A New Process for Efficient Recovery of Rhodium from Spent Carbonyl Rhodium Catalyst by Microreactor
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
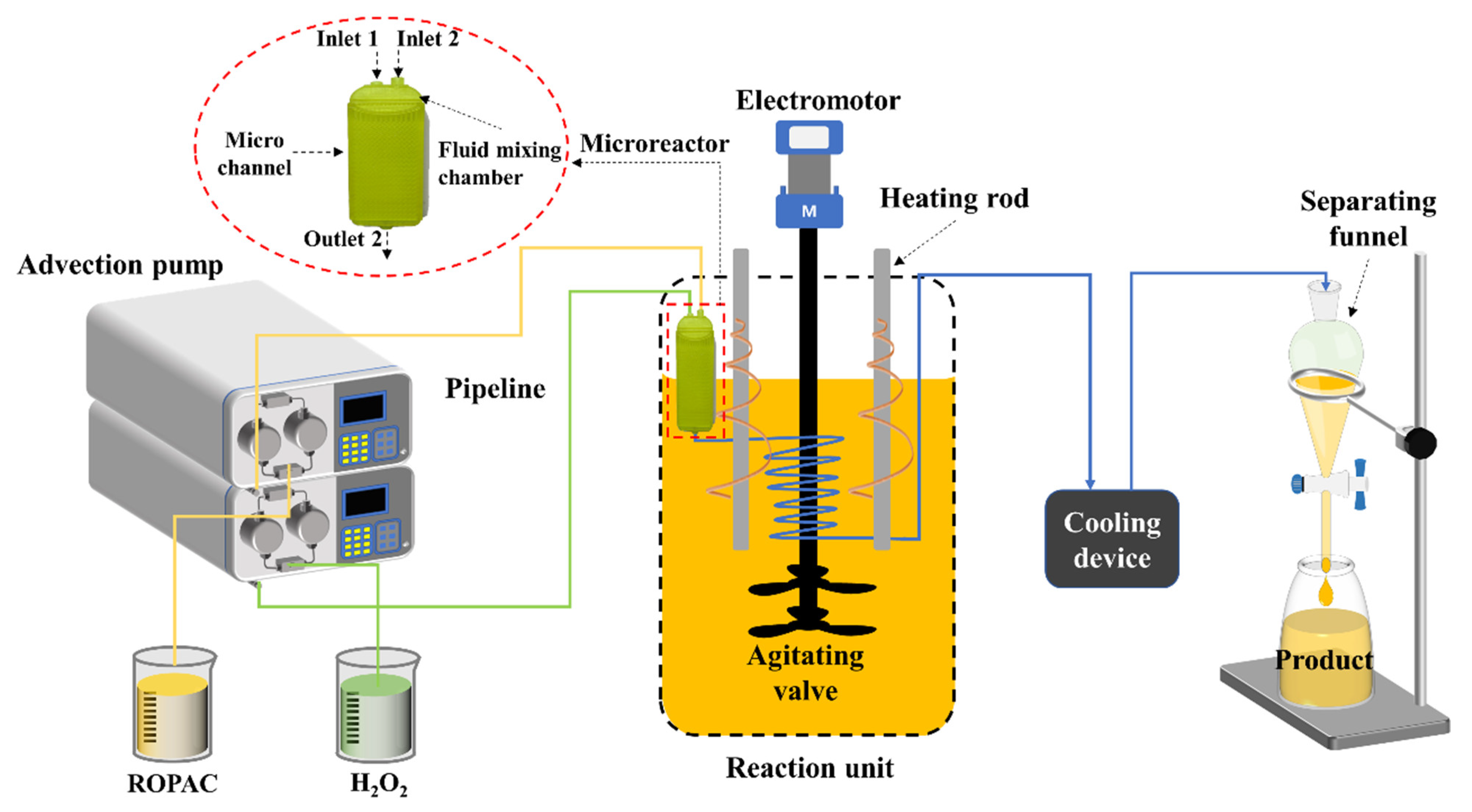
2.2. Microreactor Structure
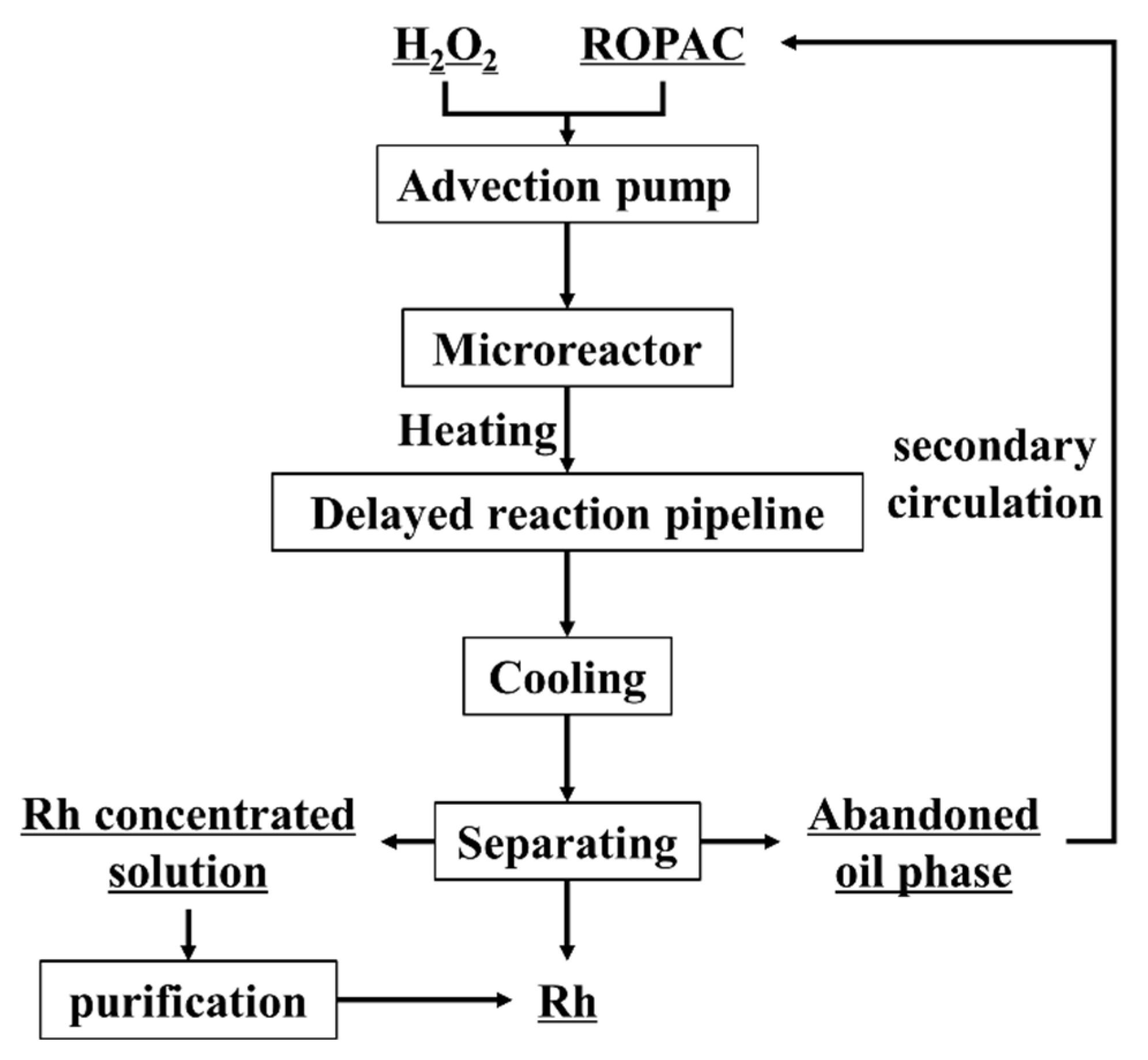
2.3. Experimental Procedure
2.4. Characterization Methods
3. Results and Discussion
3.1. Effect of Critical Process Parameters on the Recovery Rate of Rh
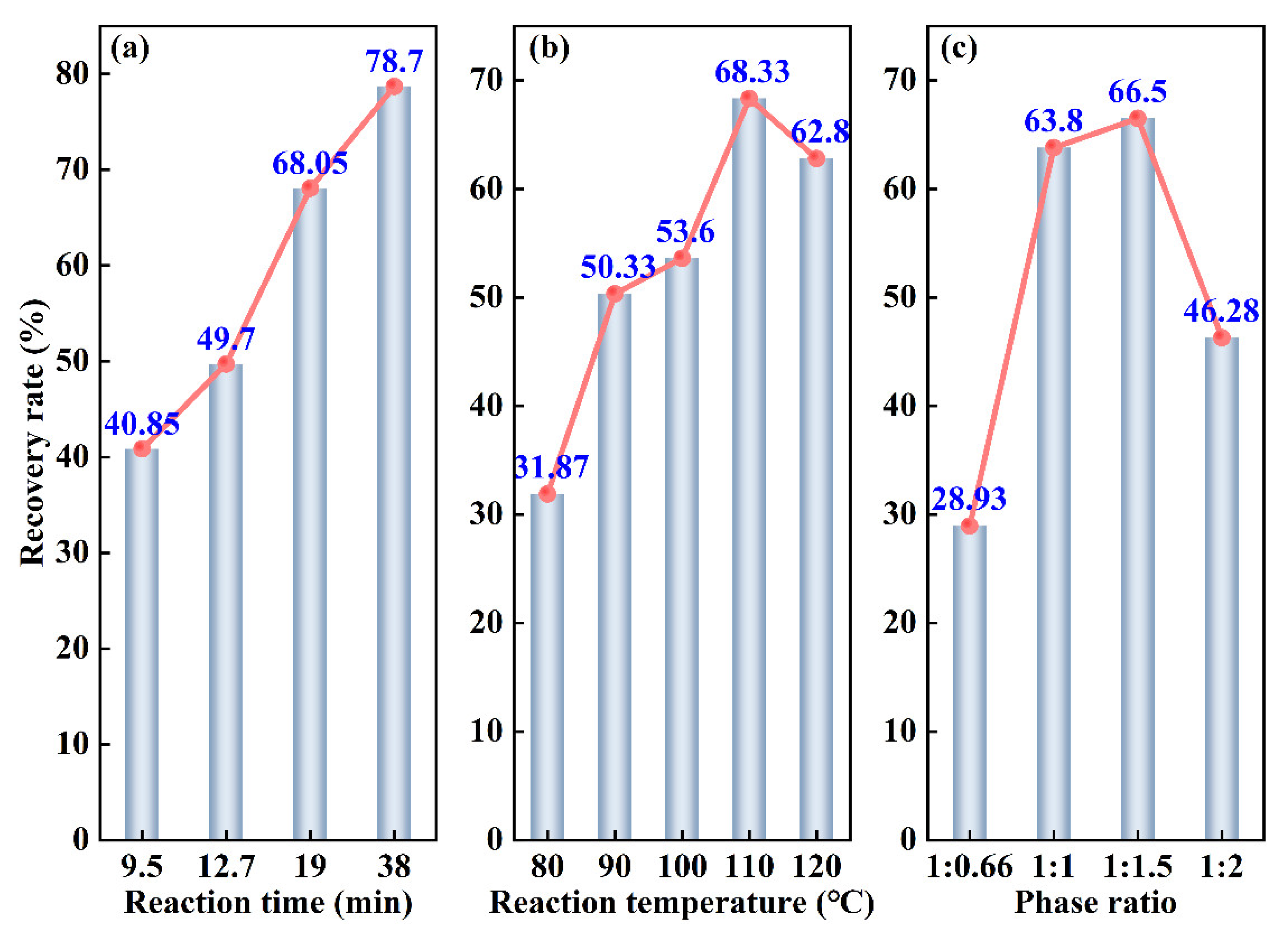
3.1.1. Reaction Time
3.1.2. Reaction Temperature
3.1.3. Phase Ratio
3.2. RSM Optimizes Experimental Design
3.2.1. Determination of RSM Factors
3.2.2. The Recovery Rate Response Surface Experiment and Variance Analysis
12.83C2
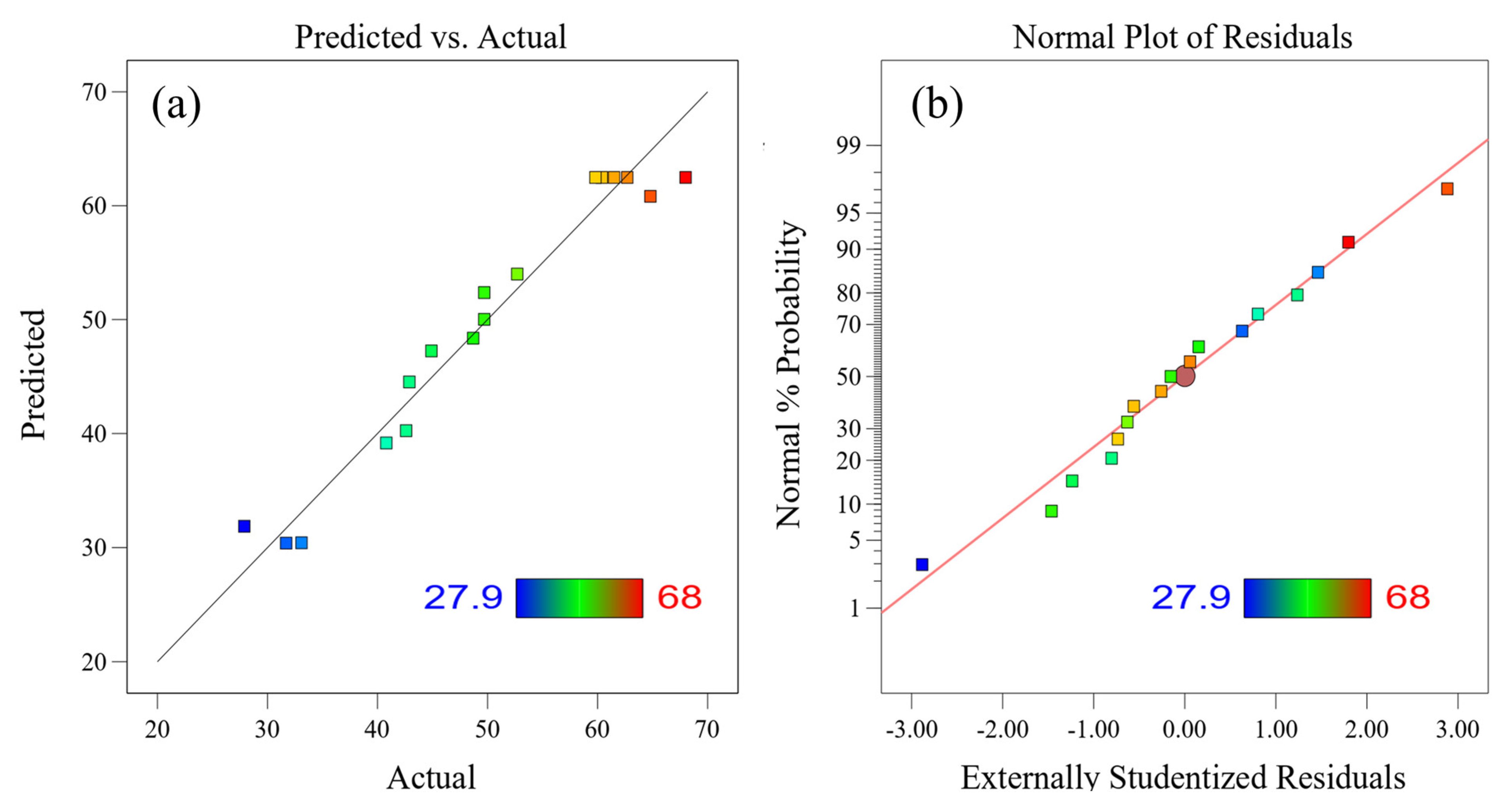
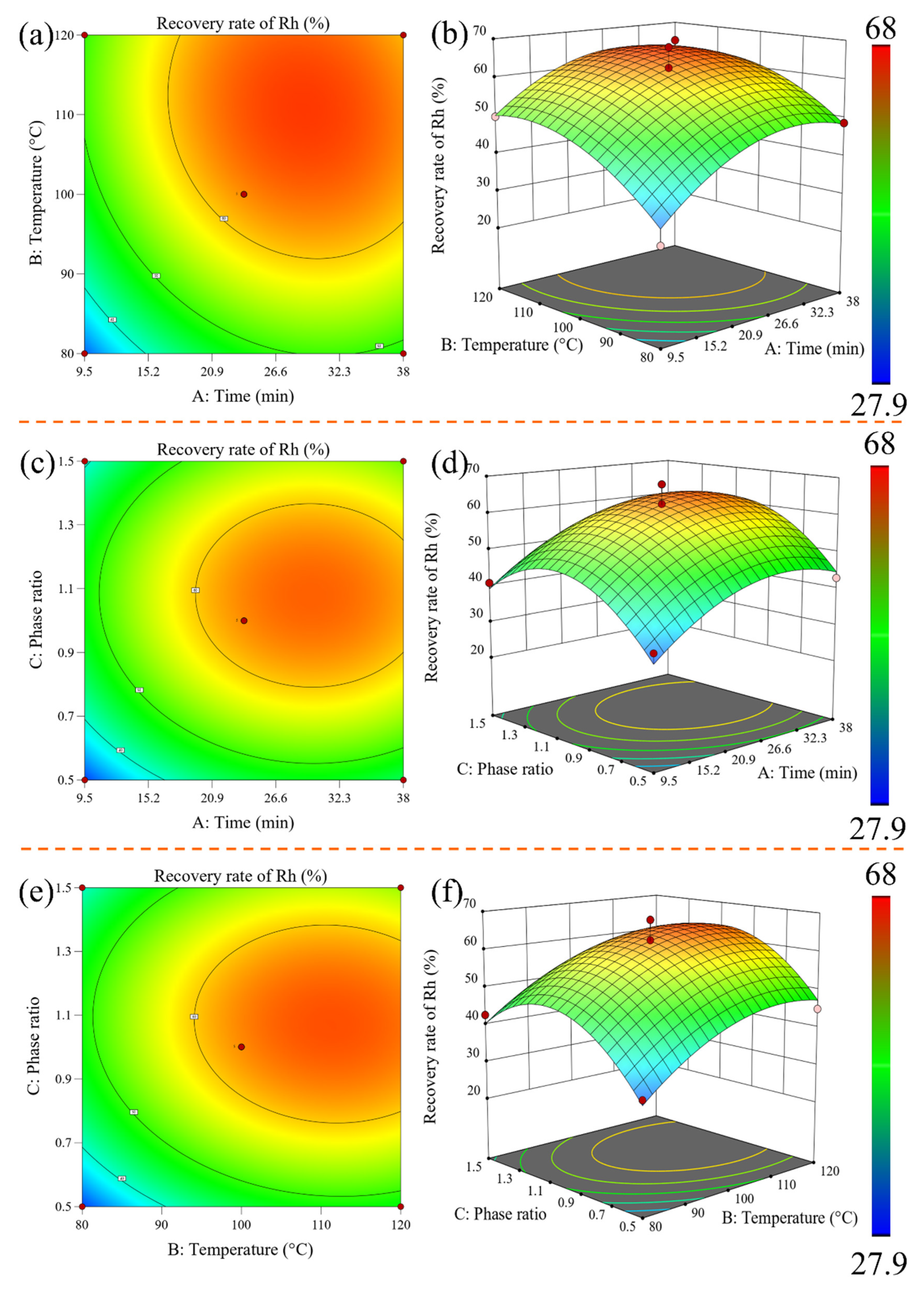
3.2.3. Response Surface Analysis, Optimization, and Verification
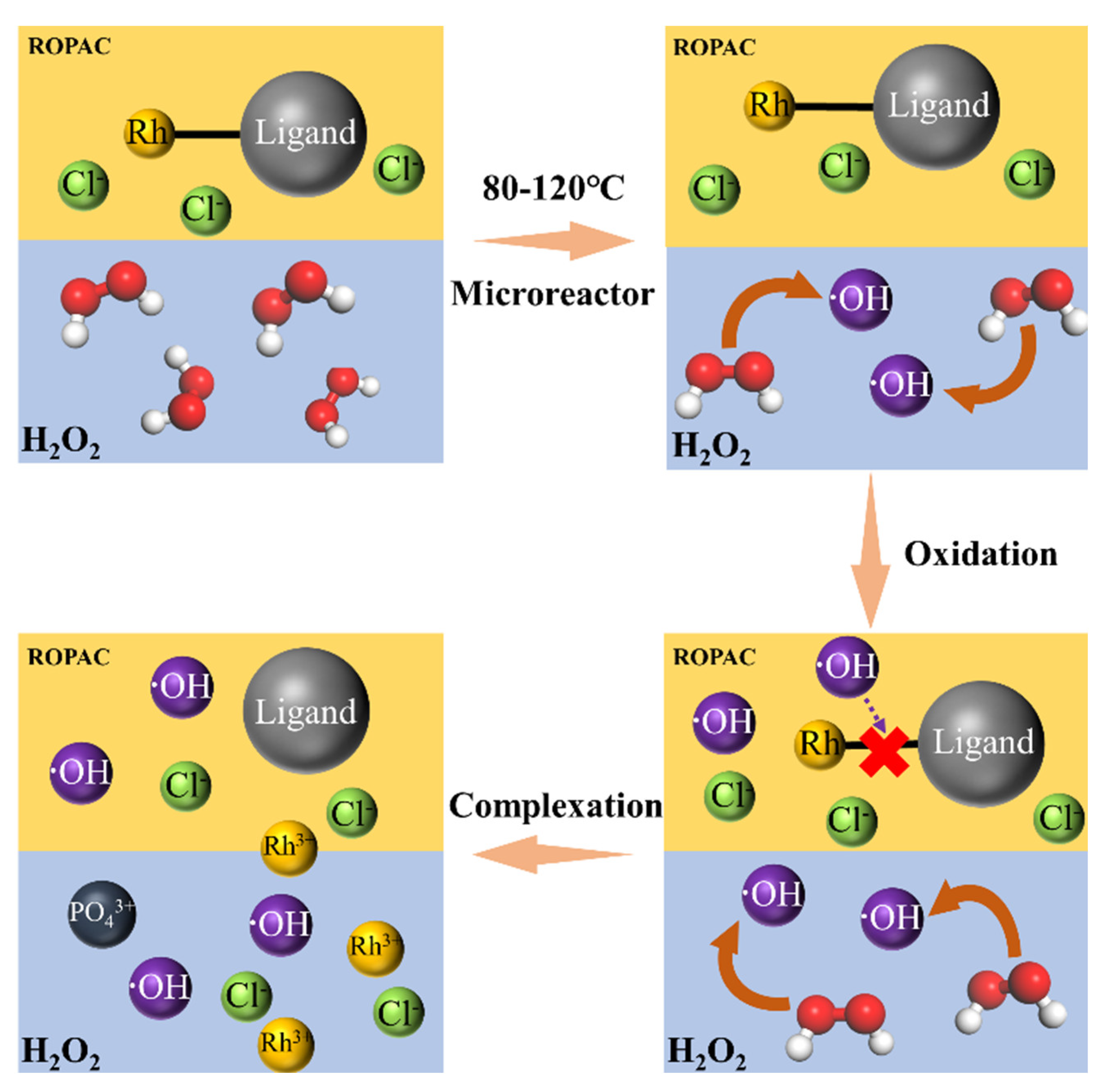
3.3. FTIR Evidence of the Rh Recovery Mechanism
4. Conclusions
- (1)
- Using microchannel reactor technology as a replacement for traditional processes in the oxidative recovery of waste rhodium homogeneous catalysts has shown significant effectiveness. This approach demonstrates remarkable improvements in reaction efficiency and process time reduction while also offering notable advantages over traditional methods in terms of environmental friendliness, sustainability, and safety considerations.
- (2)
- By oxidatively breaking the Rh-P chemical bond using H2O2, the complexation recovery of Rh was achieved. Based on the single-factor experiment, response surface optimization design was applied to obtain the optimized process conditions: reaction time of 29 min, reaction temperature of 110 °C, and phase ratio of 1:1. The results of the continuous experiment for 4 h showed a Rh recovery rate of 74.93%. Under the same conditions, secondary oxidation treatment resulted in a Rh recovery rate of 95.6%.
- (3)
- Under the optimized conditions, the Rh content in the oil phase was measured at 21.22 mg/L, while the concentration of chloride (Cl−) in the aqueous phase is 209.33 mg/L and the phosphate (PO43+) concentration is 266.01 mg/L. These findings indicate the near-complete recovery of Rh from the spent catalyst. The removal mechanism of Rh in ROPAC can be further confirmed through FTIR analysis. Simultaneously, the higher concentration of chlorine in the waste catalyst indicates the entry of rhodium into the aqueous phase, forming a rhodium chloride aqueous complex.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Xiong, X.; Sui, G.; Zhang, Y. Synthesis and Characterization of Acetylacetonatocarbonyltriphenylphosphinerhodium(I). Precious Met. 2005, 26, 43–46. [Google Scholar]
- Li, Z.; Quan, K.D.; Han, Z.M. Research progress in rhodium recovery process from spent precious metal catalysts. Petrochem. Ind. Technol. 2020, 27, 270–271. [Google Scholar]
- Jiang, L.Y.; Li, C.; Li, J.X.; Hao, T.T.; Wang, P.F.; Wang, B.L. Study on preparing high-purity rhodium chloride by using digestion solution produced in dispelling waste catalyst containing rhodium from butyl octanol unit. Inorg. Chem. Ind. 2017, 49, 76–78. [Google Scholar]
- Shi, H.X. Analysis of development status and market supply and demand of butanol and octanol industry. China Petrochem. Ind. Obs. 2019, 5, 56–60. [Google Scholar]
- Wang, B.; Zhang, G.; Jiang, L.; Li, C.; Wang, P.; Li, J.; Zang, J. Study on preparation process of rhodium trichloride by roasting recovery technology from waste rhodium solution of carbonyl synthesis. Inorg. Chem. Ind. 2023, 55, 104–108. [Google Scholar]
- Xie, X.; Jiang, D.; Zhang, B.; Liu, G.; Zhang, F.; Wang, F.; Wu, Z.; Liu, A. Study on the Process of RecoVering Rhodium from Low—Concentration Rhodium—Containing organic Waste Catalysts. China Resour. Compr. Util. 2022, 40, 10–12. [Google Scholar]
- Zhang, J.C.; Jiang, D.; Xie, X.; Liu, G.Q.; Zhang, F. Study on the Process of Recovering Rhodium from Spent Homogeneous Catalyst Containing Low Concentration Rhodium. China Resour. Compr. Util. 2022, 40, 24–27. [Google Scholar]
- Pei, H.Y.; Zhao, J.C.; Wu, Y.D.; Cheng, J.L.; Dong, H.G. Technology Progress on Recovery of Rhodium from Organic Wastes. Precious Met. 2019, 40, 85–90. [Google Scholar]
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. Jom 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Yong, Y.; Jiang, G. A Recovery Method of Rhodium Catalyst. CN103498056A, 8 January 2014. [Google Scholar]
- Dai, Q.; Zheng, Y.; Chen, L.; Cai, D.; Chen, Q.; Yao, L.; Zhou, X.; Liu, P. A Method for Recovering Rhodium Catalyst from Rhodium-Containing Wastewater Phase. CN105944768A, 21 September 2016. [Google Scholar]
- Dong, Y.; Li, J. A Method for Recovering Rhodium from Rhodium Catalyst Waste Liquid. CN110964910A, 7 April 2020. [Google Scholar]
- Yang, C.; Wang, G.; Li, Y.; Wang, F.; Wan, S.; Wang, L.; Meng, S.; Meng, R. Chi KebinA Method for Recovering Rhodium from Waste Rhodium Catalyst for Carbonyl Synthesis Reaction. CN1414125A, 17 November 2004. [Google Scholar]
- Yu, H.; Li, C.; Jiang, L.; Li, J.; Li, J. A Method for Recovering Rhodium from Rhodium-Containing Waste Liquid to Prepare Hydrated Rhodium Trichloride. CN102557155A, 31 July 2013. [Google Scholar]
- Chen, G.X.; Yuan, Z.W.; Jiang, S.Y.; Gong, H. Technical progress in recovery of noble metal rhodium from spent catalysts. Sino-Glob. Energy 2021, 26, 65–69. [Google Scholar]
- Li, Y.M.; Jiao, X.D.; Lu, X.N. Research Progress of Rh Recovery Techniques from Spent Precious Metals Catalyst. Precious Met. 2014, 35, 42–44. [Google Scholar]
- Liu, Q.; Sun, W.; Yang, J.; Peng, Y.; Wang, W. A Method for Recovering Precious Metals from Waste Catalysts Containing Precious Metals. CN1448522A, 15 October 2003. [Google Scholar]
- Li, C.; Yu, H.; Jiang, L.; Li, J.; Li, J.; Hao, T. The Method of Recovering Rhodium from Rhodium-Containing Waste Catalyst in Hydroformylation Reaction by Hydrogen Peroxide Treatment. CN102925699A, 13 February 2013. [Google Scholar]
- Li, T.; Lv, S.; Wang, S.; Liu, B.; Qin, Y. A Method for Recovering Rhodium from Deactivated Propylene Hydroformylation Catalyst. CN103509061A, 15 January 2014. [Google Scholar]
- Chen, Z.; Pei, Z.; Zhao, X.; Zhang, J.; Wei, J.; Hao, N. Acoustic microreactors for chemical engineering. Chem. Eng. J. 2022, 433, 133258. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Yue, J. Green process intensification using microreactor technology for the synthesis of biobased chemicals and fuels. Chem. Eng. Process.-Process Intensif. 2022, 177, 109002. [Google Scholar] [CrossRef]
- Yue, J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3–19. [Google Scholar] [CrossRef]
- Li, X.; Jiang, F.; Ravindra, A.V.; Zhou, J.; Zhou, A.; Le, T.; Peng, J.; Ju, S. Mixing processes in a 3D printed large-flow microstructured reactor: Finite element simulations and experimental study. Chem. Eng. J. 2019, 370, 295–304. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Ju, S.; Gu, Y.; Tan, W.; Li, X.; Wang, S. Miniaturized application of 3D-printed large-flow microreactor in extraction and separation of platinum, palladium and rhodium. J. Chem. Technol. Biotechnol. 2021, 96, 1007–1015. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Baral, A.; Ju, S.; Gu, Y. High-efficiency absorption of low NOX concentration in metallurgical flue gas using a three dimensional printed large-flow microstructured reactor. Arab. J. Chem. 2022, 15, 103711. [Google Scholar] [CrossRef]
- Ding, Y.-J.; Li, J.-Y.; Zheng, H.-D.; Cui, Y.-J.; Liu, B.; Zhang, S.-G. Oxidation-complexation leaching and kinetic study of rhodium from spent homogeneous catalysts. Chin. J. Eng. 2023, 45, 214–222. [Google Scholar]
- Assila, O.; Vilaça, N.; Bertão, A.R.; Fonseca, A.M.; Parpot, P.; Soares, O.S.G.P.; Pereira, M.F.R.; Baltazar, F.; Bañobre-López, M.; Neves, I.C. Optimization of iron-ZIF-8 catalysts for degradation of tartrazine in water by Fenton-like reaction. Chemosphere 2023, 339, 139634. [Google Scholar] [CrossRef]
- Fischbacher, A.; von Sonntag, C.; Schmidt, T.C. Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe(II) ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, N.; Liu, X.; Shi, Y.; Ling, C.; Zhang, L. Ascorbate guided conversion of hydrogen peroxide to hydroxyl radical on goethite. Appl. Catal. B Environ. 2021, 282, 119558. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ockuly, R.A.; Weese, M.L.; Smucker, B.J.; Edwards, D.J.; Chang, L. Response surface experiments: A meta-analysis. Chemom. Intell. Lab. Syst. 2017, 164, 64–75. [Google Scholar] [CrossRef]
- Gong, S.; Dong, X.; Zhang, T.; Niu, Y.; Hou, K.; Chen, W.; An, Y.; Liu, B. Preparation and characterization of micro-spherical ruthenium particles using spray drying combined with microwave calcination of ammonium hexachlororuthenate (IV). Int. J. Refract. Met. Hard Mater. 2023, 115, 106276. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Liu, J.; Wu, J.; Wang, Z.; Zhang, X. Ultrasonic-assisted alka line extraction of Plukenetia volubilis protein isolate and its process ing properties. Food Ferment. Ind. 2021, 47, 128–135. [Google Scholar]
- Jinmeng, S.; Zongcai, T.; Hui, W.; Yueming, H. Process optimization and quality analysis of mango cake prepared with fish protein gelatin. Sci. Technol. Food Ind. 2022, 43, 189–195. [Google Scholar]
- Zhang, Y.; Jiang, S.; Zhang, X. Method for Step-by-Step Recovery of Effective Components of Waste Rhodium Parker Catalyst. CN111848674A, 30 October 2020. [Google Scholar]
- Niu, Y.; Gao, P.; Ju, S.; Li, F.; Wang, S.; Xu, Z.; Lin, J.; Yang, J.; Peng, H. Hydrogen Peroxide/Phosphoric Acid Modification of Hydrochars for Sulfamethoxazole and Carbamazepine Adsorption: The Role of Oxygen-Containing Functional Groups. Langmuir ACS J. Surf. Colloids 2023, 39, 5679–5688. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, T.; Lv, L.; Chen, Y.; Zhong, B.; Tang, S. Extraction performance and mechanism of TBP in the separation of Fe3+ from wet-processing phosphoric acid. Sep. Purif. Technol. 2021, 272, 118822. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X. Green synthesis of modified polyethylene packing supported tea polyphenols-NZVI for nitrate removal from wastewater: Characterization and mechanisms. Sci. Total Environ. 2022, 806, 150596. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Mahandra, H.; Singh, R.; Gupta, B. Liquid-liquid extraction studies on Zn(II) and Cd(II) using phosphonium ionic liquid (Cyphos IL 104) and recovery of zinc from zinc plating mud. Sep. Purif. Technol. 2017, 177, 281–292. [Google Scholar] [CrossRef]


| Elements | Ca | Cr | Fe | Na | P | Rh | Si |
|---|---|---|---|---|---|---|---|
| Content | 3.47 | 3.50 | 9.68 | 14.92 | 4320.99 | 681.79 | 21.27 |
| Methods | Temperature (°C) | Time (h) | Phase Ratio | Recovery Rate (%) | Security |
|---|---|---|---|---|---|
| Kettle-type reactor | 90 | >8.00 | 1:1 | ≤95% | The danger of deflagration gas overflow |
| Microreactors | 110 | 0.66 | 1:1 | 95.6% | Safe |
| Factors | Codes | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Time/min | A | 9.5 | 23.75 | 38 |
| Temperature/°C | B | 80 | 100 | 120 |
| Phase radio | C | 0.5 | 1 | 1.5 |
| Factor 1 | Factor 2 | Factor 3 | Response Value | |
|---|---|---|---|---|
| Run | A: Time/min | B: Temperature/°C | C: Phase Radio | η/% |
| 1 | 23.75 | 100.00 | 1.00 | 69.8 |
| 2 | 9.5 | 120.00 | 1.00 | 59.7 |
| 3 | 38.00 | 80.00 | 1.00 | 58.7 |
| 4 | 23.75 | 80.00 | 1.50 | 52.6 |
| 5 | 23.75 | 100.00 | 1.00 | 72.7 |
| 6 | 9.50 | 80.00 | 1.00 | 37.9 |
| 7 | 23.75 | 100.00 | 1.00 | 78 |
| 8 | 23.75 | 120.00 | 0.50 | 54.9 |
| 9 | 23.75 | 100.00 | 1.00 | 71.5 |
| 10 | 23.75 | 100.00 | 1.00 | 70.4 |
| 11 | 38.00 | 100.00 | 1.50 | 59.7 |
| 12 | 23.75 | 80.00 | 0.50 | 41.7 |
| 13 | 38.00 | 120.00 | 1.00 | 74.8 |
| 14 | 38.00 | 100.00 | 0.50 | 52.9 |
| 15 | 9.50 | 100.00 | 1.50 | 50.8 |
| 16 | 9.50 | 100.00 | 0.50 | 43.1 |
| 17 | 23.75 | 120.00 | 1.50 | 62.7 |
| Source | SS | DF | MS | F-Value | p-Value (Prob > F) | Significance |
|---|---|---|---|---|---|---|
| Model | 2261.99 | 9 | 251.33 | 16.17 | 0.0007 | significant |
| A | 372.65 | 1 | 372.65 | 23.97 | 0.0018 | |
| B | 468.18 | 1 | 468.18 | 30.12 | 0.0009 | |
| C | 137.78 | 1 | 137.78 | 8.86 | 0.0206 | |
| AB | 8.12 | 1 | 8.12 | 0.5225 | 0.4932 | |
| AC | 0.2025 | 1 | 0.2025 | 0.0130 | 0.9123 | |
| BC | 2.40 | 1 | 2.40 | 0.1545 | 0.7059 | |
| A2 | 271.33 | 1 | 271.33 | 17.45 | 0.0041 | |
| B2 | 187.74 | 1 | 187.74 | 12.08 | 0.0103 | |
| C2 | 692.82 | 1 | 692.82 | 44.57 | 0.0003 | |
| Residual | 108.82 | 7 | 15.55 | |||
| Lack of Fit | 65.83 | 3 | 21.94 | 2.04 | 0.2507 | not significant |
| Pure error | 42.99 | 4 | 10.75 | |||
| Cor total | 2370.81 | 16 |
| Time/min | Temperature/°C | Phase Radio | Rh Recovery Rate/% |
|---|---|---|---|
| 29 | 110 | 1 | 66.00 |
| 29 | 110 | 1 | 65.85 |
| 29 | 110 | 1 | 66.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Niu, Y.; Hu, J.; Ju, S.; Gu, Y.; Tan, W. A New Process for Efficient Recovery of Rhodium from Spent Carbonyl Rhodium Catalyst by Microreactor. Materials 2023, 16, 6271. https://doi.org/10.3390/ma16186271
Guo L, Niu Y, Hu J, Ju S, Gu Y, Tan W. A New Process for Efficient Recovery of Rhodium from Spent Carbonyl Rhodium Catalyst by Microreactor. Materials. 2023; 16(18):6271. https://doi.org/10.3390/ma16186271
Chicago/Turabian StyleGuo, Lei, Yifan Niu, Jianjun Hu, Shaohua Ju, Yongwan Gu, and Wenjin Tan. 2023. "A New Process for Efficient Recovery of Rhodium from Spent Carbonyl Rhodium Catalyst by Microreactor" Materials 16, no. 18: 6271. https://doi.org/10.3390/ma16186271
APA StyleGuo, L., Niu, Y., Hu, J., Ju, S., Gu, Y., & Tan, W. (2023). A New Process for Efficient Recovery of Rhodium from Spent Carbonyl Rhodium Catalyst by Microreactor. Materials, 16(18), 6271. https://doi.org/10.3390/ma16186271






