Fe3O4 Magnetic Nanoparticles Obtained by the Novel Aerosol-Based Technique for Theranostic Applications
Abstract
1. Introduction
2. Sample Preparation and Experimental Methods
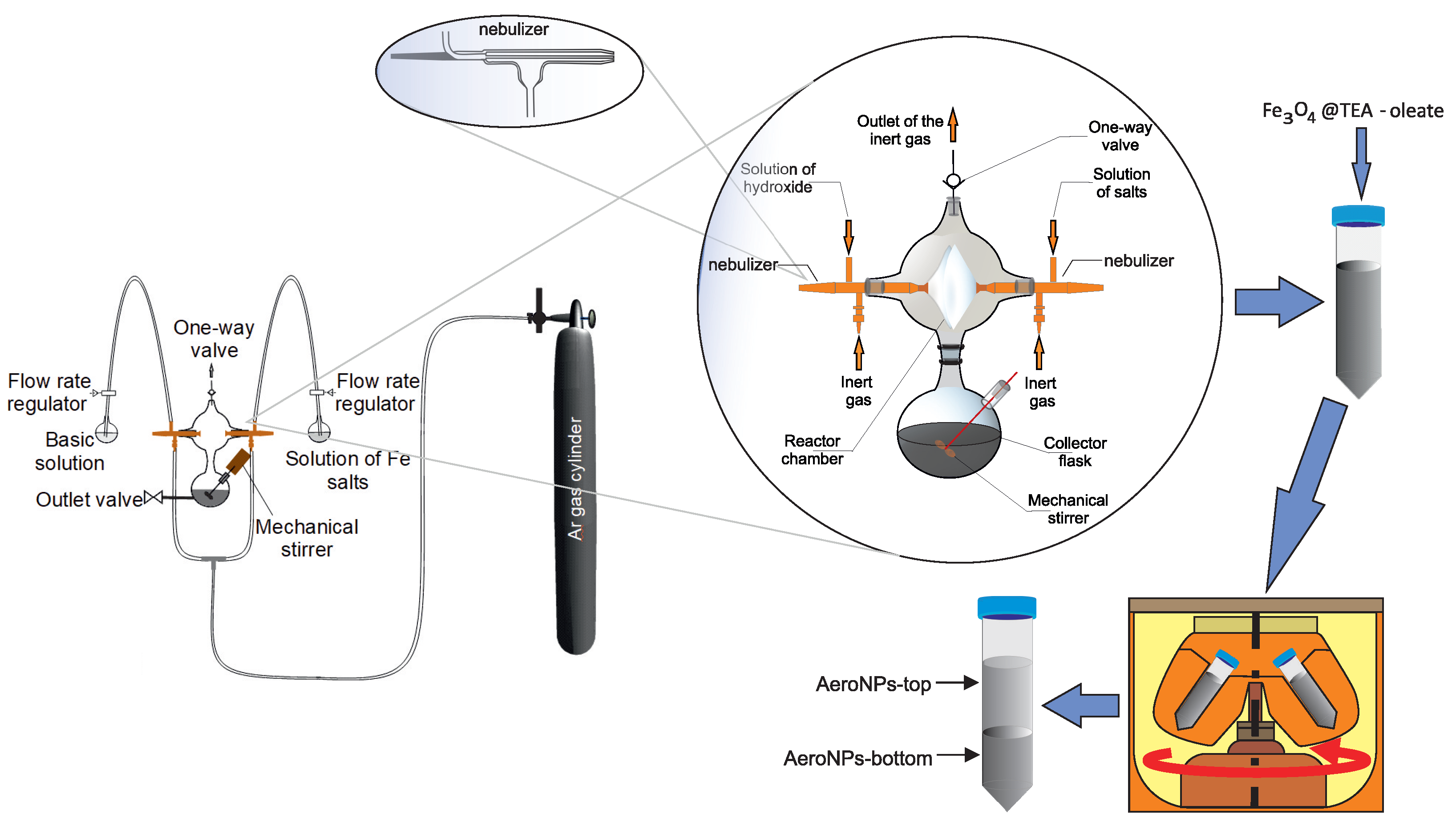
2.1. Novel Aerosol-Based Technique for the Synthesis of Iron-Oxide NPs
2.2. Stabilization of NPs with Triethanolammonium Oleate (TEA-Oleate)
2.3. Materials Characterization
2.4. Characterization of Interactions between NPs and Biological Material
2.4.1. Cell Lines and MTS Assay
2.4.2. Holotomographic Microscopy
2.5. Measurements of Relaxivity in MR Experiment
3. Results and Discussion
3.1. Tem Studies
3.2. X-ray Diffraction
3.3. The Effect of Magnetic Field on the Dispersion of NPs Stabilized by the TEA-Oleate/DLS Studies
3.4. Mössbauer Spectra Analysis
3.5. Room Temperature Magnetic Measurements
3.6. Mts Assay
3.7. Holotomographic Studies
3.8. Magnetic Heating Efficiency of AeroNPs Dispersion
3.9. MR Relaxation Measurements and MR Imaging for the 1AeroNPs and 2AeroNPs Specimens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Lee, J.W.; Byun, D.; Choi, W. Coaxial-nanostructured MnFe2O4 nanoparticles on polydopamine-coated MWCNT for anode materials in rechargeable batteries. Nanoscale 2018, 10, 18949–18960. [Google Scholar] [CrossRef] [PubMed]
- Gaire, M.; Khatoon, N.; Subedi, B.; Chrisey, D. Flexible iron oxide supercapacitor electrodes by photonic processing. J. Mater. Res. 2021, 36, 4536–4546. [Google Scholar] [CrossRef]
- Ichinose, N.; Ozaki, Y.; Kashu, S. Superfine Particle Technology; Springer: London, UK, 2012. [Google Scholar] [CrossRef]
- Mul, G.; Kapteijn, F.; Doornkamp, C.; Moulijn, J.A. Transition Metal oxide catalyzed carbon black oxidation: A study with 18O2. J. Catal. 1998, 179, 258–266. [Google Scholar] [CrossRef]
- Vinoy, K.J.; Jha, R.M. Radar Absorbing Materials: From Theory to Design and Characterization; Kluwer Academic Publishers Norwell: Boston, MA, USA, 1996. [Google Scholar] [CrossRef]
- Soares, P.I.; Laia, C.A.; Carvalho, A.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Iron oxide nanoparticles stabilized with a bilayer of oleic acid for magnetic hyperthermia and MRI applications. Appl. Surf. Sci. 2016, 383, 240–247. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Lozano Chamizo, L.; Luengo Morato, Y.; Ovejero Paredes, K.; Contreras Caceres, R.; Filice, M.; Marciello, M. Ionotropic Gelation–Based Synthesis of Chitosan–Metal Hybrid Nanoparticles Showing Combined Antimicrobial and Tissue Regenerative Activities. Polymers 2021, 13, 3910. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, P.; Błasiak, B.; Depciuch, J.; Pruba, M.; Kitala, D.; Vorobyova, S.; Stec, M.; Bushinsky, M.; Konakov, A.; Baran, J.; et al. Application of iron–based magnetic nanoparticles stabilized with triethanolammonium oleate for theranostics. J. Mater. Sci. 2022, 57, 4716–4737. [Google Scholar] [CrossRef]
- Unni, M.; Zhang, J.; George, T.J.; Segal, M.S.; Fan, Z.H.; Rinaldi, C. Engineering magnetic nanoparticles and their integration with microfluidics for cell isolation. J. Colloid Interface Sci. 2020, 564, 204–215. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef]
- Hewlin, R.L., Jr.; Tindall, J.M. Computational Assessment of Magnetic Nanoparticle Targeting Efficiency in a Simplified Circle of Willis Arterial Model. Int. J. Mol. Sci. 2023, 24, 2545. [Google Scholar] [CrossRef]
- Dash, A.; Błasiak, B.; Tomanek, B.; Banerjee, A.; Trudel, S.; Latta, P.; van Veggel, F.C. Colloidally Stable Monodisperse Fe Nanoparticles as T2 Contrast Agents High-Field Clin. Preclin. Magn. Reson. Imaging. ACS Appl. Nano Mater. 2021, 4, 1235–1242. [Google Scholar] [CrossRef]
- Montet, X.; Weissleder, R.; Josephson, L. Imaging pancreatic cancer with a peptide- nanoparticle conjugate targeted to normal pancreas. Bioconjugate Chem. 2006, 17, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, S.; Li, Y.; Zeng, L.; Lian, G.; Li, J.; Chen, S.; Huang, K.; Chen, Y. Nanoparticles modified by triple single chain antibodies for MRI examination and targeted therapy in pancreatic cancer. Nanoscale 2020, 12, 4473–4490. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Bardeesy, N.; Anbazhagan, R.; Gurumurthy, S.; Berger, J.; Alencar, H.; DePinho, R.A.; Mahmood, U.; Weissleder, R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008, 5, e85. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, M.; Shahbazi-Gahrouei, D.; Mehrgardi, M.A.; Aghaei, M.; Khoshfetrat, S.M. Synthesis and cytotoxicity assessment of gold-coated magnetic iron oxide nanoparticles. J. Biomed. Phys. Eng. 2018, 8, 357. [Google Scholar]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Keshtkar, M.; Shahbazi-Gahrouei, D.; Khoshfetrat, S.M.; Mehrgardi, M.A.; Aghaei, M. Aptamer-conjugated magnetic nanoparticles as targeted magnetic resonance imaging contrast agent for breast cancer. J. Med. Signals Sensors 2016, 6, 243. [Google Scholar]
- Tomanek, B.; Iqbal, U.; Blasiak, B.; Abulrob, A.; Albaghdadi, H.; Matyas, J.R.; Ponjevic, D.; Sutherland, G.R. Evaluation of brain tumor vessels specific contrast agents for glioblastoma imaging. Neuro-Oncology 2012, 14, 53–63. [Google Scholar] [CrossRef][Green Version]
- Adiseshaiah, P.P.; Crist, R.M.; Hook, S.S.; McNeil, S.E. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat. Rev. Clin. Oncol. 2016, 13, 750–765. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem. Soc. Rev. 2015, 44, 6733–6748. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron oxide nanoparticles: An alternative for positive contrast in magnetic resonance imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic nanoparticles as MRI contrast agents. In Surface-Modified Nanobiomaterials for Electrochemical and Biomedicine Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 49–91. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Fung, K.B.; Zhou, X.Y.; Cui, W.; Colson, C.; Mai, D.; Jeffris, K.; Huynh, Q.; Saayujya, C.; Kabuli, L.; et al. Non-radioactive and sensitive tracking of neutrophils towards inflammation using antibody functionalized magnetic particle imaging tracers. Nanotheranostics 2021, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Kaiser, W.A. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine 2012, 7, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Rapp, A.; Greulich, K.O.; Kaiser, W.A. Assessment of DNA damage in target tumor cells after thermoablation in mice. Radiology 2005, 237, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperth. 2003, 19, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. 2002, 43, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Schildkopf, P.; Ott, O.J.; Frey, B.; Wadepohl, M.; Sauer, R.; Fietkau, R.; S Gaipl, U. Biological rationales and clinical applications of temperature controlled hyperthermia-implications for multimodal cancer treatments. Curr. Med. Chem. 2010, 17, 3045–3057. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; Von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neuro-Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldöfner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef]
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite nanoparticles: Synthesis and applications in optics and nanophotonics. Materials 2022, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.M.; Lachaize, S.; Falqui, A.; Respaud, M.; Chaudret, B. Iron nanoparticle growth in organic superstructures. J. Am. Chem. Soc. 2009, 131, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Izydorzak-Wozniak, M.; Leonowicz, M. Carbon matrix based magnetic nanocomposites for potential biomedical applications. J. Nanosci. Nanotechnol. 2014, 14, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Meiser, F.; Möhwald, H. Nanoengineering of iron oxide and iron oxide/silica hollow spheres by sequential layering combined with a sol–gel process. J. Colloid Interface Sci. 2005, 288, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Pruba, M.; Smolkova, I.; Pawlik, K.; Zubko, M.; Pawlik, P. Phase structure and heating efficiency of as-synthesised iron–oxide nanoparticles and after long time storage in glycerol. Acta Phys. Pol. A 2019, 135, 229–231. [Google Scholar] [CrossRef]
- Pawlik, P.; Pruba, M.; Pawlik, K.; Kotynia, K. Phase Structure and Heat Generation in the Co–Precipitated Magnetite Nanoparticles. Acta Phys. Pol. A 2017, 131, 1217–1221. [Google Scholar] [CrossRef]
- Boistelle, R.; Astier, J. Crystallization mechanisms in solution. J. Cryst. Growth 1988, 90, 14–30. [Google Scholar] [CrossRef]
- Sugimoto, T. Formation of Monodispersed Nano– and Micro–Particles Controlled in Size, Shape, and Internal Structure. Chem. Eng. Technol. 2003, 26, 313–321. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; Gonzalez-Carreño, T.; Serna, C.J. Synthesis, properties and biomedical applications of magnetic nanoparticles. Handb. Magn. Mater. 2006, 16, 403–482. [Google Scholar] [CrossRef]
- Smolkova, I.S.; Kazantseva, N.E.; Babayan, V.; Vilcakova, J.; Pizurova, N.; Saha, P. The role of diffusion–controlled growth in the formation of uniform iron oxide nanoparticles with a link to magnetic hyperthermia. Cryst. Growth Des. 2017, 17, 2323–2332. [Google Scholar] [CrossRef]
- Smolkova, I.S.; Kazantseva, N.E.; Babayan, V.; Smolka, P.; Parmar, H.; Vilcakova, J.; Schneeweiss, O.; Pizurova, N. Alternating magnetic field energy absorption in the dispersion of iron oxide nanoparticles in a viscous medium. J. Magn. Magn. Mater. 2015, 374, 508–515. [Google Scholar] [CrossRef]
- Bala, T.; Sankar, C.R.; Baidakova, M.; Osipov, V.; Enoki, T.; Joy, P.; Prasad, B.; Sastry, M. Cobalt and magnesium ferrite nanoparticles: Preparation using liquid foams as templates and their magnetic characteristics. Langmuir 2005, 21, 10638–10643. [Google Scholar] [CrossRef] [PubMed]
- Abiev, R.; Almyasheva, O.; Izotova, S.; Gusarov, V. Synthesis of cobalt ferrite nanoparticles by means of confined impinging—Jets reactors. J. Chem. Technol. Appl. 2017, 1, 7–13. [Google Scholar] [CrossRef]
- Abiev, R.; Almyasheva, O.; Gusarov, V.; Izotova, S. Method of Producing Nanopowder of Cobalt Ferrite and Microreactor to this End. RF Patent 2625981, 29 September 2017. [Google Scholar]
- Lin, M.T. Apparatus and Method for Making Nanoparticles. US Patent US20060292056A1; App. 11/442,409, 26 June 2006. [Google Scholar]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Cruz, M.M.; Thanh, N.T.K.; et al. Co–precipitation synthesis of stable iron oxide nanoparticles with NaOH: New insights and continuous production via flow chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Pawlik, P.; Pawlik, K.; Maximenko, A. Glass Reactor and Method of Obtaining Homogeneous Iron Oxide Nanoparticles Based on Aerosol. reg. no. P.438156. 2021.
- Dash, A.; Błasiak, B.; Tomanek, B.; van Veggel, F.C. Validation of inner, second, and outer sphere contributions to T1T2 Relax. Gd3+- Nanoparticles Using Eu3+ Lifetime Decay A Probe. J. Phys. Chem. C 2018, 122, 11557–11569. [Google Scholar] [CrossRef]
- Soares, P.I.; Alves, A.M.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Effects of surfactants on the magnetic properties of iron oxide colloids. J. Colloid Interface Sci. 2014, 419, 46–51. [Google Scholar] [CrossRef]
- Savliwala, S.; Liu, S.; Rinaldi-Ramos, C.M. Particle motion artifacts in equilibrium magnetization measurements of large iron oxide nanoparticles. J. Magn. Magn. Mater. 2022, 547, 168889. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Sun, Q.; Chen, Q. One–step hydrothermal process to prepare highly crystalline Fe3O4 nanoparticles with improved magnetic properties. Mater. Res. Bull. 2003, 38, 1113–1118. [Google Scholar] [CrossRef]
- Lemine, O.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol–gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Wang, Y.X.J.; Leung, K.C.F.; Lee, S.F.; Zhao, F.; Wang, D.W.; Lai, J.M.; Wan, C.; Cheng, C.H.; Ahuja, A.T. Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Gu, N. In vitro biological effects of magnetic nanoparticles. Chin. Sci. Bull. 2012, 57, 3972–3978. [Google Scholar] [CrossRef][Green Version]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the intersections between metabolism and cancer biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Wildeboer, R.; Southern, P.; Pankhurst, Q. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]

















| Sample Type | FeCl · 6HO (g) | FeSO · 7HO (g) | NHOH (25%) (g) | Feeding Rate of Salts (mL/s) | Feeding Rate of Ammonia (mL/s) |
|---|---|---|---|---|---|
| 1AeroNPs | 0.459 | 0.24 | 11 | 0.035 | 0.017 |
| 2AeroNPs | 0.918 | 0.48 | 18 | 0.035 | 0.017 |
| Sample | a (Å) | Space Group | (nm) | GOF | ||
|---|---|---|---|---|---|---|
| Starting values | 8.397 | |||||
| 1AeroNPs | 8.382 | cubic | 10.4 | 0.422 | 0.497 | 1.177 |
| 2AeroNPs-bottom | 8.373 | Fdm | 8.4 | 0.781 | 0.804 | 1.030 |
| 2AeroNPs-top | 8.377 | 6.8 | 0.954 | 0.991 | 1.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, P.; Błasiak, B.; Pruba, M.; Miaskowski, A.; Moraczyński, O.; Miszczyk, J.; Tomanek, B.; Depciuch, J. Fe3O4 Magnetic Nanoparticles Obtained by the Novel Aerosol-Based Technique for Theranostic Applications. Materials 2023, 16, 6483. https://doi.org/10.3390/ma16196483
Pawlik P, Błasiak B, Pruba M, Miaskowski A, Moraczyński O, Miszczyk J, Tomanek B, Depciuch J. Fe3O4 Magnetic Nanoparticles Obtained by the Novel Aerosol-Based Technique for Theranostic Applications. Materials. 2023; 16(19):6483. https://doi.org/10.3390/ma16196483
Chicago/Turabian StylePawlik, Piotr, Barbara Błasiak, Marcin Pruba, Arkadiusz Miaskowski, Oskar Moraczyński, Justyna Miszczyk, Boguslaw Tomanek, and Joanna Depciuch. 2023. "Fe3O4 Magnetic Nanoparticles Obtained by the Novel Aerosol-Based Technique for Theranostic Applications" Materials 16, no. 19: 6483. https://doi.org/10.3390/ma16196483
APA StylePawlik, P., Błasiak, B., Pruba, M., Miaskowski, A., Moraczyński, O., Miszczyk, J., Tomanek, B., & Depciuch, J. (2023). Fe3O4 Magnetic Nanoparticles Obtained by the Novel Aerosol-Based Technique for Theranostic Applications. Materials, 16(19), 6483. https://doi.org/10.3390/ma16196483







