRef]
- Indu Siva Ranjani, G.; Ramamurthy, K. Behaviour of foam concrete under sulphate environments. Cem. Concr. Compos. 2012, 34, 825–834. [Google Scholar] [CrossRef]
- Neramitkornburi, A.; Horpibulsuk, S.; Shen, S.L.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Durability against wetting-drying cycles of sustainable lightweight cellular cemented construction material comprising clay and fly ash wastes. Constr. Build. Mater. 2015, 77, 41–49. [Google Scholar] [CrossRef]
- Liu, K.; Li, R.M.; Du, Y.; Wei, M. Durability experimental study of lightweight soil subjected to wetting-drying cycles and sodium sulfate soaking. Rock Soil Mech. 2015, 36, 362–366. [Google Scholar]
- Mamun, M.; Bindiganavile, V. Sulphate resistance of fibre reinforced cement-based foams. Constr. Build. Mater. 2011, 25, 3427–3442. [Google Scholar] [CrossRef]
- Wang, X.Y.; Han, P.J.; Bai, X.H.; Li, X. Influences of slag on properties of lightweight cement-treated soils subjected to sulfate corrosion. Constr. Build. Mater. 2019, 205, 511–518. [Google Scholar]
- Raj, B.; Sathyan, D.; Madhavan, M.K.; Raj, A. Mechanical and durability properties of hybrid fiber reinforced foam concrete. Constr. Build. Mater. 2020, 245, 118373. [Google Scholar] [CrossRef]
- Othman, R.; Muthusamy, K.; Duraisamy, Y.; Sulaiman, M.A.; Jaya, R.P.; Ghani, N.A.A.A.; Mangi, S.A. Evaluation of the sulphate resistance of foamed concrete containing processed spent bleaching earth. Eur. J. Environ. Civ. Eng. 2022, 26, 3632–3647. [Google Scholar] [CrossRef]
- Al-Shwaiter, A.; Awang, H.; Khalaf, M.A. Performance of sustainable lightweight foam concrete prepared using palm oil ash as a sand replacement. Constr. Build. Mater. 2022, 322, 126482. [Google Scholar] [CrossRef]
- Liu, X.; Ni, C.; Zhang, L.; Sheng, K.; Hong, B. Durability assessment of lightweight cellular concrete in subgrade by the method of analytic hierarchy process combined with fuzzy comprehensive evaluation. Math. Probl. Eng. 2019, 2019, 4915870. [Google Scholar] [CrossRef]
- Rao, F.; Zhang, Z.; Ye, G.; Liu, J. Mechanical behavior and assessment of foamed cement paste under staged cyclic loading. Mater. Struct. 2021, 54, 182–193. [Google Scholar] [CrossRef]
- Nguyen, T.; Kashani, A.; Ngo, T.; Bordas, S. Deep neural network with high-order neuron for the prediction of foamed concrete strength. Comput. Aided Civ. Infrastruct. Eng. 2019, 34, 316–332. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Ma, G.; Yuan, Y.; Nener, B. Automating the mixture design of lightweight foamed concrete using multi-objective firefly algorithm and support vector regression. Cem. Concr. Compos. 2021, 121, 104103. [Google Scholar] [CrossRef]
- Pan, P.; Yang, W.; Zhang, Y.; Li, P.-P. Optimized strength modelling of foamed concrete using principal component analysis featurized regressors. Structures 2023, 48, 1730–1745. [Google Scholar] [CrossRef]
- CJJ/T177(2012); Technical Specification for Filling Engineering of Bubble Mixed Lightweight Soil. Industry Standards of the People’s Republic of China: Beijing, China, 2012.
- GB/T50081(2022); The Standard for Test Methods of Mechanical Properties of Ordinary Concrete. Industry standards of the People’s Republic of China: Beijing, China, 2002.
- Tian, X.; Zhu, N. Durability prediction method of concrete soil based on deep belief network. Adv. Civ. Eng. 2022, 2022, 38306. [Google Scholar] [CrossRef]
- Liu, X.F.; Mei, G.D.; Li, J.X.; Lun, Y.X. Orthogonal test of flexural toughness for hybrid fiber reinforced concretes. Adv. Mater. Res. 2011, 239–242, 2006–2010. [Google Scholar]
- Liu, H.L.; Deng, A.; Chu, J. Effect of different mixing ratios of polystyrene pre-puff beads and cement on the mechanical behaviour of lightweight fill. Geotext. Geomembr. 2006, 24, 331–338. [Google Scholar] [CrossRef]
- Gomis-Yagues, V.; Boluda-Botella, N.; Ruiz-Bevia, F. Gypsum precipitation/ dissolution as an explanation of the decrease of sulphate concentration during seawater intrusion. J. Hydrol. 2000, 28, 48–55. [Google Scholar] [CrossRef]
- Kearsley, E.P.; Wainwright, P.J. The effect of high fly ash content on the compressive strength of foamed concrete. Cem. Concr. Res. 2001, 31, 105–112. [Google Scholar] [CrossRef]
- Park, H.I.; Kim, Y.T. Prediction of strength of reinforced lightweight soil using an artificial neural network. Eng. Comput. 2011, 28, 600–615. [Google Scholar] [CrossRef]
- Neramitkornburi, A.; Horpibulsuk, S.; Shen, S.; Arulrajah, A.; Disfani, M.M. Engineering properties of lightweight cellular cemented clay-fly ash material. Soils Found. 2015, 55, 471–483. [Google Scholar] [CrossRef]
- La Rubia-García, M.D.; Yebra-Rodríguez, Á.; Eliche-Quesada, D.; Corpas-Iglesias, F.A.; López-Galindo, A. Assessment of olive mill solid residue (pomace) as an additive in lightweight brick production. Constr. Build. Mater. 2012, 36, 495–500. [Google Scholar] [CrossRef]
- Teerawattanasuk, C.; Voottipruex, P.; Horpibulsuk, S. Mix design charts for lightweight cellular cemented bangkok clay. Appl. Clay Sci. 2015, 104, 318–323. [Google Scholar] [CrossRef]
- Mydin, M.A.O. Investigating the effect of sisal fibre content on durability properties of lightweight foamed concrete. Adv. Sci. Technol. Res. J. 2022, 16, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gencel, O.; Bayraktar, O.Y.; Kaplan, G. Lightweight foam concrete containing expanded perlite and glass sand: Physico-mechanical, durability, and insulation properties. Constr. Build. Mater. 2022, 320, 126187. [Google Scholar] [CrossRef]






| Density of the FLS (g/cm3) | Water-Cement Ratio | Cement Quality (kg) | Water Quality (kg) | Foam Volume (L) | Foam Volume Ratio (Vfoam/Vwater) |
|---|---|---|---|---|---|
| 800 | 0.45 | 4.4 | 2 | 8 | 4 |
| 900 | 0.45 | 4.4 | 2 | 7 | 3.5 |
| Test No. | ρ/g/cm3 | m | c/% | p/d | N |
|---|---|---|---|---|---|
| D0.8 | 0.8 | N/A | N/A | N/A | N/A |
| D0.9 | 0.9 | N/A | N/A | N/A | N/A |
| D0.8-C0.5Na-SA28/56 | 0.8 | Na2SO4 | 0.5 | 28, 56 | 0 |
| D0.8-C5Na-SA28/56 | 0.8 | Na2SO4 | 5.0 | 28, 56 | 0 |
| D0.8-C4.24Mg-SA28/56 | 0.8 | MgSO4 | 4.24 | 28, 56 | 0 |
| D0.9-C5Na-SA28/56 | 0.9 | Na2SO4 | 5.0 | 28, 56 | 0 |
| D0.9-C4.24Mg-SA28/56 | 0.9 | MgSO4 | 5.0 | 28, 56 | 0 |
| D0.8-C0.5Na-DW5/10 | 0.8 | Na2SO4 | 0.5 | N/A | 5, 10 |
| D0.8-C5Na-DW5/10 | 0.8 | Na2SO4 | 5.0 | N/A | 5, 10 |
| D0.8-C4.24Mg-DW5/10 | 0.8 | MgSO4 | 4.24 | N/A | 5, 10 |
| D0.9-C5Na-DW5/10 | 0.9 | Na2SO4 | 5.0 | N/A | 5, 10 |
| D0.9-C4.24Mg-DW5/10 | 0.9 | MgSO4 | 4.24 | N/A | 5, 10 |
| Test No. | c/% | ρ/g/cm3 | CRC Prediction | |
|---|---|---|---|---|
| Equation (5) | Equation (6) | |||
| 1 | A(0.338) | A(0.6) | 0 | 0.068 |
| 2 | A(0.338) | B(1.1) | 0.963 | 0.592 |
| 3 | A(0.338) | C(1.6) | 0.999 | 0.826 |
| 4 | B(1.69) | A(0.6) | 0 | 0 |
| 5 | B(1.69) | B(1.1) | 0.827 | 0.073 |
| 6 | B(1.69) | C(1.6) | 0.995 | 0.385 |
| 7 | C(3.38) | A(0.6) | 0 | 0 |
| 8 | C(3.38) | B(1.1) | 0.685 | 0.050 |
| 9 | C(3.38) | C(1.6) | 0.990 | 0.148 |
| Rs | 0.096 | 0.995 | N/A | N/A |
| Rc | 0.429 | 0.430 | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, Y.; Ye, G.; Zhang, S.; Shen, H.; Chen, Y. Strength Degradation of Foamed Lightweight Soil Due to Chemical Erosion and Wet-Dry Cycle and Its Empirical Model. Materials 2023, 16, 6505. https://doi.org/10.3390/ma16196505
Zhang Z, Zhang Y, Ye G, Zhang S, Shen H, Chen Y. Strength Degradation of Foamed Lightweight Soil Due to Chemical Erosion and Wet-Dry Cycle and Its Empirical Model. Materials. 2023; 16(19):6505. https://doi.org/10.3390/ma16196505
Chicago/Turabian StyleZhang, Zhen, Yonggang Zhang, Guanbao Ye, Shenyi Zhang, Honghui Shen, and Yonggui Chen. 2023. "Strength Degradation of Foamed Lightweight Soil Due to Chemical Erosion and Wet-Dry Cycle and Its Empirical Model" Materials 16, no. 19: 6505. https://doi.org/10.3390/ma16196505
APA StyleZhang, Z., Zhang, Y., Ye, G., Zhang, S., Shen, H., & Chen, Y. (2023). Strength Degradation of Foamed Lightweight Soil Due to Chemical Erosion and Wet-Dry Cycle and Its Empirical Model. Materials, 16(19), 6505. https://doi.org/10.3390/ma16196505






