Intermolecular Interactions as a Measure of Dapsone Solubility in Neat Solvents and Binary Solvent Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Measurements
2.3. Solubility Dataset Curation
2.4. Computations Protocol
2.4.1. COSMO-RS Solubility Computations
2.4.2. Affinity Characteristic of Solute–Solvent Systems
2.4.3. Machine Learning Protocol
3. Results and Discussion
3.1. New Data of Dapsone Solubility
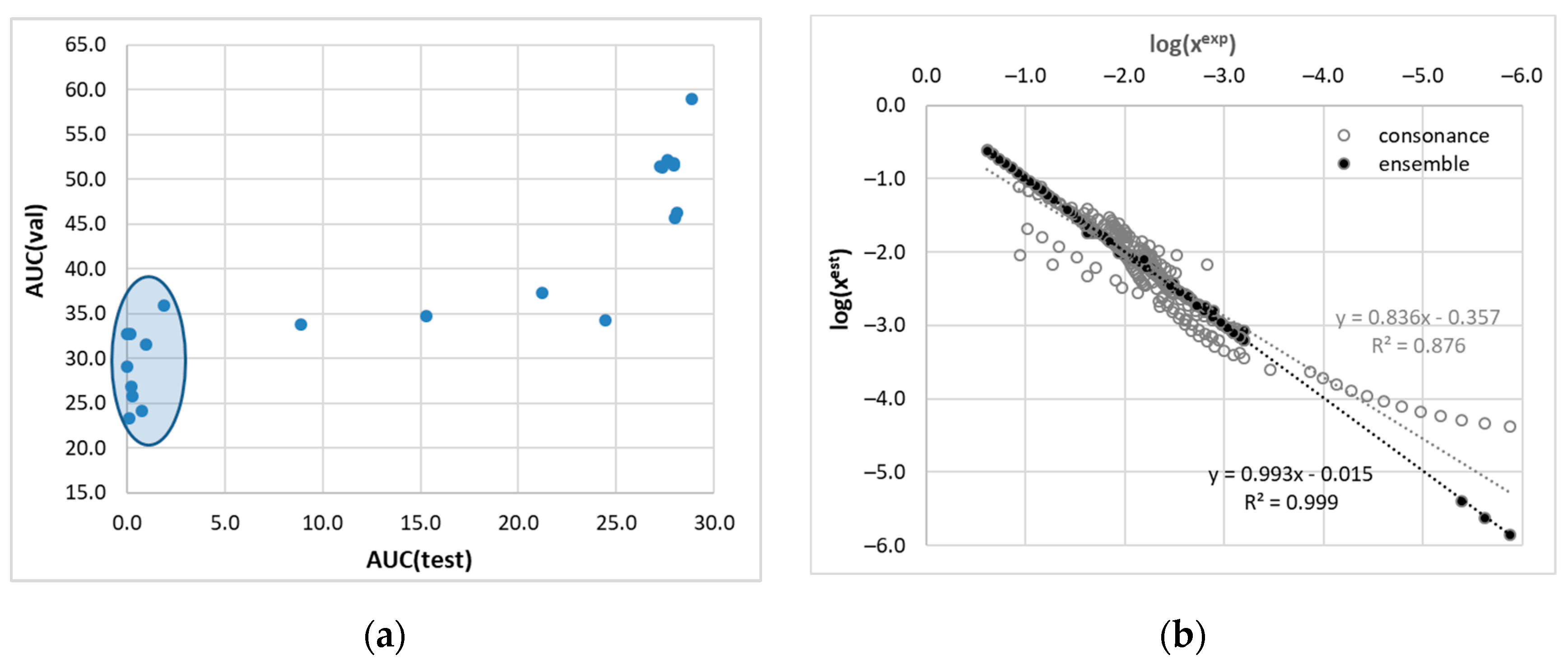
3.2. Solubility Prediction Using Consonance Solvents
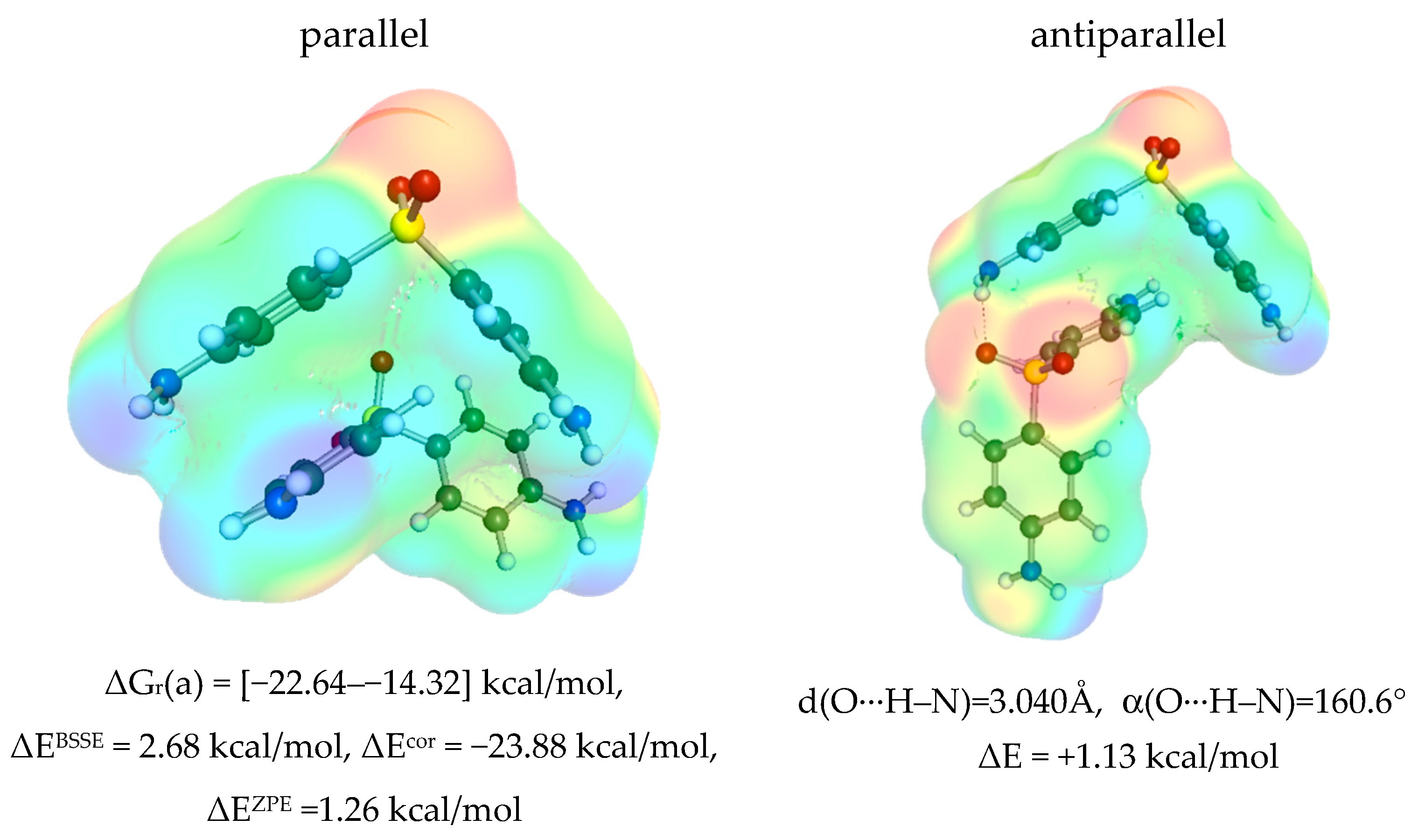
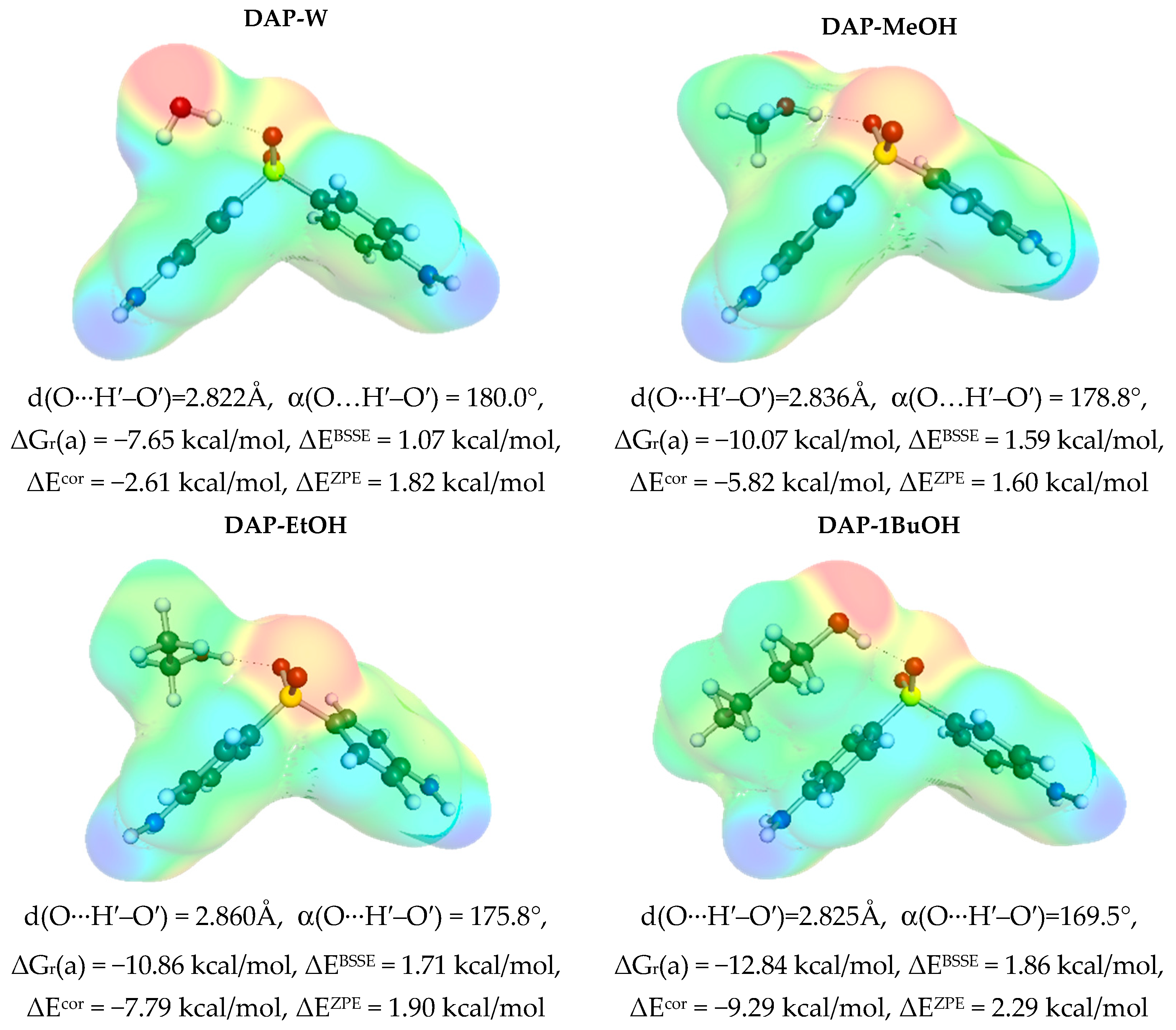
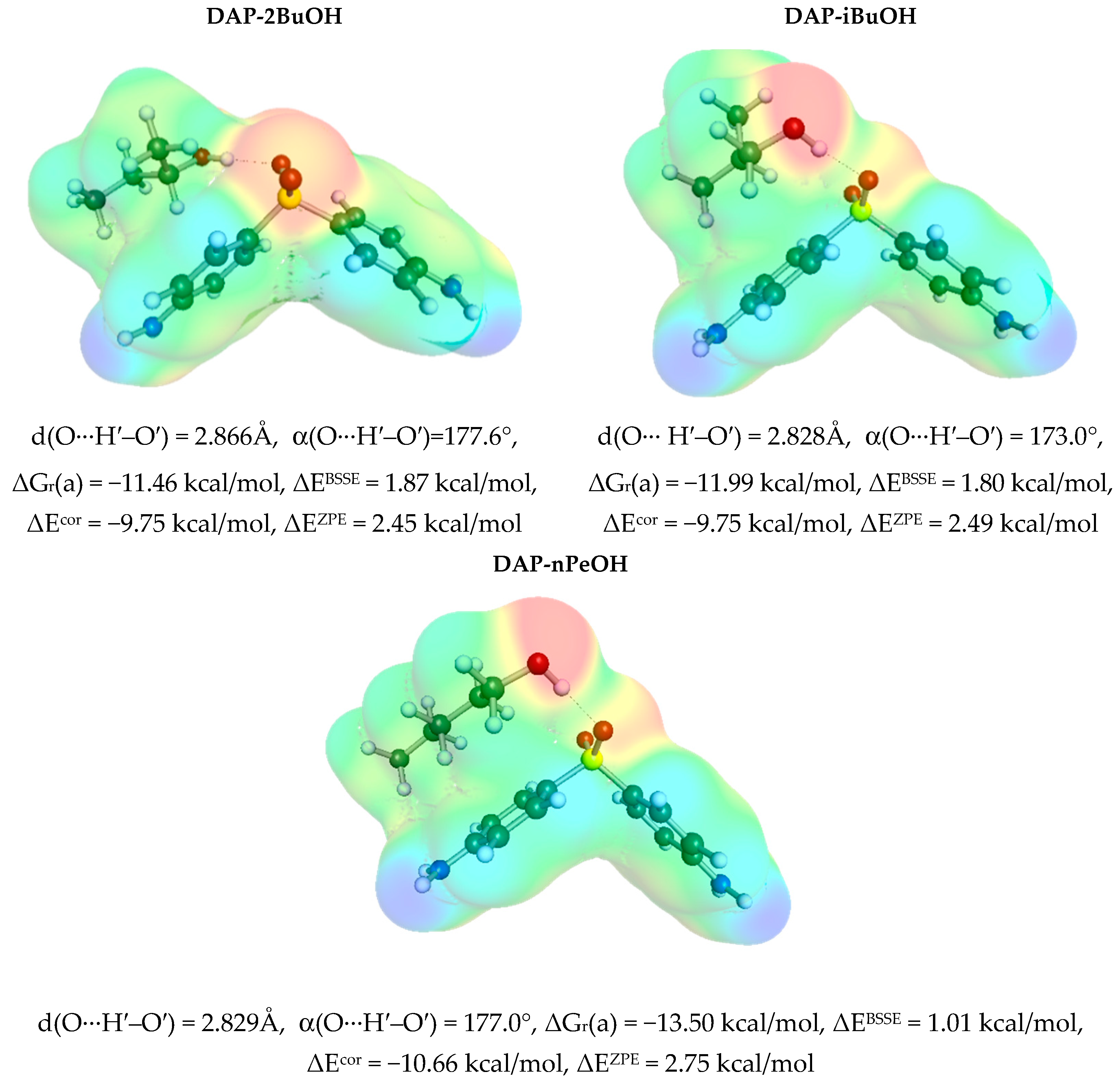
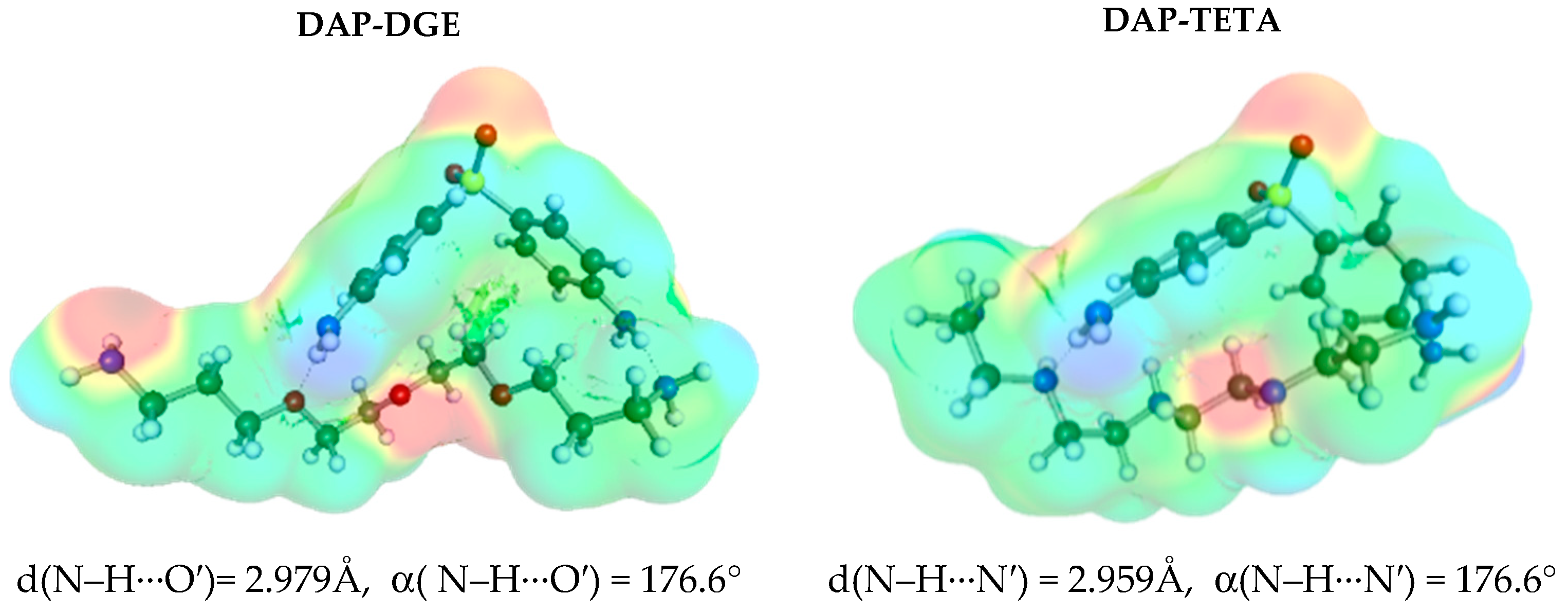
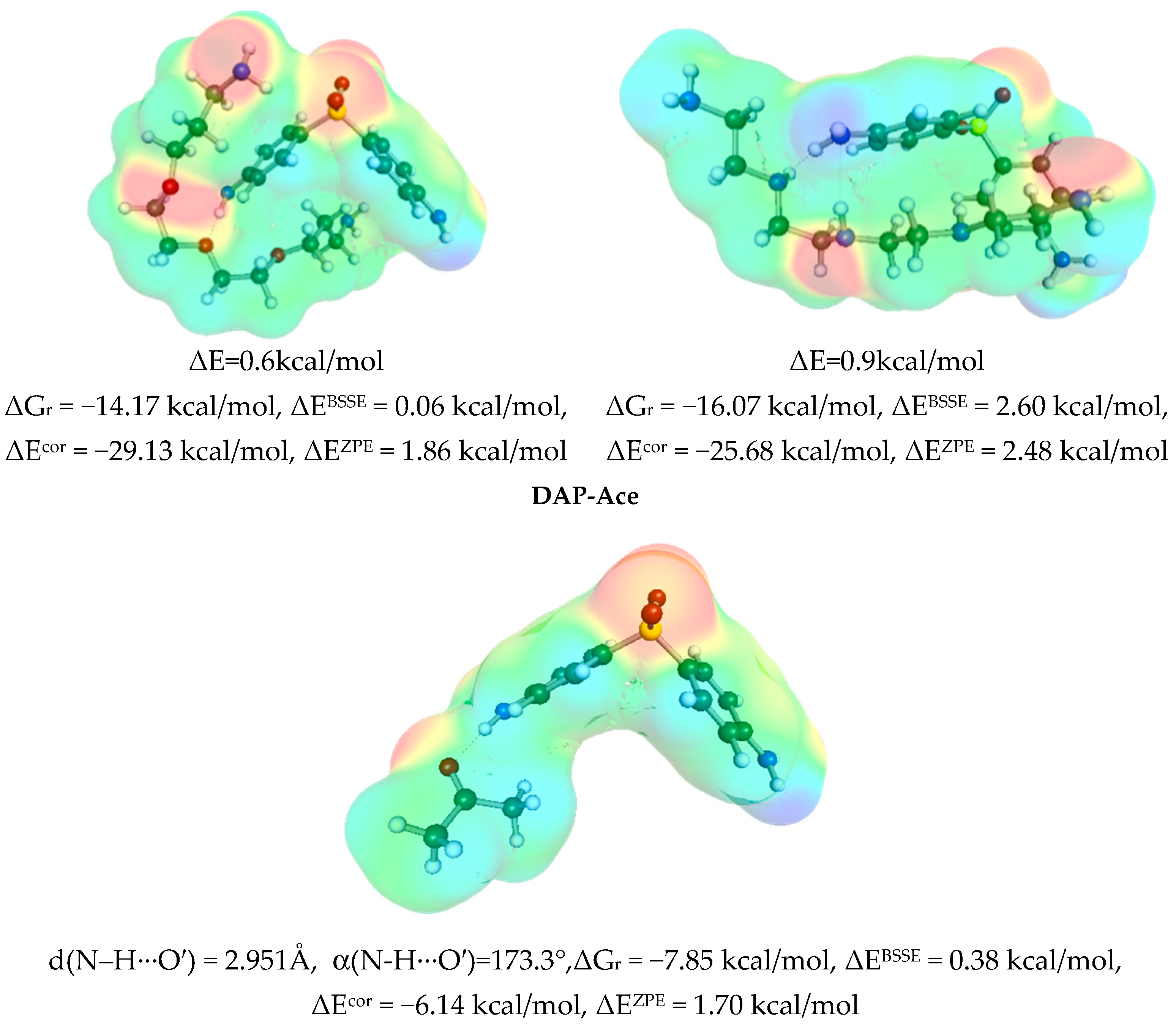
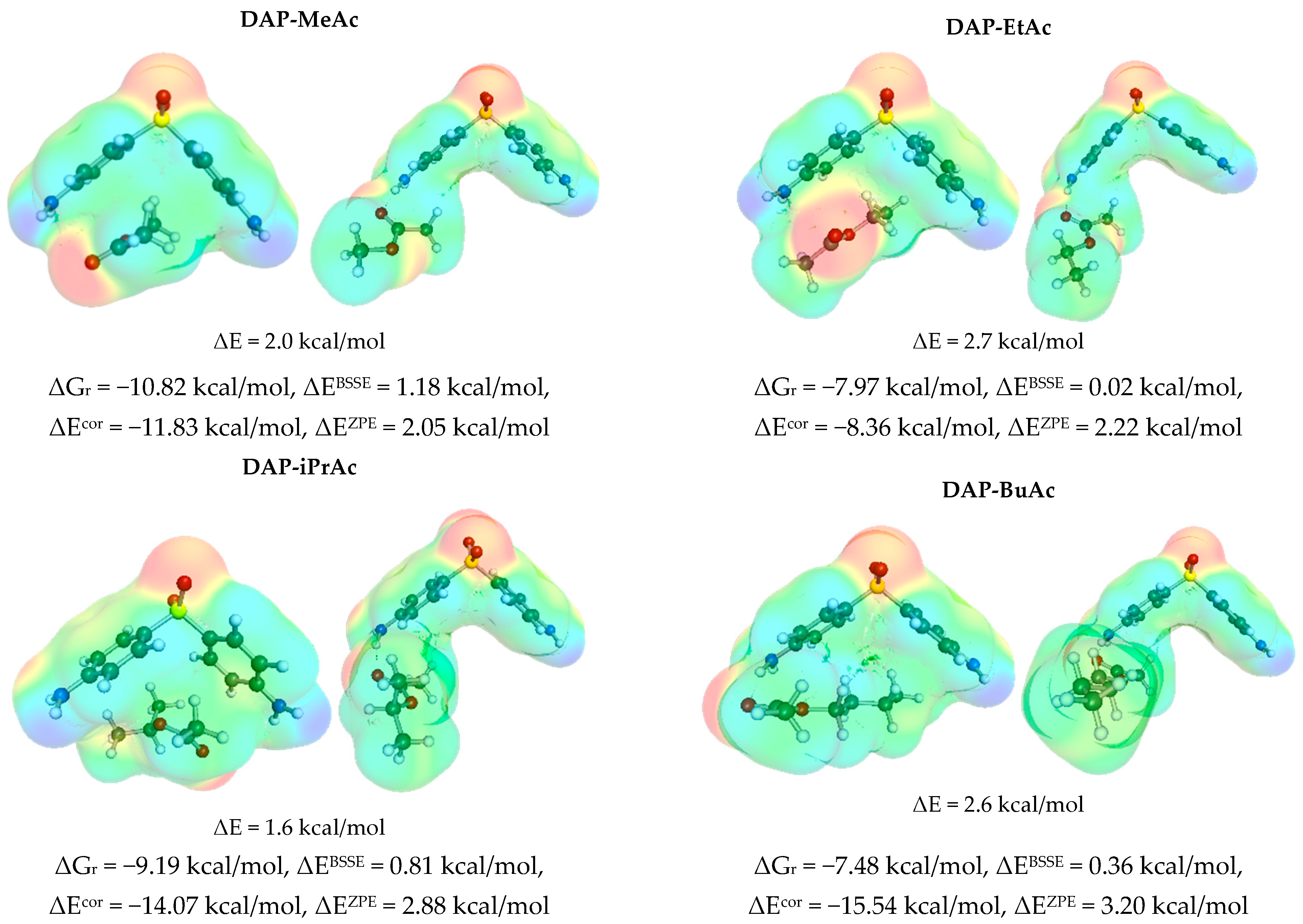
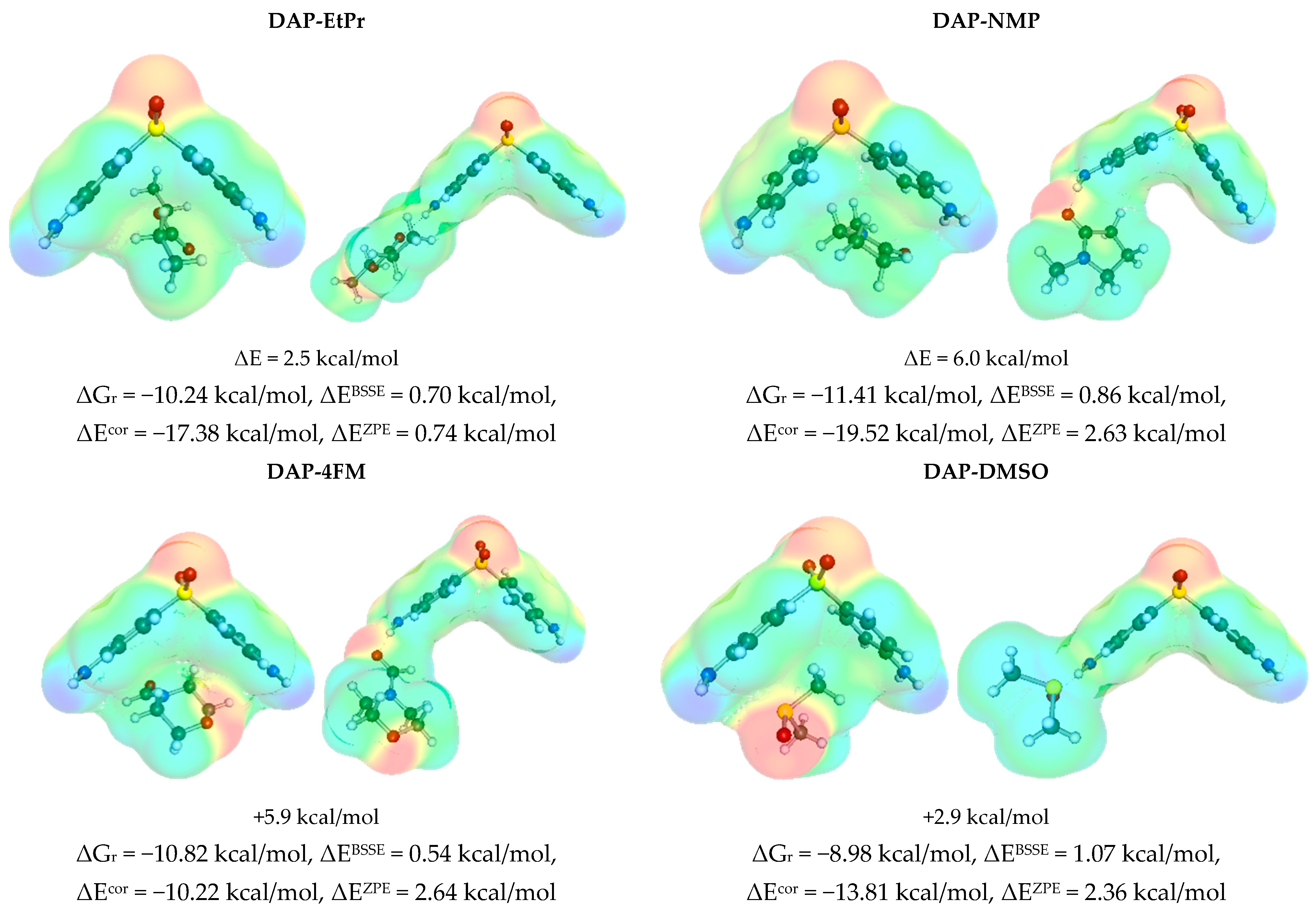
3.3. Solute–Solvent Intermolecular Interactions
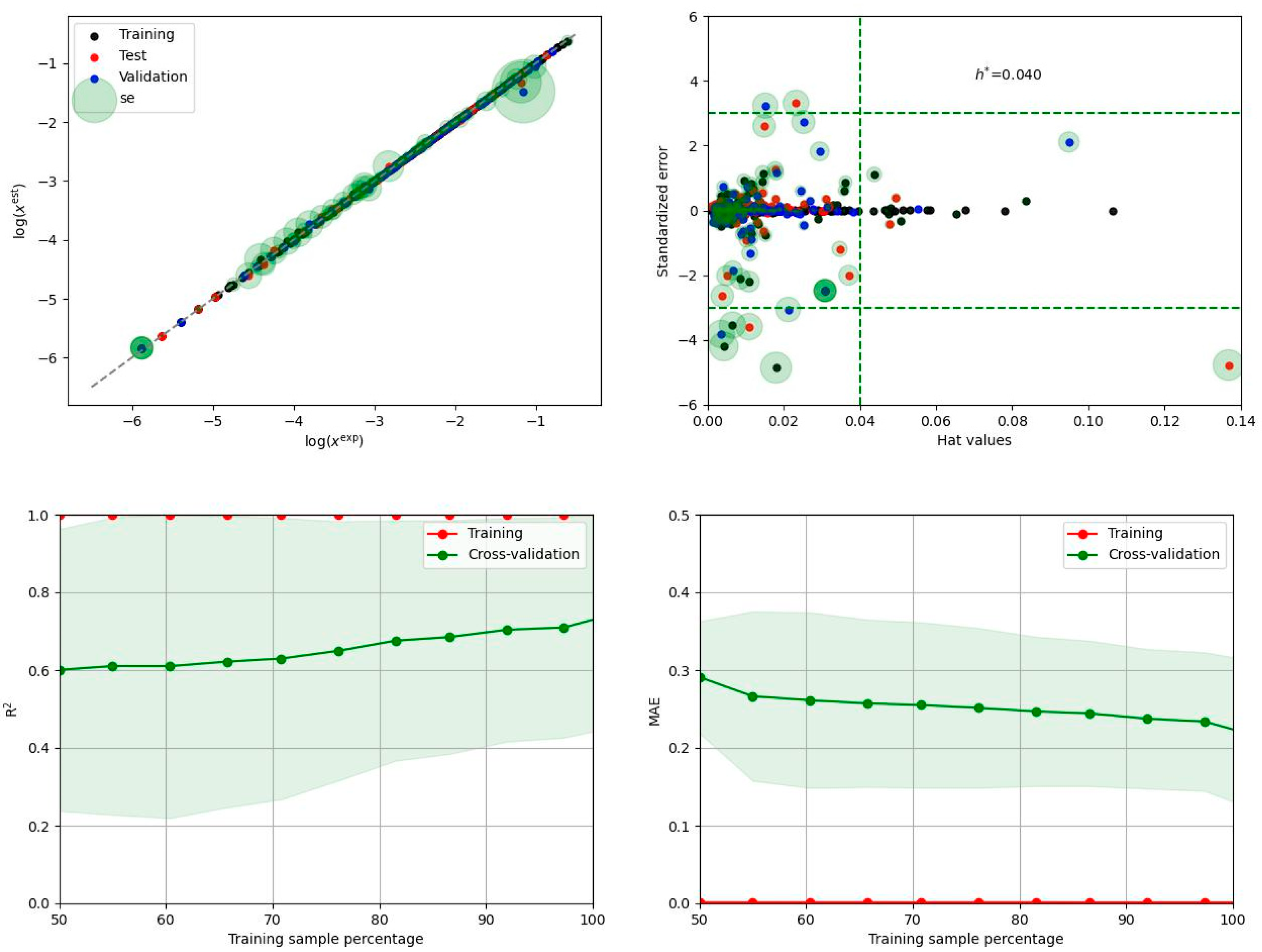
3.4. Ensemble Model for Solubility Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madanipour, M.R.; Fatehi-zardalou, M.; Rahimi, N.; Hemmati, S.; Alaeddini, M.; Etemad-Moghadam, S.; Shayan, M.; Dabiri, S.; Dehpour, A.R. The anti-inflammatory effect of dapsone on ovalbumin-induced allergic rhinitis in balb/c mice. Life Sci. 2022, 297, 120449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.I.; Stiller, M.J. Dapsone and sulfones in dermatology: Overview and update. J. Am. Acad. Dermatol. 2001, 45, 420–434. [Google Scholar] [CrossRef] [PubMed]
- May, S.M.; Motosue, M.S.; Park, M.A. Dapsone is often tolerated in HIV-infected patients with history of sulfonamide antibiotic intolerance. J. Allergy Clin. Immunol. Pract. 2017, 5, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103. [Google Scholar] [CrossRef]
- Moreno, E.; Calvo, A.; Schwartz, J.; Navarro-Blasco, I.; González-Peñas, E.; Sanmartín, C.; Irache, J.; Espuelas, S. Evaluation of Skin Permeation and Retention of Topical Dapsone in Murine Cutaneous Leishmaniasis Lesions. Pharmaceutics 2019, 11, 607. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Dehury, B.; Sahoo, J.; Vedithi, S.C.; Mahapatra, N.; Hussain, T.; Padhy, R.N. Molecular docking and simulation study for synthesis of alternative dapsone derivative as a newer antileprosy drug in multidrug therapy. J. Cell. Biochem. 2018, 119, 9838–9852. [Google Scholar] [CrossRef]
- Roman, C.; Dima, B.; Muyshont, L.; Schurmans, T.; Gilliaux, O. Indications and efficiency of dapsone in IgA vasculitis (Henoch-Schonlein purpura): Case series and a review of the literature. Eur. J. Pediatr. 2019, 178, 1275–1281. [Google Scholar] [CrossRef]
- Ghaoui, N.; Hanna, E.; Abbas, O.; Kibbi, A.G.; Kurban, M. Update on the use of dapsone in dermatology. Int. J. Dermatol. 2020, 59, 787–795. [Google Scholar] [CrossRef]
- Ríos, C.; Orozco-Suarez, S.; Salgado-Ceballos, H.; Mendez-Armenta, M.; Nava-Ruiz, C.; Santander, I.; Barón-Flores, V.; Caram-Salas, N.; Diaz-Ruiz, A. Anti-Apoptotic Effects of Dapsone After Spinal Cord Injury in Rats. Neurochem. Res. 2015, 40, 1243–1251. [Google Scholar] [CrossRef]
- Tingle, M.; Mahmud, R.; Maggs, J.; Pirmohamed, M.; Park, B. Comparison of the metabolism and toxicity of dapsone in rat, mouse and man. J. Pharmacol. Exp. Ther. 1997, 283, 817–823. [Google Scholar]
- Mitra, A.K.; Thummel, K.E.; Kalhorn, T.F.; Kharasch, E.D.; Unadkat, J.D.; Slattery, J.T. Metabolism of dapsone to its hydroxylamine by CYP2E1 in vitro and in vivo. Clin. Pharmacol. Ther. 1995, 58, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Paolinelli, M.; Campanati, A.; Brisigotti, V.; Offidani, A. Metabolic, pharmacokinetic, and toxicological issues surrounding dapsone. Expert Opin. Drug Metab. Toxicol. 2019, 15, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Rahimpour, E.; Karimzadeh, Z.; Zhao, H. Simulation of dapsone solubility data in mono- and mixed-solvents at various temperatures. J. Mol. Liq. 2022, 345, 118223. [Google Scholar] [CrossRef]
- Schneider-Rauber, G.; Argenta, D.F.; Caon, T. Emerging Technologies to Target Drug Delivery to the Skin—The Role of Crystals and Carrier-Based Systems in the Case Study of Dapsone. Pharm. Res. 2020, 37, 240. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, X.; Li, J.; Guan, A.; Zhou, Z.; Guo, F. New insight into improving the solubility of poorly soluble drugs by preventing the formation of their hydrogen-bonds: A case of dapsone salts with camphorsulfonic and 5-sulfosalicylic acid. CrystEngComm 2021, 23, 6191–6198. [Google Scholar] [CrossRef]
- Paredes da Rocha, N.; de Souza, A.; Nishitani Yukuyama, M.; Lopes Barreto, T.; Macedo, L.D.O.; Löbenberg, R.; Lima Barros de Araújo, G.; Ishida, K.; Araci Bou-Chacra, N. Highly water-soluble dapsone nanocrystals: Towards innovative preparations for an undermined drug. Int. J. Pharm. 2023, 630, 122428. [Google Scholar] [CrossRef]
- Trombino, S.; Siciliano, C.; Procopio, D.; Curcio, F.; Laganà, A.S.; Di Gioia, M.L.; Cassano, R. Deep Eutectic Solvents for Improving the Solubilization and Delivery of Dapsone. Pharmaceutics 2022, 14, 333. [Google Scholar] [CrossRef]
- Martínez, F.; Jouyban, A.; Acree, W.E. Pharmaceuticals solubility is still nowadays widely studied everywhere. Pharm. Sci. 2017, 23, 1–2. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Yaseen, G.; Ahmad, M.; Zafar, M.; Akram, A.; Sultana, S.; Kilic, O.; Sonmez, G.D. Current status of solvents used in the pharmaceutical industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 195–219. [Google Scholar]
- Parmentier, M.; Gabriel, C.M.; Guo, P.; Isley, N.A.; Zhou, J.; Gallou, F. Switching from organic solvents to water at an industrial scale. Curr. Opin. Green Sustain. Chem. 2017, 7, 13–17. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Lovette, M.A. Solubility Model to Guide Solvent Selection in Synthetic Process Development. Cryst. Growth Des. 2022, 22, 4404–4420. [Google Scholar] [CrossRef]
- Modarresi, H.; Conte, E.; Abildskov, J.; Gani, R.; Crafts, P. Model-Based Calculation of Solid Solubility for Solvent Selection—A Review. Ind. Eng. Chem. Res. 2008, 47, 5234–5242. [Google Scholar] [CrossRef]
- Moodley, K.; Rarey, J.; Ramjugernath, D. Model evaluation for the prediction of solubility of active pharmaceutical ingredients (APIs) to guide solid–liquid separator design. Asian J. Pharm. Sci. 2018, 13, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Eckert, F. Fast Solvent Screening via Quantum Chemistry: COSMO-RS Approach. AIChE J. 2002, 48, 369–385. [Google Scholar]
- Klamt, A. Solvent-screening and co-crystal screening for drug development with COSMO-RS. J. Cheminform. 2012, 4, O14. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 799–805. [Google Scholar] [CrossRef]
- Palmelund, H.; Andersson, M.P.; Asgreen, C.J.; Boyd, B.J.; Rantanen, J.; Löbmann, K. Tailor-made solvents for pharmaceutical use? Experimental and computational approach for determining solubility in deep eutectic solvents (DES). Int. J. Pharm. X 2019, 1, 100034. [Google Scholar] [CrossRef]
- Klajmon, M. Purely Predicting the Pharmaceutical Solubility: What to Expect from PC-SAFT and COSMO-RS? Mol. Pharm. 2022, 19, 4212–4232. [Google Scholar] [CrossRef]
- Klamt, A.; Eckert, F.; Hornig, M.; Beck, M.E.; Bürger, T. Prediction of aqueous solubility of drugs and pesticides with COSMO-RS. J. Comput. Chem. 2002, 23, 275–281. [Google Scholar] [CrossRef]
- Przybyłek, M.; Miernicka, A.; Nowak, M.; Cysewski, P. New Screening Protocol for Effective Green Solvents Selection of Benzamide, Salicylamide and Ethenzamide. Molecules 2022, 27, 3323. [Google Scholar] [CrossRef]
- Loschen, C.; Klamt, A. Prediction of Solubilities and Partition Coefficients in Polymers Using COSMO-RS. Ind. Eng. Chem. Res. 2014, 53, 11478–11487. [Google Scholar] [CrossRef]
- Buggert, M.; Cadena, C.; Mokrushina, L.; Smirnova, I.; Maginn, E.J.; Arlt, W. COSMO-RS Calculations of Partition Coefficients: Different Tools for Conformation Search. Chem. Eng. Technol. 2009, 32, 977–986. [Google Scholar] [CrossRef]
- Roy, D.; Patel, C. Revisiting the Use of Quantum Chemical Calculations in LogPoctanol-water Prediction. Molecules 2023, 28, 801. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Accurate prediction of basicity in aqueous solution with COSMO-RS. J. Comput. Chem. 2006, 27, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Radović, M.; Cvjetko Bubalo, M.; Radošević, K.; Rogošić, M.; Coutinho, J.A.P.; Radojčić Redovniković, I.; Jurinjak Tušek, A. Prediction of pH Value of Aqueous Acidic and Basic Deep Eutectic Solvent Using COSMO-RS σ Profiles’ Molecular Descriptors. Molecules 2022, 27, 4489. [Google Scholar] [CrossRef]
- Andersson, M.P.; Jensen, J.H.; Stipp, S.L.S. Predicting pK a for proteins using COSMO-RS. PeerJ 2013, 1, e198. [Google Scholar] [CrossRef]
- Guidetti, M.; Hilfiker, R.; Kuentz, M.; Bauer-Brandl, A.; Blatter, F. Exploring the Cocrystal Landscape of Posaconazole by Combining High-Throughput Screening Experimentation with Computational Chemistry. Cryst. Growth Des. 2023, 23, 842–852. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, S.; Jiang, Y.; Martins, I.C.B.; Rades, T. Recent Advances in Co-Former Screening and Formation Prediction of Multicomponent Solid Forms of Low Molecular Weight Drugs. Pharmaceutics 2023, 15, 2174. [Google Scholar] [CrossRef]
- Przybyłek, M.; Ziółkowska, D.; Mroczyńska, K.; Cysewski, P. Applicability of Phenolic Acids as Effective Enhancers of Cocrystal Solubility of Methylxanthines. Cryst. Growth Des. 2017, 17, 2186–2193. [Google Scholar] [CrossRef]
- Li, C.; Wu, D.; Li, J.; Ji, X.; Qi, L.; Sun, Q.; Wang, A.; Xie, C.; Gong, J.; Chen, W. Multicomponent crystals of clotrimazole: A combined theoretical and experimental study. CrystEngComm 2021, 23, 6977–6993. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Xiao, Y.; Li, C.; Ji, X.; Sun, Q.; Chang, D.; Zhou, L.; Jing, D.; Gong, J.; et al. Salts of 2-hydroxybenzylamine with improvements on solubility and stability: Virtual and experimental screening. Eur. J. Pharm. Sci. 2022, 169, 106091. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.; Gyak, K.-W.; Kim, S.D.; Kim, M.-J.; Min, K. Novel Solubility Prediction Models: Molecular Fingerprints and Physicochemical Features vs Graph Convolutional Neural Networks. ACS Omega 2022, 7, 12268–12277. [Google Scholar] [CrossRef] [PubMed]
- Panapitiya, G.; Girard, M.; Hollas, A.; Sepulveda, J.; Murugesan, V.; Wang, W.; Saldanha, E. Evaluation of Deep Learning Architectures for Aqueous Solubility Prediction. ACS Omega 2022, 7, 15695–15710. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, F.H.; Chung, Y.; Green, W.H. Predicting Solubility Limits of Organic Solutes for a Wide Range of Solvents and Temperatures. J. Am. Chem. Soc. 2022, 144, 10785–10797. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Przybyłek, M. Finding the Right Solvent: A Novel Screening Protocol for Identifying Environmentally Friendly and Cost-Effective Options for Benzenesulfonamide. Molecules 2023, 28, 5008. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Nowak, W.; Olczak, M. Solubility Characteristics of Acetaminophen and Phenacetin in Binary Mixtures of Aqueous Organic Solvents: Experimental and Deep Machine Learning Screening of Green Dissolution Media. Pharmaceutics 2022, 14, 2828. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M.; Rozalski, R. Experimental and Theoretical Screening for Green Solvents Improving Sulfamethizole Solubility. Materials 2021, 14, 5915. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Cymerman, P.; Przybyłek, M. Solvent Screening for Solubility Enhancement of Theophylline in Neat, Binary and Ternary NADES Solvents: New Measurements and Ensemble Machine Learning. Int. J. Mol. Sci. 2021, 22, 7347. [Google Scholar] [CrossRef]
- Jeliński, T.; Bugalska, N.; Koszucka, K.; Przybyłek, M.; Cysewski, P. Solubility of sulfanilamide in binary solvents containing water: Measurements and prediction using Buchowski-Ksiazczak solubility model. J. Mol. Liq. 2020, 319, 114342. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M.; Kowalska, A.; Tymorek, N. Thermodynamics and intermolecular interactions of nicotinamide in neat and binary solutions: Experimental measurements and COSMO-RS concentration dependent reactions investigations. Int. J. Mol. Sci. 2021, 22, 7365. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, M.; Kowalska, A.; Tymorek, N.; Dziaman, T.; Cysewski, P. Thermodynamic Characteristics of Phenacetin in Solid State and Saturated Solutions in Several Neat and Binary Solvents. Molecules 2021, 26, 4078. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Jeliński, T.; Przybyłek, M. Application of COSMO-RS-DARE as a Tool for Testing Consistency of Solubility Data: Case of Coumarin in Neat Alcohols. Molecules 2022, 27, 5274. [Google Scholar] [CrossRef]
- Li, W.; Ma, Y.; Yang, Y.; Xu, S.; Shi, P.; Wu, S. Solubility measurement, correlation and mixing thermodynamics properties of dapsone in twelve mono solvents. J. Mol. Liq. 2019, 280, 175–181. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Xue, Y.; Zhu, P.; Zhao, H. Comprehensive insight into solubility, dissolution properties and solvation behaviour of dapsone in co-solvent solutions. J. Mol. Liq. 2021, 341, 117403. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.; Wang, J.; Liu, T.; Qu, Y. Solubility Determination and Thermodynamic Modeling of Amitriptyline Hydrochloride in 13 Pure Solvents at Temperatures of 283.15–323.15 K. J. Chem. Eng. Data 2021, 66, 1877–1889. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, X.; Liu, B.; Du, Y.; Wang, J. Temperature dependent solubility of sodium cyclamate in selected pure solvents and binary methanol+water mixed solvents. Fluid Phase Equilib. 2015, 390, 1–6. [Google Scholar] [CrossRef]
- Liang, A.; Wang, S.; Qu, Y. Determination and Correlation of Solubility of Phenylbutazone in Monosolvents and Binary Solvent Mixtures. J. Chem. Eng. Data 2017, 62, 864–871. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Ma, M.; Qu, Y. Determination and Modeling of Artesunate Solubility in 13 Pure Solvents at 283.15–323.15 K. J. Chem. Eng. Data 2022, 67, 3734–3747. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Wang, J. Measurement and Correlation of Solubility of Loratadine in Different Pure Solvents and Binary Mixtures. J. Chem. Eng. Data 2017, 62, 391–397. [Google Scholar] [CrossRef]
- Galaon, T.; David, V. Deviation from van’t Hoff dependence in RP-LC induced by tautomeric interconversion observed for four compounds. J. Sep. Sci. 2011, 34, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes. Biovia COSMOtherm, version 22.0.0; Dassault Systèmes: Vélizy-Villacoublay, France, 2022. [Google Scholar]
- Cysewski, P. Application of the consonance solvent concept for accurate prediction of buckminster solubility in 180 net solvents using COSMO-RS approach. Symmetry 2019, 11, 828. [Google Scholar] [CrossRef]
- Dassault Systèmes. Biovia COSMOconf, version 20.0.0; Dassault Systèmes: Vélizy-Villacoublay, France, 2020. [Google Scholar]
- TURBOMOLE GmbH. TURBOMOLE, version 7.5.1; Dassault Systèmes: Vélizy-Villacoublay, France, 2020. [Google Scholar]
- Dassault Systèmes. Biovia TmoleX, version 21.0.1; Dassault Systèmes: Vélizy-Villacoublay, France, 2020. [Google Scholar]
- Jeliński, T.; Kubsik, M.; Cysewski, P. Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations. Materials 2022, 15, 2472. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Witte, J.; Neaton, J.B.; Head-Gordon, M. Effective empirical corrections for basis set superposition error in the def2-SVPD basis: gCP and DFT-C. J. Chem. Phys. 2017, 146, 234105. [Google Scholar] [CrossRef]
- Akiba, T.; Sano, S.; Yanase, T.; Ohta, T.; Koyama, M. Optuna: A Next-generation Hyperparameter Optimization Framework. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019; pp. 2623–2631. [Google Scholar]
- Ueda, H.; Osaki, H.; Miyano, T. Baloxavir Marboxil Shows Anomalous Conversion of Crystal Forms from Stable to Metastable through Formation of Specific Solvate Form. J. Pharm. Sci. 2023, 112, 158–165. [Google Scholar] [CrossRef]
- Do, H.T.; Chua, Y.Z.; Kumar, A.; Pabsch, D.; Hallermann, M.; Zaitsau, D.; Schick, C.; Held, C. Melting properties of amino acids and their solubility in water. RSC Adv. 2020, 10, 44205–44215. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Tsivintzelis, I. On the Thermodynamic Thermal Properties of Quercetin and Similar Pharmaceuticals. Molecules 2022, 27, 6630. [Google Scholar] [CrossRef]
- Braun, D.E.; Krüger, H.; Kahlenberg, V.; Griesser, U.J. Molecular level understanding of the reversible phase transformation between forms III and II of dapsone. Cryst. Growth Des. 2017, 17, 5054–5060. [Google Scholar] [CrossRef]
- Braun, D.E.; Vickers, M.; Griesser, U.J. Dapsone Form V: A Late Appearing Thermodynamic Polymorph of a Pharmaceutical. Mol. Pharm. 2019, 16, 3221–3236. [Google Scholar] [CrossRef]
- Keyvanpour, M.R.; Shirzad, M.B. An Analysis of QSAR Research Based on Machine Learning Concepts. Curr. Drug Discov. Technol. 2021, 18, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, P.; Ji, X.; Li, M.; Lu, W. Feature Selection in Machine Learning for Perovskite Materials Design and Discovery. Materials 2023, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- How, W.B.; Wang, B.; Chu, W.; Tkatchenko, A.; Prezhdo, O.V. Significance of the Chemical Environment of an Element in Nonadiabatic Molecular Dynamics: Feature Selection and Dimensionality Reduction with Machine Learning. J. Phys. Chem. Lett. 2021, 12, 12026–12032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, H. Machine Learning Modeling of Environmentally Relevant Chemical Reactions for Organic Compounds. ACS EST Water 2022. [Google Scholar] [CrossRef]
- Dhal, P.; Azad, C. A comprehensive survey on feature selection in the various fields of machine learning. Appl. Intell. 2022, 52, 4543–4581. [Google Scholar] [CrossRef]
- RMG. Available online: https://rmg.mit.edu/database/solvation/searchSolubility/ (accessed on 4 September 2023).
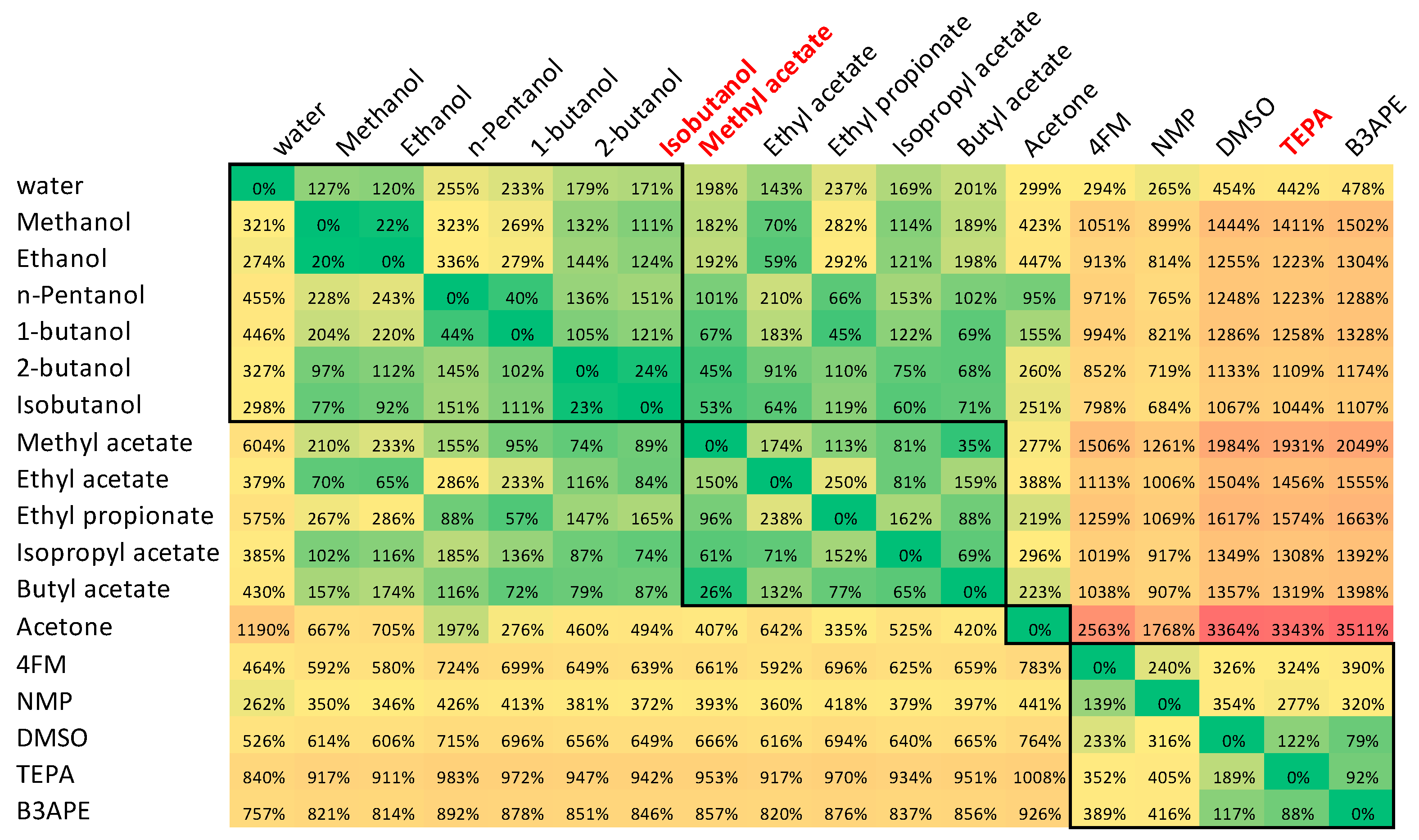
| T (K) | sDAP (mol/dm3) | xDAP∙104 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4FM | DMSO | TEPA | NMP | B3APE | 4FM | DMSO | TEPA | NMP | B3APE | |
| 298.15 | 0.042 (±0.000) | 0.256 (±0.009) | 0.056 (±0.001) | 0.108 (±0.003) | 0.068 (±0.001) | 43.11 (±0.25) | 187.57 (±7.02) | 105.69 (±2.23) | 105.13 (±3.20) | 147.51 (±1.43) |
| 303.15 | 0.074 (±0.004) | 0.344 (±0.002) | 0.079 (±0.002) | 0.243 (±0.005) | 0.109 (±0.011) | 73.90 (±4.18) | 251.58 (±1.54) | 149.90 (±4.00) | 236.59 (±5.16) | 231.14 (±22.69) |
| 308.15 | 0.126 (±0.001) | 0.462 (±0.012) | 0.104 (±0.000) | 0.525 (±0.009) | 0.142 (±0.002) | 125.57 (±0.89) | 342.88 (±9.24) | 196.54 (±0.47) | 524.74 (±10.19) | 297.60 (±4.48) |
| 313.15 | 0.191 (±0.001) | 0.598 (±0.011) | 0.141 (±0.002) | 1.040 (±0.118) | 0.191 (±0.003) | 191.48 (±0.55) | 444.27 (±9.20) | 262.29 (±4.27) | 1113.63 (±17.53) | 397.51 (±6.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cysewski, P.; Przybyłek, M.; Jeliński, T. Intermolecular Interactions as a Measure of Dapsone Solubility in Neat Solvents and Binary Solvent Mixtures. Materials 2023, 16, 6336. https://doi.org/10.3390/ma16186336
Cysewski P, Przybyłek M, Jeliński T. Intermolecular Interactions as a Measure of Dapsone Solubility in Neat Solvents and Binary Solvent Mixtures. Materials. 2023; 16(18):6336. https://doi.org/10.3390/ma16186336
Chicago/Turabian StyleCysewski, Piotr, Maciej Przybyłek, and Tomasz Jeliński. 2023. "Intermolecular Interactions as a Measure of Dapsone Solubility in Neat Solvents and Binary Solvent Mixtures" Materials 16, no. 18: 6336. https://doi.org/10.3390/ma16186336
APA StyleCysewski, P., Przybyłek, M., & Jeliński, T. (2023). Intermolecular Interactions as a Measure of Dapsone Solubility in Neat Solvents and Binary Solvent Mixtures. Materials, 16(18), 6336. https://doi.org/10.3390/ma16186336








