Influence of Solid Solutions on the Al2024 High-Temperature Deformation Behavior
Abstract
:1. Introduction
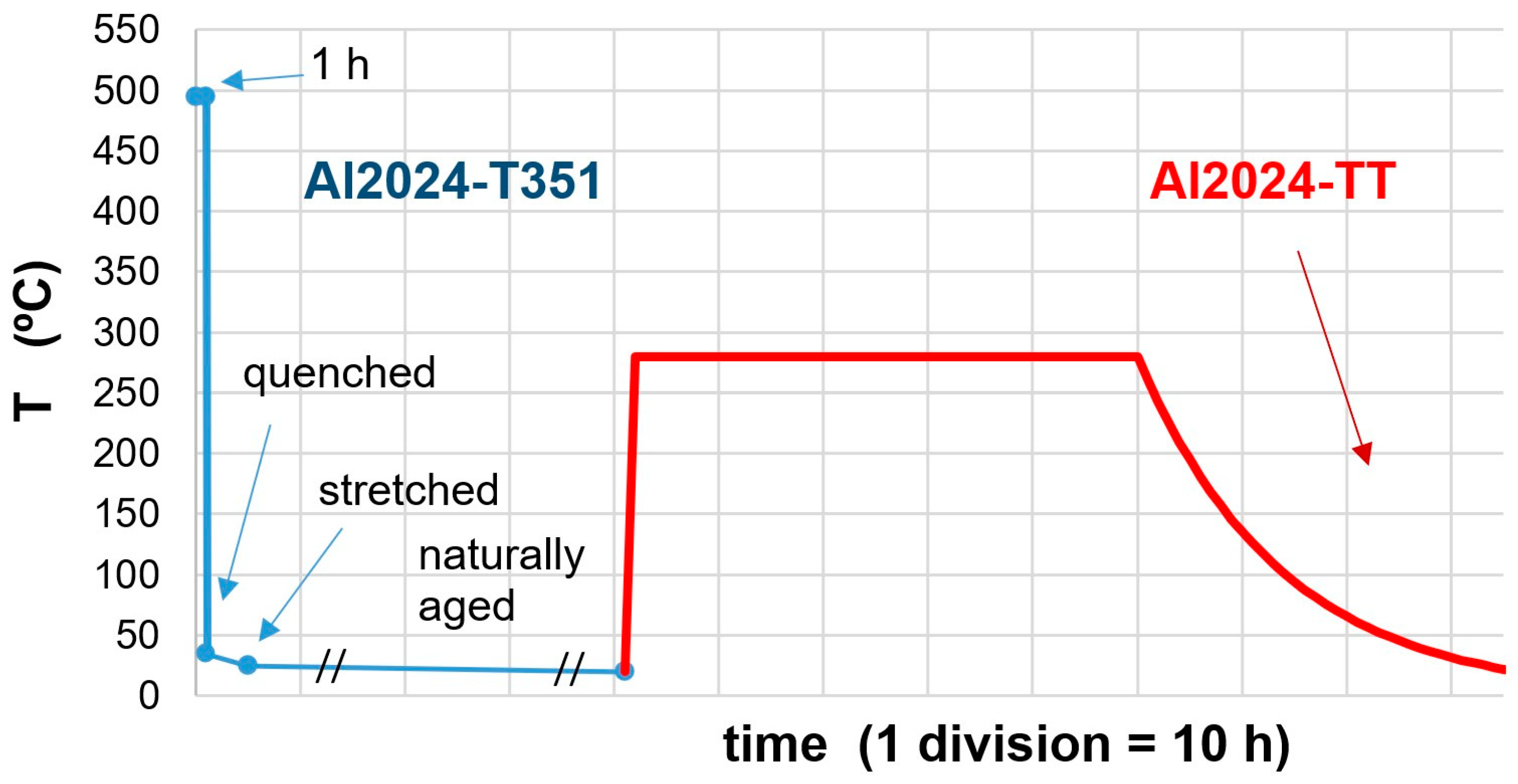
2. Materials and Methods
3. Results and Discussion
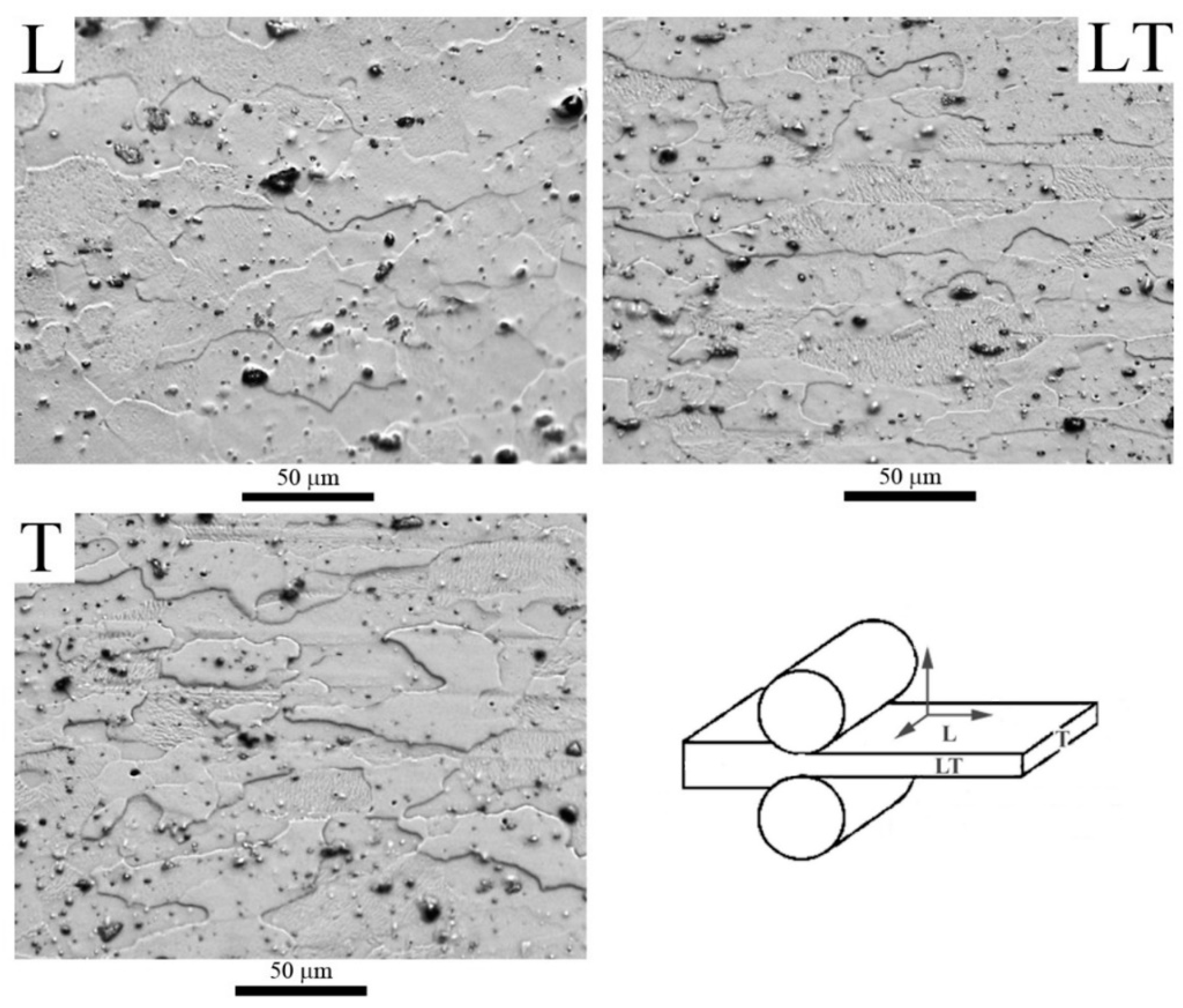
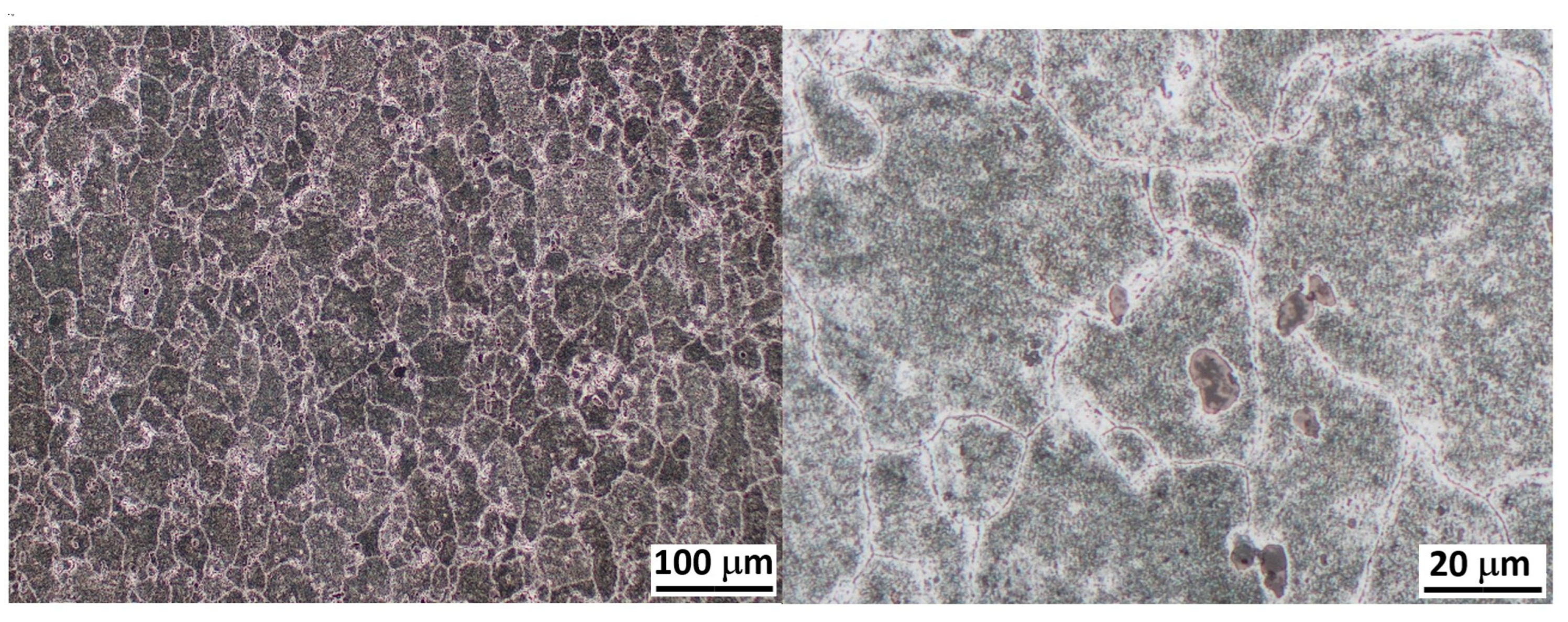
3.1. Microstructures
3.2. Ultramicrohardness
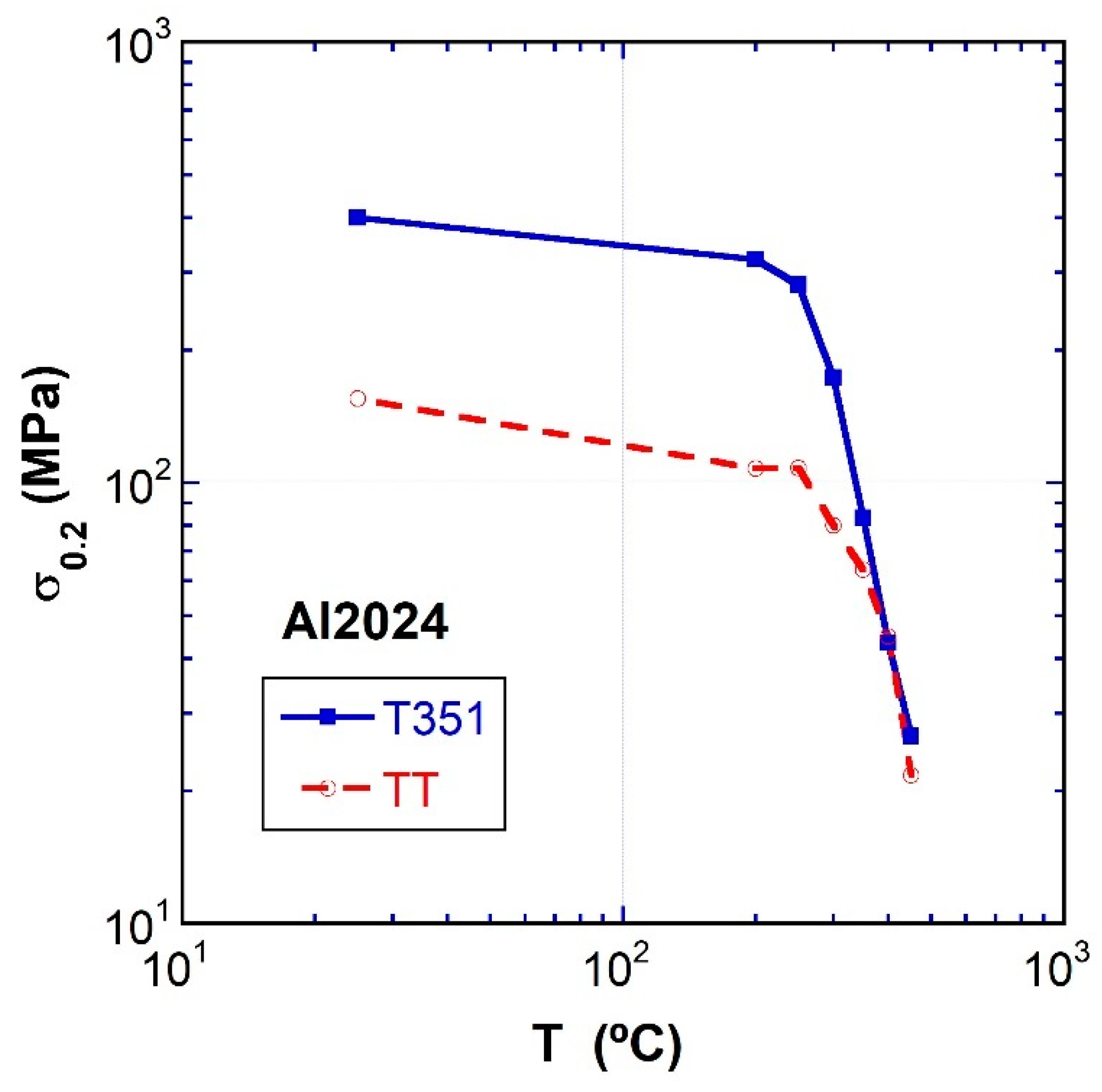
3.3. Tensile Tests at Intermediate and High Temperatures
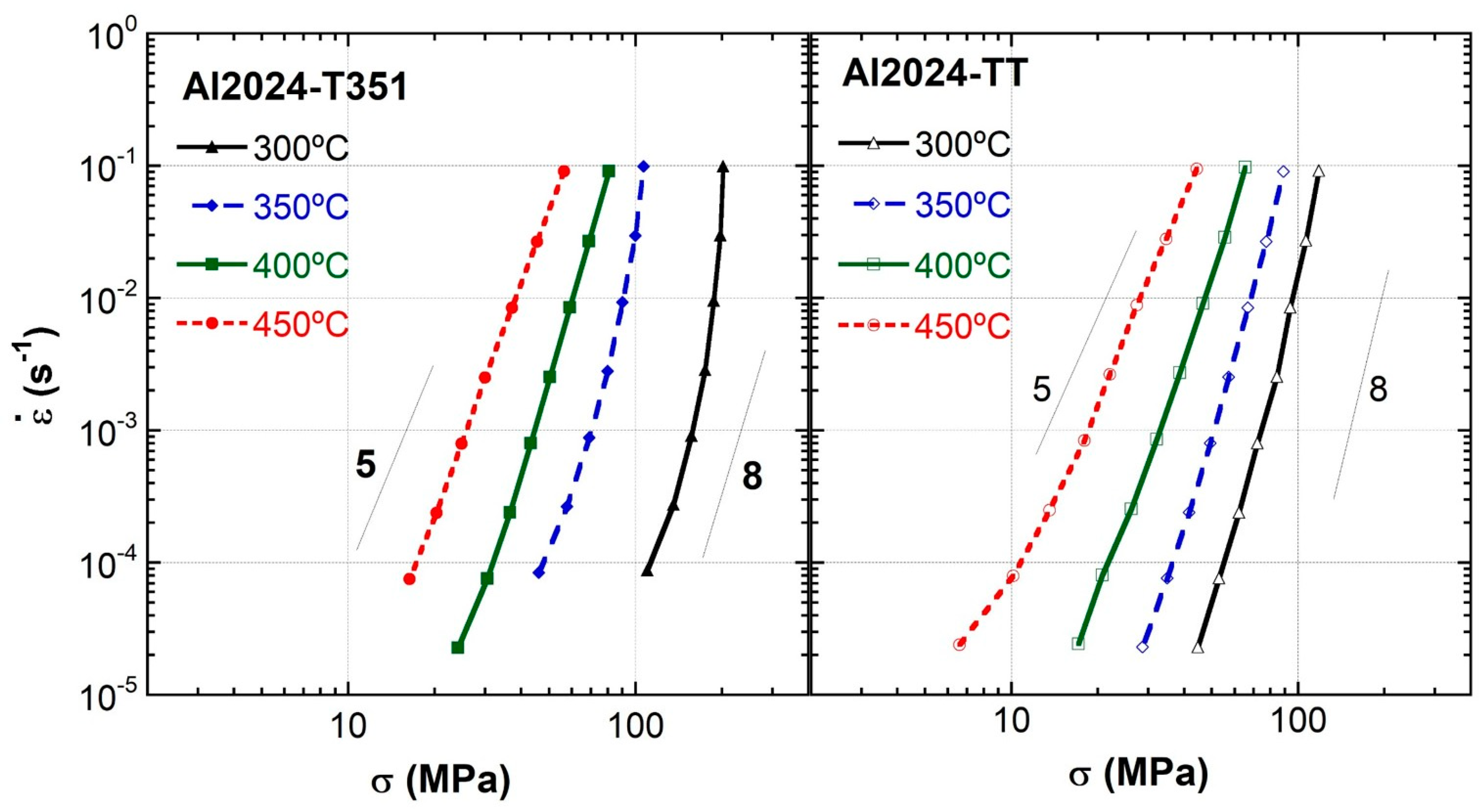
3.4. Strain Rate Change Tensile Tests
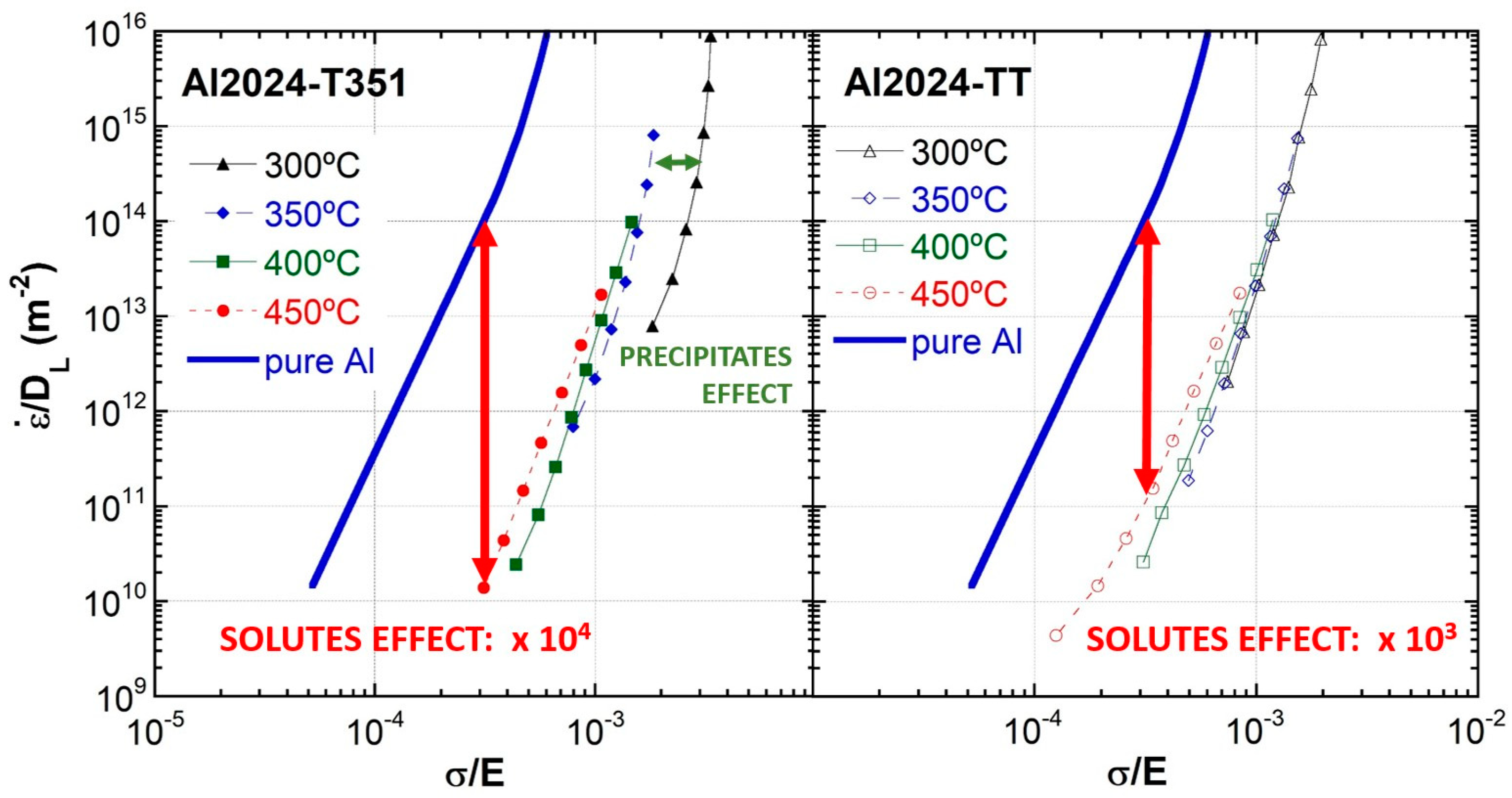
3.5. Deformation Mechanisms
4. Conclusions
- (1)
- Minimum hardness is reached for the Al2024 TT temper after a very slow cooling rate in the furnace, which results in coarse precipitates and reduction of solid solution atoms.
- (2)
- The flow stress is higher for the T351 temper at all temperatures. A strong decrease occurs at temperatures higher than 300 °C for both materials. In contrast, the elongation to failure for the TT temper is higher than for the T351 temper, and a strong increase occurs for both materials above 300 °C.
- (3)
- Differences in flow rate at a given stress are observed at all temperatures for the T351 and TT tempers and are especially high compared with pure aluminum, being about 3 and 4 orders of magnitude at the highest temperatures for which slip creep is the rate-controlling mechanism. At these high temperatures, most precipitates have dissolved, and the main reinforcing mechanism is attributed to solutes in solid solutions.
- (4)
- The differences in high-temperature flow rate between Al2024 T351, Al2024 TT tempers, and pure aluminum are attributed to the influence of solutes in solid solutions on both glide and climb processes. Solutes in solid solutions affect stacking fault energies (SFE) and decrease both glide and climb rates for a given stress. However, the SFE alone does not explain the differences among the three materials.
- (5)
- The synergic effect of various solutes and their influence on both glide and climb processes is important in this alloy and should be further investigated in other materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.C.; Li, Q.-F.; Xia, Y.-C.; Li, L.-T. A phenomenological constitutive model for high temperature flow stress prediction of Al–Cu–Mg alloy. Mater. Sci. Eng. A 2012, 534, 654–662. [Google Scholar] [CrossRef]
- Lin, Y.C.; Xia, Y.-C.; Jiang, Y.-Q.; Zhou, H.-M.; Li, L.-T. Precipitation hardening of 2024-T3 aluminum alloy during creep aging. Mater. Sci. Eng. A 2013, 565, 420–429. [Google Scholar] [CrossRef]
- Bai, S.; Yi, X.; Liu, Z. On the role of Ag additions on the initial solute hardening and competitive precipitation of Al-Cu-Mg alloys. J. Alloys Compd. 2023, 945, 169339. [Google Scholar] [CrossRef]
- Farajollahi, R.; Jamshidi Aval, H.; Jamaati, R.; Hájovská, Z.; Nagy, Š. Effects of pre- and post-friction surfacing heat treatment on microstructure and corrosion behavior of nickel-aluminide reinforced Al-Cu-Mg alloy. J. Alloys Compd. 2022, 906, 164211. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Z.; Bai, S.; Ou, L.; Zhang, J.; He, G.; Zhao, J. Effect of rolling temperature on mechanical properties and corrosion resistance of Al-Cu-Mg-Ag alloy. J. Alloys Compd. 2022, 897, 163168. [Google Scholar] [CrossRef]
- He, G.; Liu, Z.; Liu, F. Effects of dislocation slip behaviour and second-phase particles on hot rolled texture of an Al-Cu-Mg alloy with a high Cu/Mg ratio. J. Alloys Compd. 2022, 911, 165085. [Google Scholar] [CrossRef]
- Ozdemir, F.; Witharamage, C.S.; Darwish, A.A.; Okuyucu, H.; Gupta, R.K. Corrosion behavior of age hardening aluminum alloys produced by high-energy ball milling. J. Alloys Compd. 2022, 900, 163488. [Google Scholar] [CrossRef]
- Sun, T.; Chen, J.; Wu, Y.; Wang, M.; Fu, Y.; Wang, H.; Wang, H. Achieving excellent strength of the LPBF additively manufactured Al–Cu–Mg composite via in-situ mixing TiB2 and solution treatment. Mater. Sci. Eng. A 2022, 850, 143531. [Google Scholar] [CrossRef]
- Farajollahi, R.; Jamshidi Aval, H.; Jamaati, R. Effects of Ni on the microstructure, mechanical and tribological properties of AA2024-Al3NiCu composite fabricated by stir casting process. J. Alloys Compd. 2021, 887, 161433. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, S.; Li, X.; Yan, H.; Yi, D.; Wang, J.; Yang, X.; Liu, B.; Xu, H.; Bai, P. Composition design method of Al-Cu alloy for laser powder bed fusion. J. Alloys Compd. 2022, 914, 165298. [Google Scholar] [CrossRef]
- Ma, S.; Li, Y.; Kan, W.; Zhang, J.; Wang, M.; Wang, L.; Wu, Y.; Wang, H.; Chen, Z. Enhancement of grain refinement and heat resistance in TiB2-reinforced Al-Cu-Mg-Fe-Ni matrix composite additive manufactured by electron beam melting. J. Alloys Compd. 2022, 924, 166395. [Google Scholar] [CrossRef]
- Song, Y.; Du, W.; Zhao, L.; Zeng, L.; Liu, W.; Chen, Y.; Zhu, B.; Zhang, X.; Ding, X. The coupling influences and corresponding mechanisms of high efficiency thermal-magnetic treatments on the dimensional stability of Al-Cu-Mg alloy. J. Alloys Compd. 2022, 928, 167187. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.; Kang, N.; Wang, Z.; Wang, Q.; Liu, Y.; Huang, W. Laser powder bed fusion of Zr-modified Al–Cu–Mg alloy: Crack-inhibiting, grain refinement, and mechanical properties. Mater. Sci. Eng. A 2022, 838, 142618. [Google Scholar] [CrossRef]
- Esin, V.A.; Briez, L.; Sennour, M.; Köster, A.; Gratiot, E.; Crépin, J. Precipitation-hardness map for Al–Cu–Mg alloy (AA2024-T3). J. Alloys Compd. 2021, 854, 157164. [Google Scholar] [CrossRef]
- Zhu, Z.; Qin, R.; Sun, Y.; Tang, J.; Jiang, F.; You, C. Improvement in Mechanical Properties of Al2024 Alloy Using Mechanical Working and Heat Treatment. Materials 2023, 16, 5568. [Google Scholar] [CrossRef]
- Ruano, O.A.; Sherby, O.D. On constitutive equations for various diffusion-controlled creep mechanisms. Rev. Phys. Appliquée 1988, 23, 625–637. [Google Scholar] [CrossRef]
- Sherby, O.D.; Burke, P.M. Mechanical behavior of crystalline solids at elevated temperature. Prog. Mater. Sci. 1968, 13, 323–390. [Google Scholar] [CrossRef]
- Barrett, C.R.; Ardell, A.J.; Sherby, O.D. Influence of modulus on the temperature dependence of the activation energy for creep at high temperatures. Trans. AIME 1964, 230, 200–212. [Google Scholar]
- Pahutová, M.; Hostinský, T.; Čadek, J. On the influence of stacking fault energy on high temperature creep of Cu-Al solid solutions. Scr. Metall. 1969, 3, 293–296. [Google Scholar] [CrossRef]
- Burton, B. The influence of stacking fault energy on creep. Acta Metall. 1982, 30, 905–910. [Google Scholar] [CrossRef]
- Chaudhury, P.K.; Mohamed, F.A. Creep and ductility in an Al-Cu solid-solution alloy. Metall. Trans. A 1987, 18, 2105–2114. [Google Scholar] [CrossRef]
- Soliman, M.S. Effect of Cu concentration on the high-temperature creep behavior of Al-Cu solid solution alloys. Mater. Sci. Eng. A 1995, 201, 111–117. [Google Scholar] [CrossRef]
- Venables, J.A. The electron microscopy of deformation twinning. J. Phys. Chem. Solids 1964, 25, 685–692. [Google Scholar] [CrossRef]
- Thornton, P.R.; Mitchell, T.E.; Hirsch, P.B. The dependence of cross-slip on stacking-fault energy in face-centred cubic metals and alloys. Philos. Mag. 1962, 7, 1349–1369. [Google Scholar] [CrossRef]
- Barrett, C.R.; Sherby, O.D. Influence of stacking-fault energy on high-temperature creep of pure meals. Trans. AIME 1965, 233, 1116–1119. [Google Scholar]
- Davies, C.K.L.; Davies, P.W.; Wilshire, B. The effect of variations in stacking-fault energy on the creep of nickel-cobalt alloys. Philos. Mag. 1965, 12, 827–839. [Google Scholar] [CrossRef]
- Singh Deo, N.N.; Barrett, C.R. High-temperature creep of some dilute copper silicon alloys. Trans. AIME 1969, 245, 2467–2473. [Google Scholar]
- Vandervoort, R.R. On the possible influence of stacking fault energy on the creep of pure BCC metals. Trans. AIME 1969, 245, 2269–2272. [Google Scholar]
- Shalayev, V.I.; Tkachenko, I.B.; Pavlov, V.A. Influence of stacking fault energy on creep of FCC Metals. Phys. Met. Met. Engl. Trans. 1969, 27, 136–143. [Google Scholar]
- Oikawa, H.; Karashima, S. Effect of stacking-fault energy on steady-state creep rates in copper-base solid solutions. Scr. Metall. 1971, 5, 909–914. [Google Scholar] [CrossRef]
- Johnson, W.R.; Barrett, C.R.; Nix, W.D. The high-temperature creep behavior of nickel-rich Ni-W solid solutions. Metall. Trans. 1972, 3, 963–969. [Google Scholar] [CrossRef]
- Kozyrskiy, G.Y.; Okrainets, P.N.; Pischak, V.K. Dependence of the structural changes and mechanical properties of metals with an F. C. C. lattice on the energy of stacking faults. Phys. Met. Met. Engl. Trans. 1972, 33, 173–178. [Google Scholar]
- Mohamed, F.A.; Langdon, T.G. The transition from dislocation climb to viscous glide in creep of solid solution alloys. Acta Metall. 1974, 22, 779–788. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, Y.; Shih, C. High Temperature Creep of Ni-Cr-Co Alloys and the Effect of Stacking Fault Energy. Int. J. Mater. Res. 1987, 78, 339–343. [Google Scholar] [CrossRef]
- Weertman, J. Theory of the influence of stacking-fault width of split dislocations on high-temperature creep rate. Trans. AIME 1965, 233, 2069–2075. [Google Scholar]
- Argon, A.S.; Moffatt, W.C. Climb of extended edge dislocations. Acta Metall. 1981, 29, 293–299. [Google Scholar] [CrossRef]
- Kong, Q.P.; Li, Y. Investigation of the climb of extended dislocations during high-temperature creep. Philos. Mag. A 1993, 68, 113–119. [Google Scholar] [CrossRef]
- Argon, A.S.; Takeuchi, S. Internal stresses in power-law creep. Acta Metall. 1981, 29, 1877–1884. [Google Scholar] [CrossRef]
- Orozco-Caballero, A.; Álvarez-Leal, M.; Carreño, F.; Ruano, O.A. Superplastic Behavior of Overaged 2024 Aluminum Alloy after Friction Stir Processing. Metals 2022, 12, 1880. [Google Scholar] [CrossRef]
- Totten, G.E.; MacKenzie, D.S. Handbook of Aluminum Vol. 1 Physical Metallurgy and Processes; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Genevois, C.; Deschamps, A.; Denquin, A.; Doisneaucottignies, B. Quantitative investigation of precipitation and mechanical behaviour for AA2024 friction stir welds. Acta Mater. 2005, 53, 2447–2458. [Google Scholar] [CrossRef]
- Álvarez-Leal, M. Superplasticidad de las Aleaciones Aeronáuticas de Aluminio 2024 y Magnesio WE54 Mediante Procesado Severo por Fricción-Agitación (FSP). Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2019. [Google Scholar]
- Orozco-Caballero, A. Optimización de propiedades mecánicas de las aleaciones de aluminio Al-7%Si y Al7075 mediante deformación plástica severa: Procesado por fricción batida (FSP) y extrusión en canal angular constante (ECAP). Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Glenn, A.M.; Hughes, A.E.; Torpy, A.; Nolze, G.; Birbilis, N. Defect density associated with constituent particles in AA2024-T3 and its role in corrosion. Surf. Interface Anal. 2016, 48, 780–788. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, G.; Cao, Y.; Zhu, Y.; Huang, X.; Zhou, Y.; Li, Q.; Zeng, Q.; Liu, Q. Microstructure evolution of thermo-mechanically affected zone in dissimilar AA2024/7075 joint produced by friction stir welding. Vacuum 2020, 179, 109515. [Google Scholar] [CrossRef]
- Shih, H.-C.; Ho, N.-J.; Huang, J.C. Precipitation behaviors in Al-Cu-Mg and 2024 aluminum alloys. Metall. Mater. Trans. A 1996, 27, 2479–2494. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Hidalgo, P.; Carsí, M.; Ruano, O.A.; Carreño, F. Microstructural characterization by electron backscatter diffraction of a hot worked Al-Cu-Mg alloy. Mater. Sci. Eng. A 2011, 528, 3161–3168. [Google Scholar] [CrossRef]
- Shin, D.; Roy, S.; Watkins, T.R.; Shyam, A. Lattice mismatch modeling of aluminum alloys. Comput. Mater. Sci. 2017, 138, 149–159. [Google Scholar] [CrossRef]
- Carreño, F.; Ruano, O.A. Separated contribution of particles and matrix on the creep behavior of dispersion strengthened materials. Acta Mater. 1998, 46, 159–167. [Google Scholar] [CrossRef]
- Carreño, F.; Ruano, O.A. High-temperature deformation behavior of an Al-8.4Fe-3.6Ce dispersion-strengthened material. Metall. Mater. Trans. A 1999, 30, 371–376. [Google Scholar] [CrossRef]
- Servi, I.S.; Grant, N.J. Creep and Stress Rupture Behavior of Aluminum As a Function of Purity. JOM 1951, 3, 909–916. [Google Scholar] [CrossRef]
- Luthy, H.; Miller, K.; Sherby, O.D. The stress and temperature dependence of steady-state flow at intermediate temperatures for pure polycrystalline aluminium. Acta Metall. 1980, 28, 169–178. [Google Scholar] [CrossRef]
- Rohatgi, A.; Vecchio, K.S.; Gray, G.T. The influence of stacking fault energy on the mechanical behavior of Cu and Cu-Al alloys: Deformation twinning, work hardening, and dynamic recovery. Metall. Mater. Trans. A 2001, 32, 135–145. [Google Scholar] [CrossRef]
- Muzyk, M.; Pakiela, Z.; Kurzydlowski, K.J. Ab initio calculations of the generalized stacking fault energy in aluminium alloys. Scr. Mater. 2011, 64, 916–918. [Google Scholar] [CrossRef]
- Muzyk, M.; Pakieła, Z.; Kurzydłowski, K.J. Generalized Stacking Fault Energies of Aluminum Alloys–Density Functional Theory Calculations. Metals 2018, 8, 823. [Google Scholar] [CrossRef]
- Liu, L.H.; Chen, J.H.; Fan, T.W.; Liu, Z.R.; Zhang, Y.; Yuan, D.W. The possibilities to lower the stacking fault energies of aluminum materials investigated by first-principles energy calculations. Comput. Mater. Sci. 2015, 108A, 136–146. [Google Scholar] [CrossRef]
- Liu, L.-H.; Fan, T.-W.; Wu, C.-L.; Xie, P.; Yuan, D.-W.; Chen, J.-H. Synergistic Effect of Alloying Atoms on In-trinsic Stacking-Fault Energy in Austenitic Steels. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 272–279. [Google Scholar] [CrossRef]
- Raj, S.V.; Iskovitz, I.S.; Freed, A.D. Chapter 8. Modeling the role of dislocation substructure during Class M and exponential creep. In Unified Constitutive Laws of Plastic Deformation; Academic Press: San Diego, CA, USA, 1996; pp. 343–439. [Google Scholar]
- Kritzinger, S.; Dobson, P.S.; Smallman, R.E. The influence of a dilute magnesium addition on the growth ant shrinkage of dislocation loops in aluminium. Philos. Mag. 1967, 16, 217–229. [Google Scholar] [CrossRef]
- Ruano, O.A.; Álvarez-Leal, M.; Orozco-Caballero, A.; Carreño, F. Large elongations in WE54 magnesium alloy by solute-drag creep controlling the deformation behavior. Mater. Sci. Eng. A 2020, 791, 139757. [Google Scholar] [CrossRef]
- Ruano, O.A.; Álvarez-Leal, M.; Orozco-Caballero, A.; Carreño, F. Superplasticity of a friction stir processed overaged WE54 magnesium alloy. J. Magnes. Alloys 2022, 10, 3156–3166. [Google Scholar] [CrossRef]
- Taleff, E.M.; Henshall, G.A.; Nieh, T.G.; Lesuer, D.R.; Wadsworth, J. Warm-temperature tensile ductility in Al−Mg alloys. Metall. Mater. Trans. A 1998, 29, 1081–1091. [Google Scholar] [CrossRef]



| Element | Cu | Mg | Mn | Si | Fe | Zn | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | 4.3 | 1.5 | 0.6 | <0.5 | <0.5 | 0.15 | 0.03 | 0.007 | balance |
| Al2024-T351 | Al2024-TT | |
|---|---|---|
| H (GPa): | 1.34 ± 0.02 | 0.65 ± 0.02 |
| T (°C) | σ0.2 (MPa) | σmax (MPa) | eu (%) | eF (%) | |
|---|---|---|---|---|---|
| Al2024-T351 | 25 | 398.8 | 606.2 | 17.3 | 28.3 |
| 200 | 320.9 | 433.5 | 16.0 | 24.1 | |
| 250 | 280.9 | 295.9 | 3.5 | 24.4 | |
| 300 | 173.3 | 189.7 | 3.9 | 33.6 | |
| 350 | 83.1 | 97.2 | 4.3 | 58.6 | |
| 400 | 43.4 | 53.8 | 7.9 | 107.4 | |
| 450 | 26.6 | 32.5 | 24.5 | 122.2 | |
| Al2024-TT | 25 | 154.5 | 279.3 | 14.8 | 26.9 |
| 200 | 107.6 | 158.3 | 10.7 | 57.6 | |
| 250 | 108.1 | 131.1 | 7.4 | 57.2 | |
| 300 | 79.6 | 89.1 | 5.3 | 62.3 | |
| 350 | 63.5 | 69.6 | 5.0 | 79.9 | |
| 400 | 44.8 | 48.7 | 5.8 | 91.6 | |
| 450 | 21.7 | 30.5 | 28.2 | 98.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruano, O.A.; Orozco-Caballero, A.; Álvarez-Leal, M.; Carreño, F. Influence of Solid Solutions on the Al2024 High-Temperature Deformation Behavior. Materials 2023, 16, 6251. https://doi.org/10.3390/ma16186251
Ruano OA, Orozco-Caballero A, Álvarez-Leal M, Carreño F. Influence of Solid Solutions on the Al2024 High-Temperature Deformation Behavior. Materials. 2023; 16(18):6251. https://doi.org/10.3390/ma16186251
Chicago/Turabian StyleRuano, Oscar A., Alberto Orozco-Caballero, Marta Álvarez-Leal, and Fernando Carreño. 2023. "Influence of Solid Solutions on the Al2024 High-Temperature Deformation Behavior" Materials 16, no. 18: 6251. https://doi.org/10.3390/ma16186251
APA StyleRuano, O. A., Orozco-Caballero, A., Álvarez-Leal, M., & Carreño, F. (2023). Influence of Solid Solutions on the Al2024 High-Temperature Deformation Behavior. Materials, 16(18), 6251. https://doi.org/10.3390/ma16186251








