TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of TiO2 NTs and TiO2 NTs Decorated with Mo2C
2.3. Characterization
2.4. PEC Property Measurements
3. Results and Discussion
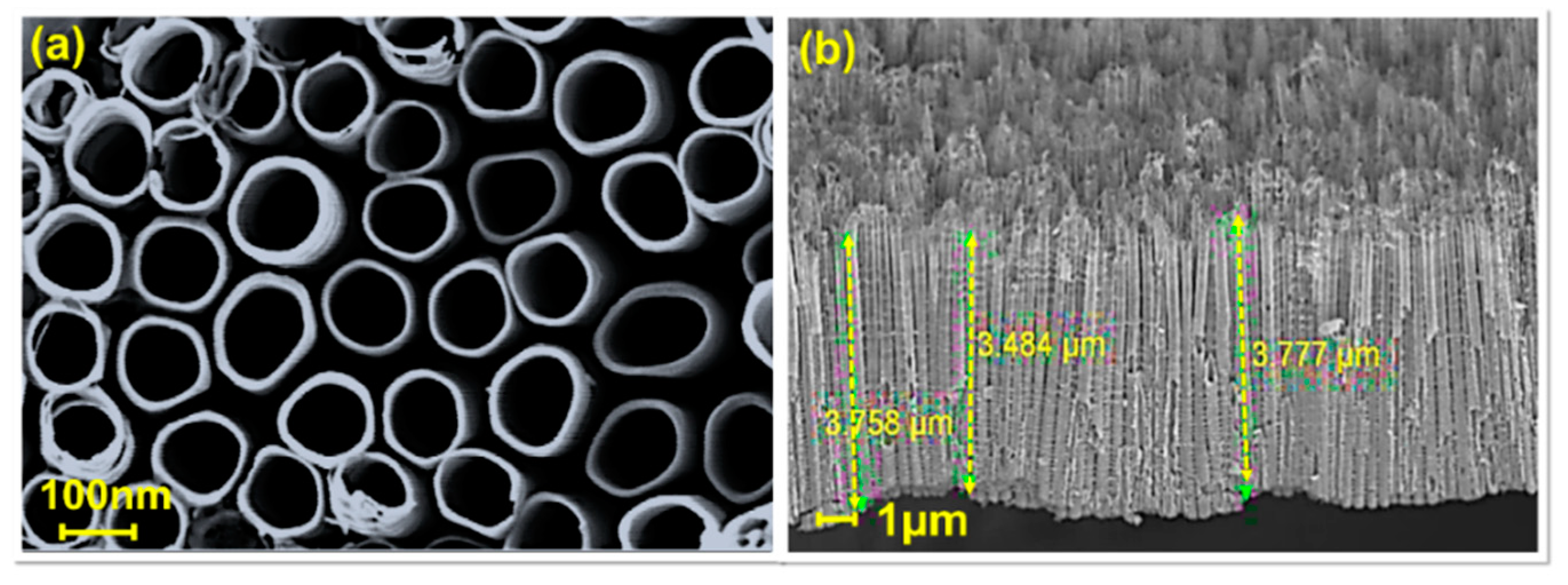
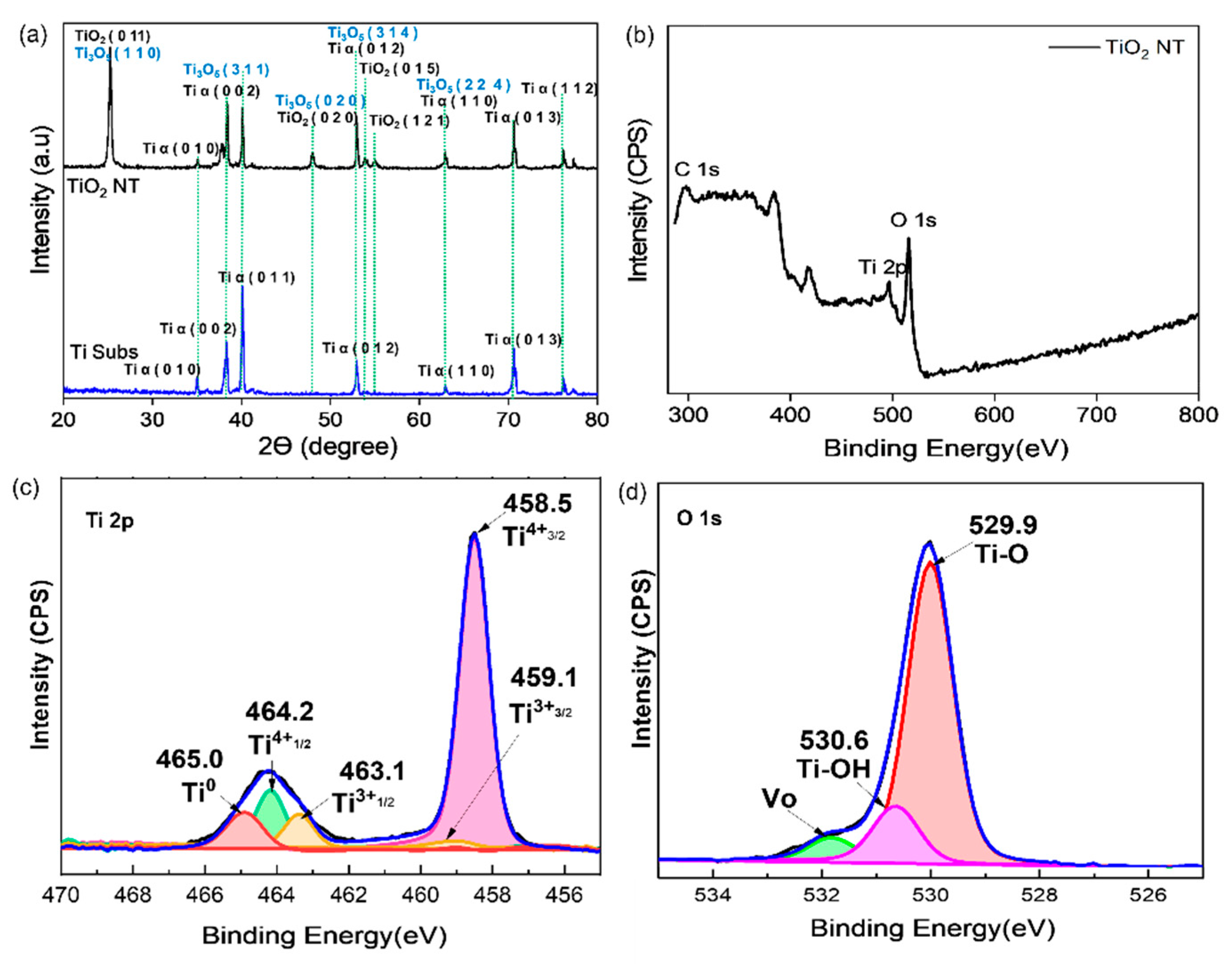
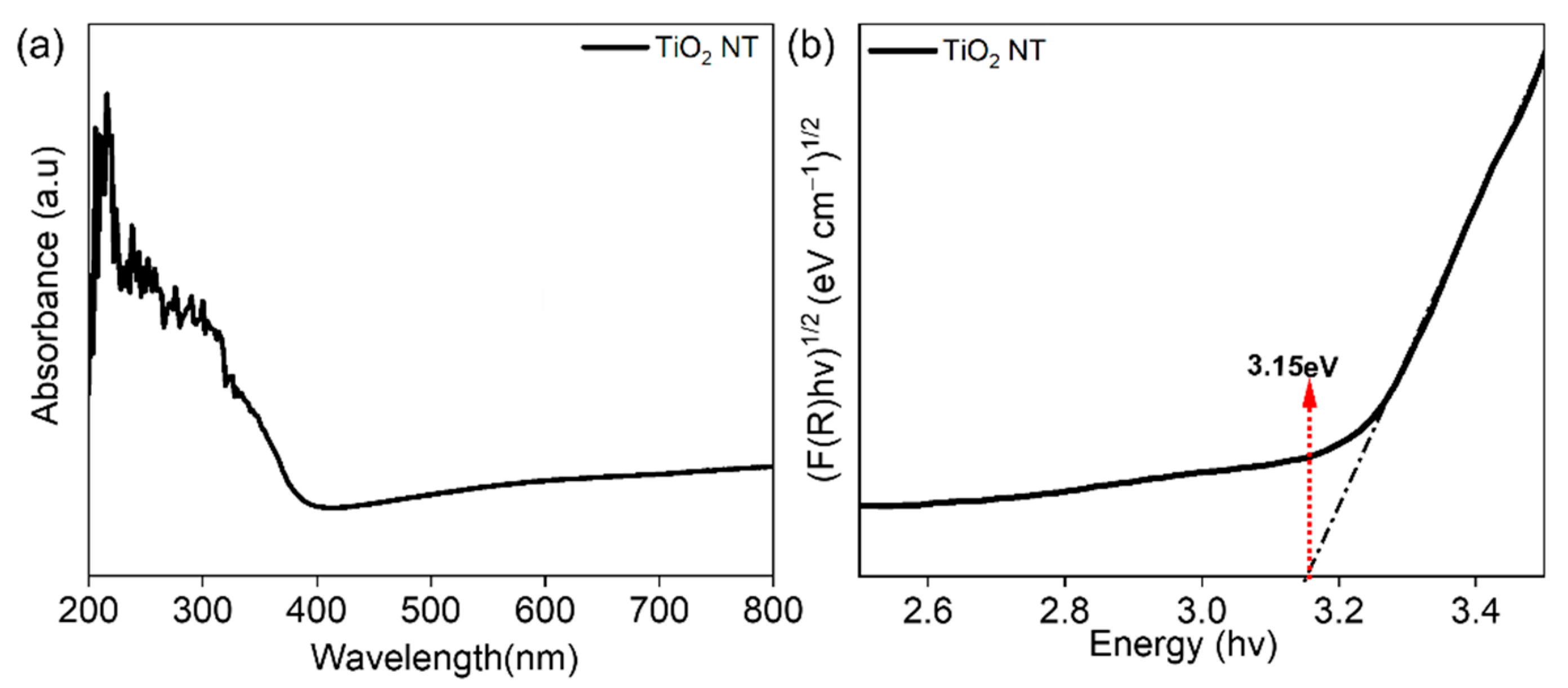
3.1. Physical Characterization of TiO2 NTs
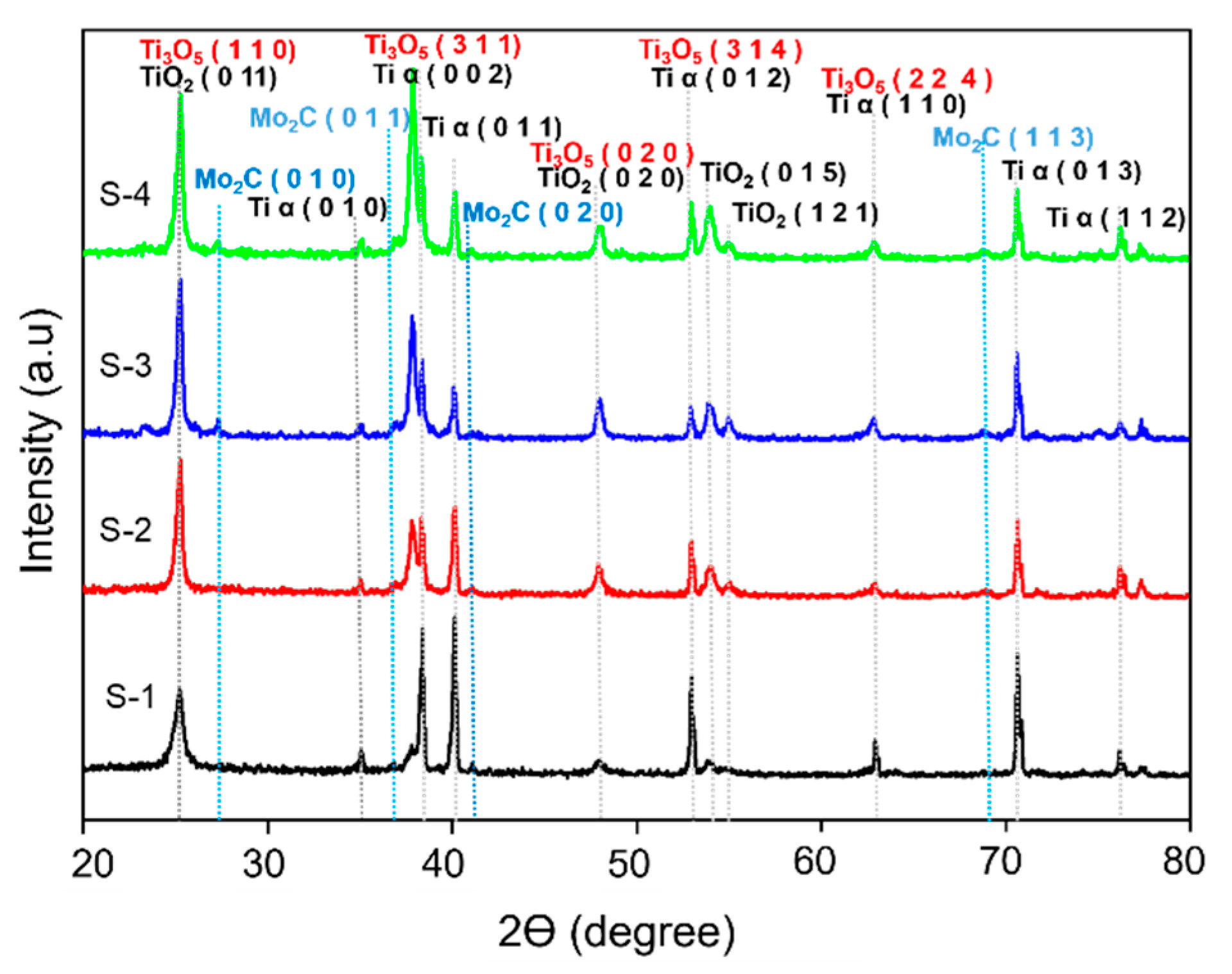
3.2. Physical Characterization of Mo2C as a Cocatalyst Decorated on TiO2 NTs
3.3. PEC Properties of TiO2 NTs and Mo2C as a Cocatalyst Decorated on TiO2 NTs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoud, M.; El-Kalliny, A.S.; Squadrito, G. Stacked titanium dioxide nanotubes photoanode facilitates unbiased hydrogen production in a solar-driven photoelectrochemical cell powered with a microbial fuel cell treating animal manure wastewater. Energy Convers. Manag. 2022, 254, 115225. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Xu, P.; Xue, W.; Lei, L.; Cheng, M.; Wang, R.; Liu, X.; Deng, R. Semiconductor-based photocatalysts for photocatalytic and photoelectrochemical water splitting: Will we stop with photocorrosion? J. Mater. Chem. A 2020, 8, 2286–2322. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 nanotubes for photoelectrochemical water splitting: Review. Int. J. Hydrogen Energy 2021, 46, 4998–5024. [Google Scholar] [CrossRef]
- Lin, S.; Ren, H.; Wu, Z.; Sun, L.; Zhang, X.-G.; Lin, Y.-M.; Zhang, K.H.L.; Lin, C.-J.; Tian, Z.-Q.; Li, J.-F. Direct Z-scheme WO3−x nanowire-bridged TiO2 nanorod arrays for highly efficient photoelectrochemical overall water splitting. J. Energy Chem. 2021, 59, 721–729. [Google Scholar] [CrossRef]
- Arifin, K.; Daud, W.R.W.; Kassim, M.B. Optical and photoelectrochemical properties of a TiO2 thin film doped with a ruthenium–tungsten bimetallic complex. Ceram. Int. 2013, 39, 2699–2707. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Salehmin, M.I.; Mohamed, M.A.; Arifin, K.; Minggu, L.J.; Kassim, M.B. Cobalt oxide as photocatalyst for water splitting: Temperature-dependent phase structures. Int. J. Hydrogen Energy 2019, 44, 25495–25504. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Yuan, B.; Wang, Y.; Sun, P.; Wang, D.; Wei, Y.; Liu, Y. Multi-heterojunction photocatalysts based on WO3 nanorods: Structural design and optimization for enhanced photocatalytic activity under visible light. Chem. Eng. J. 2013, 237, 29–37. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Minggu, L.J.; Mark-Lee, W.F.; Mohamed, M.A.; Arifin, K.; Jumali, M.H.H.; Kassim, M.B. Highly photoactive Cu2O nanowire film prepared with modified scalable synthesis method for enhanced photoelectrochemical performance. Sol. Energy Mater. Sol. Cells 2018, 182, 237–245. [Google Scholar] [CrossRef]
- Ng, K.H.; Minggu, L.J.; Mark-Lee, W.F.; Arifin, K.; Jumali, M.H.H.; Kassim, M.B. A new method for the fabrication of a bilayer WO3/Fe2O3 photoelectrode for enhanced photoelectrochemical performance. Mater. Res. Bull. 2018, 98, 47–52. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Gao, X.; Yu, B.; Gao, Y.; Han, Z.-G. TiO2-rutile/anatase homojunction with enhanced charge separation for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 26358–26366. [Google Scholar] [CrossRef]
- Liang, S.; He, J.; Sun, Z.; Liu, Q.; Jiang, Y.; Cheng, H.; He, B.; Xie, Z.; Wei, S. Improving Photoelectrochemical Water Splitting Activity of TiO2 Nanotube Arrays by Tuning Geometrical Parameters. J. Phys. Chem. C 2012, 116, 9049–9053. [Google Scholar] [CrossRef]
- Lee, J.; Tan, L.-L.; Chai, S.-P. Heterojunction photocatalysts for artificial nitrogen fixation: Fundamentals, latest advances and future perspectives. Nanoscale 2021, 13, 7011–7033. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qian, Q.; Chen, Z.; Feng, D.; Tuan, P.D.; Yin, F. Surface Modification of TiO2 Nanotubes Prepared by Porous Titanium Anodization via Hydrothermal Reaction: A Method for Synthesis High-Efficiency Adsorbents of Recovering Sr Ions. Langmuir 2022, 38, 11354–11361. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanareddy, N.; Rao, V.N.; Cheralathan, K.K.; Subramaniam, E.P.; Shankar, M.V. Pt/TiO2 nanotube photocatalyst—Effect of synthesis methods on valance state of Pt and its influence on hydrogen production and dye degradation. J. Colloid Interface Sci. 2019, 538, 83–98. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, A.; Tao, H.; Li, R.; Han, J.; Huang, M. Ni-doped hybrids of TiO2 and two-dimensional Ti3C2 MXene for enhanced photocatalytic performance. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 145, 115476. [Google Scholar] [CrossRef]
- Wang, X.; Dai, M.; Chen, Q.; Cheng, K.; Xu, H.; Zhang, J.; Song, H. Enhanced photoelectrochemical performance of NiO-doped TiO2 nanotubes prepared by an impregnation–calcination method. J. Chem. Res. 2021, 45, 1076–1082. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, J.; Xiang, Q.; Li, Y.; Liu, M.; Liao, Y. Transition-Metal-Ion (Fe, Co, Cr, Mn, Etc.) Doping of TiO2 Nanotubes: A General Approach. Inorg. Chem. 2019, 58, 12511–12515. [Google Scholar] [CrossRef]
- Cao, F.; Xiong, J.; Wu, F.; Liu, Q.; Shi, Z.; Yu, Y.; Wang, X.; Li, L. Enhanced Photoelectrochemical Performance from Rationally Designed Anatase/Rutile TiO2 Heterostructures. ACS Appl. Mater. Interfaces 2016, 8, 12239–12245. [Google Scholar] [CrossRef]
- Anitha, V.C.; Goswami, A.; Sopha, H.; Nandan, D.; Gawande, M.B.; Cepe, K.; Ng, S.; Zboril, R.; Macak, J.M. Pt nanoparticles decorated TiO2 nanotubes for the reduction of olefins. Appl. Mater. Today 2018, 10, 86–92. [Google Scholar] [CrossRef]
- Cho, S.; Yim, G.; Park, J.T.; Jang, H. Surfactant-free one-pot synthesis of Au-TiO2 core-shell nanostars by inter-cation redox reaction for photoelectrochemical water splitting. Energy Convers. Manag. 2022, 252, 115038. [Google Scholar] [CrossRef]
- Inoue, K.; Matsuda, A.; Kawamura, G. Tube length optimization of titania nanotube array for efficient photoelectrochemical water splitting. Sci. Rep. 2023, 13, 103. [Google Scholar] [CrossRef]
- Yuan, S.; Xia, M.; Liu, Z.; Wang, K.; Xiang, L.; Huang, G.; Zhang, J.; Li, N. Dual synergistic effects between Co and Mo2C in Co/Mo2C heterostructure for electrocatalytic overall water splitting. Chem. Eng. J. 2022, 430, 132697. [Google Scholar] [CrossRef]
- Yue, X.; Yi, S.; Wang, R.; Zhang, Z.; Qiu, S. A novel architecture of dandelion-like Mo2C/TiO2 heterojunction photocatalysts towards high-performance photocatalytic hydrogen production from water splitting. J. Mater. Chem. A 2017, 5, 10591–10598. [Google Scholar] [CrossRef]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Optimized Spacing between TiO2 Nanotubes for Enhanced Light Harvesting and Charge Transfer. Chemelectrochem 2018, 5, 3183–3190. [Google Scholar] [CrossRef]
- Habibi-Hagh, F.; Foruzin, L.J.; Nasirpouri, F. Remarkable improvement of photoelectrochemical water splitting in pristine and black anodic TiO2 nanotubes by enhancing microstructural ordering and uniformity. Int. J. Hydrogen Energy 2023, 48, 11225–11236. [Google Scholar] [CrossRef]
- Onoda, M. Phase Transitions of Ti3O5. J. Solid State Chem. 1998, 136, 67–73. [Google Scholar] [CrossRef]
- Babu, S.J.; Rao, V.N.; Murthy, D.H.K.; Shastri, M.; Murthy, M.; Shetty, M.; Raju, K.A.; Shivaramu, P.D.; Kumar, C.S.A.; Shankar, M.; et al. Significantly enhanced cocatalyst-free H2 evolution from defect-engineered Brown TiO2. Ceram. Int. 2021, 47, 14821–14828. [Google Scholar] [CrossRef]
- Zhao, P.-F.; Li, G.-S.; Li, W.-L.; Cheng, P.; Pang, Z.-Y.; Xiong, X.-L.; Zou, X.-L.; Xu, Q.; Lu, X.-G. Progress in Ti3O5: Synthesis, properties and applications. Trans. Nonferrous Met. Soc. China 2021, 31, 3310–3327. [Google Scholar] [CrossRef]
- Divyasri, Y.V.; Reddy, N.L.; Lee, K.; Sakar, M.; Rao, V.N.; Venkatramu, V.; Shankar, M.V.; Reddy, N.C.G. Optimization of N doping in TiO2 nanotubes for the enhanced solar light mediated photocatalytic H2 production and dye degradation. Environ. Pollut. 2021, 269, 116170. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Huang, J.; Li, L.; Gu, Y.; Jia, S.; Qiu, R.; Li, S.; Kim, B.H. Homostructured rutile TiO2 nanotree arrays thin film electrodes with nitrogen doping for enhanced photoelectrochemical performance. J. Mater. Sci. Mater. Electron. 2019, 30, 16030–16040. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Huo, J.; Li, Y.; Kang, W.; Zou, C.; Jia, L. Designing novel 0D/1D/2D NiO@La(OH)3/g-C3N4 heterojunction for enhanced photocatalytic hydrogen production. Chem. Eng. J. 2023, 460, 141667. [Google Scholar] [CrossRef]
- Li, Z.; Bian, H.; Xu, Z.; Lu, J.; Li, Y.Y. Solution-Based Comproportionation Reaction for Facile Synthesis of Black TiO2 Nanotubes and Nanoparticles. ACS Appl. Energy Mater. 2020, 3, 6087–6092. [Google Scholar] [CrossRef]
- Wu, C.; Gao, Z.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y. Ti3+ self-doped TiO2 photoelectrodes for photoelectrochemical water splitting and photoelectrocatalytic pollutant degradation. J. Energy Chem. 2016, 25, 726–733. [Google Scholar] [CrossRef]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.Y.; Wang, X. Molybdenum Carbide-Based Electrocatalysts for Hydrogen Evolution Reaction. Chem. Eur. J. 2017, 23, 10947–10961. [Google Scholar] [CrossRef] [PubMed]

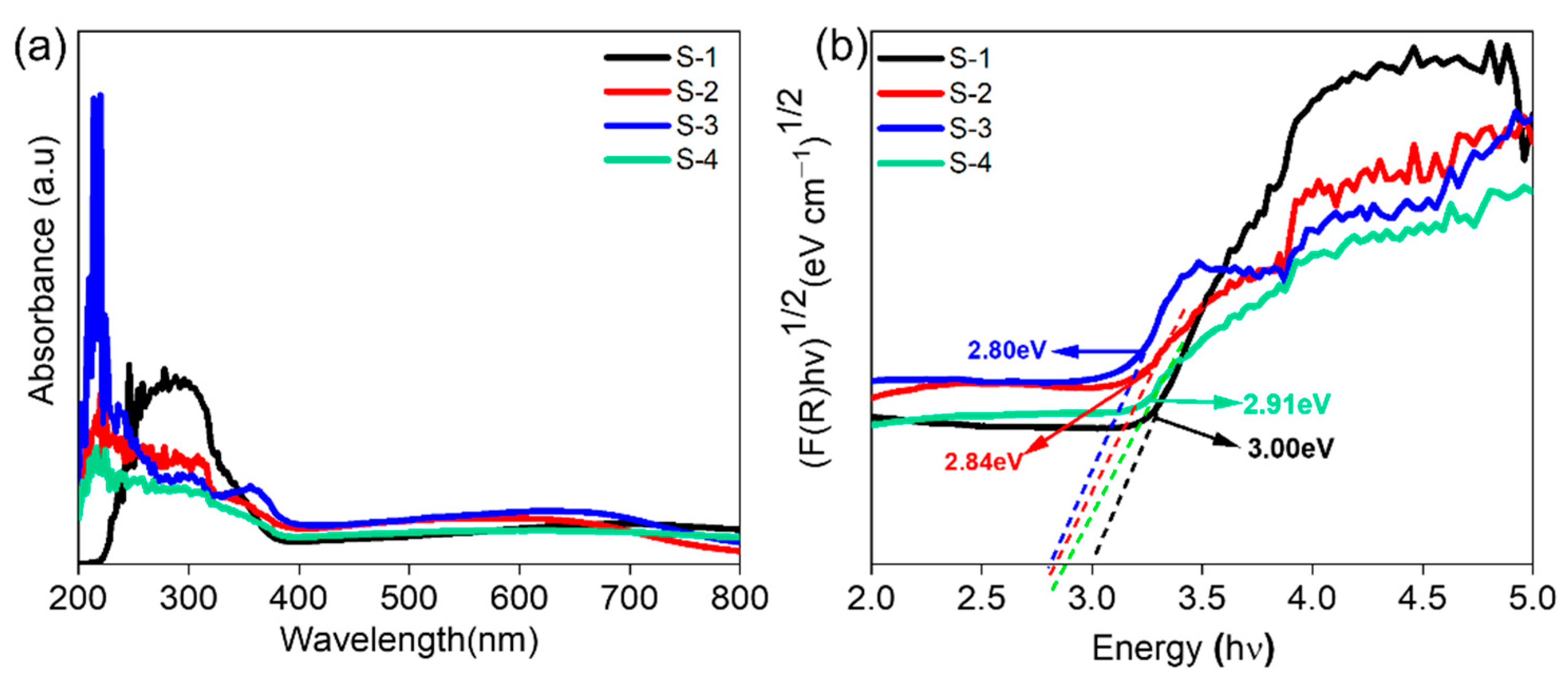
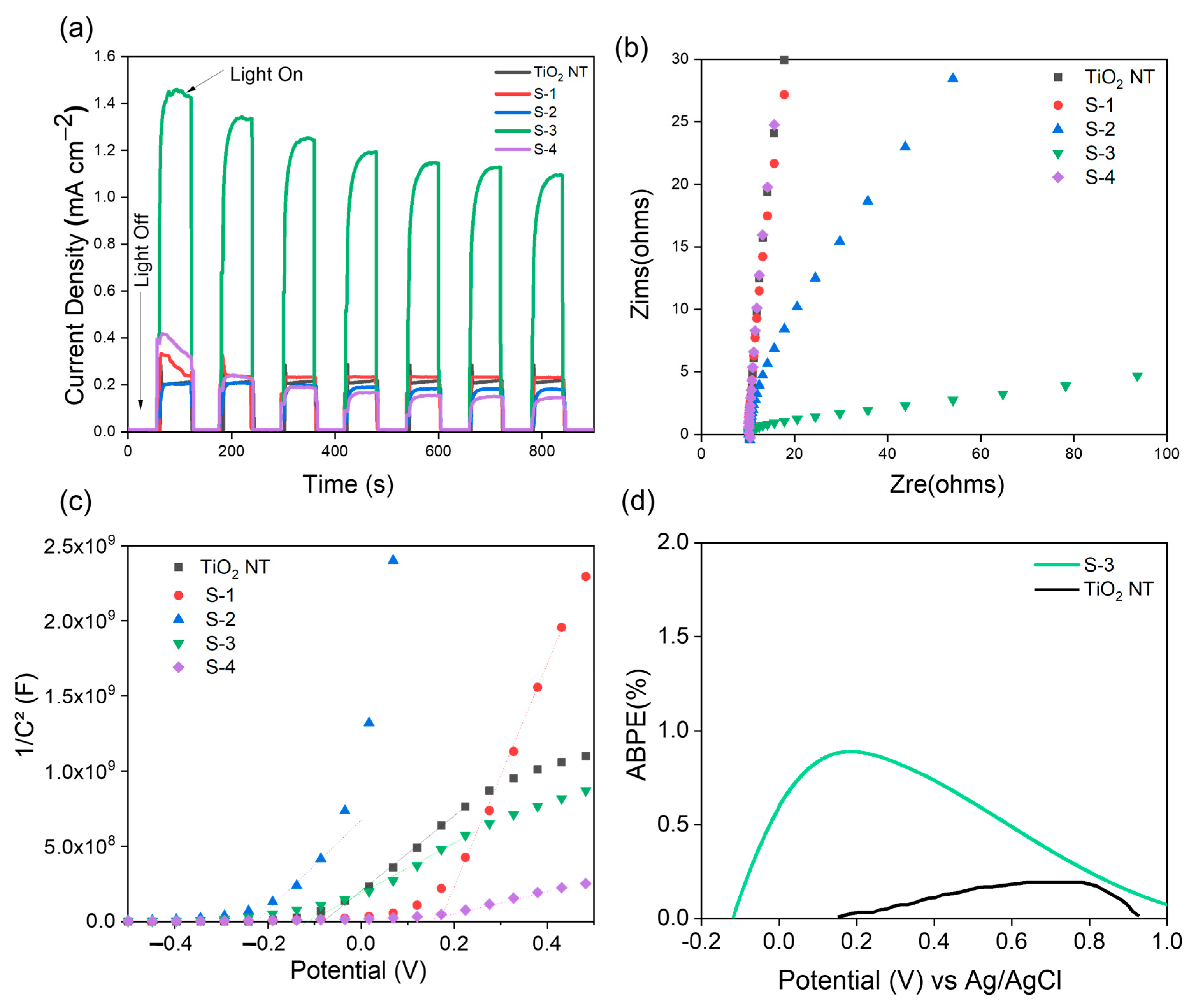
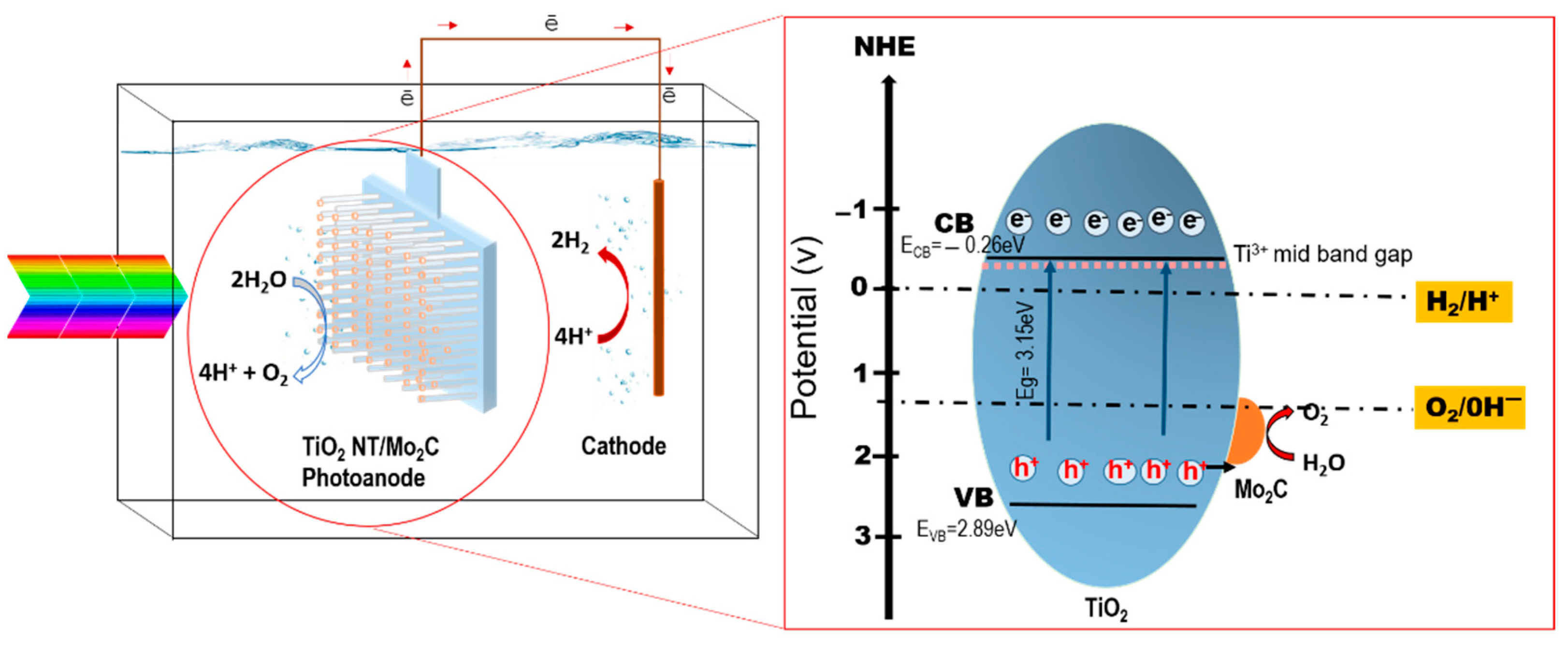
| Sample | ND (×109 cm−3) | Efb (V) | Band Gap (eV) |
|---|---|---|---|
| TiO2 NT | 3.7918 | −0.08 | 3.17 |
| S-3 | 3.2629 | −0.12 | 2.80 |
| Sample Details | Photocurrent Density (mA cm−2) at 1 V vs. Ag/AgCl | Solar to Hydrogen Conversion Efficiency, (η %) |
|---|---|---|
| TiO2 NT | 0.23 | 0.05 |
| S-3 | 1.4 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moridon, S.N.F.; Arifin, K.; Mohamed, M.A.; Minggu, L.J.; Mohamad Yunus, R.; Kassim, M.B. TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties. Materials 2023, 16, 6261. https://doi.org/10.3390/ma16186261
Moridon SNF, Arifin K, Mohamed MA, Minggu LJ, Mohamad Yunus R, Kassim MB. TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties. Materials. 2023; 16(18):6261. https://doi.org/10.3390/ma16186261
Chicago/Turabian StyleMoridon, Siti Nurul Falaein, Khuzaimah Arifin, Mohamad Azuwa Mohamed, Lorna Jeffery Minggu, Rozan Mohamad Yunus, and Mohammad B. Kassim. 2023. "TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties" Materials 16, no. 18: 6261. https://doi.org/10.3390/ma16186261
APA StyleMoridon, S. N. F., Arifin, K., Mohamed, M. A., Minggu, L. J., Mohamad Yunus, R., & Kassim, M. B. (2023). TiO2 Nanotubes Decorated with Mo2C for Enhanced Photoelectrochemical Water-Splitting Properties. Materials, 16(18), 6261. https://doi.org/10.3390/ma16186261








