The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
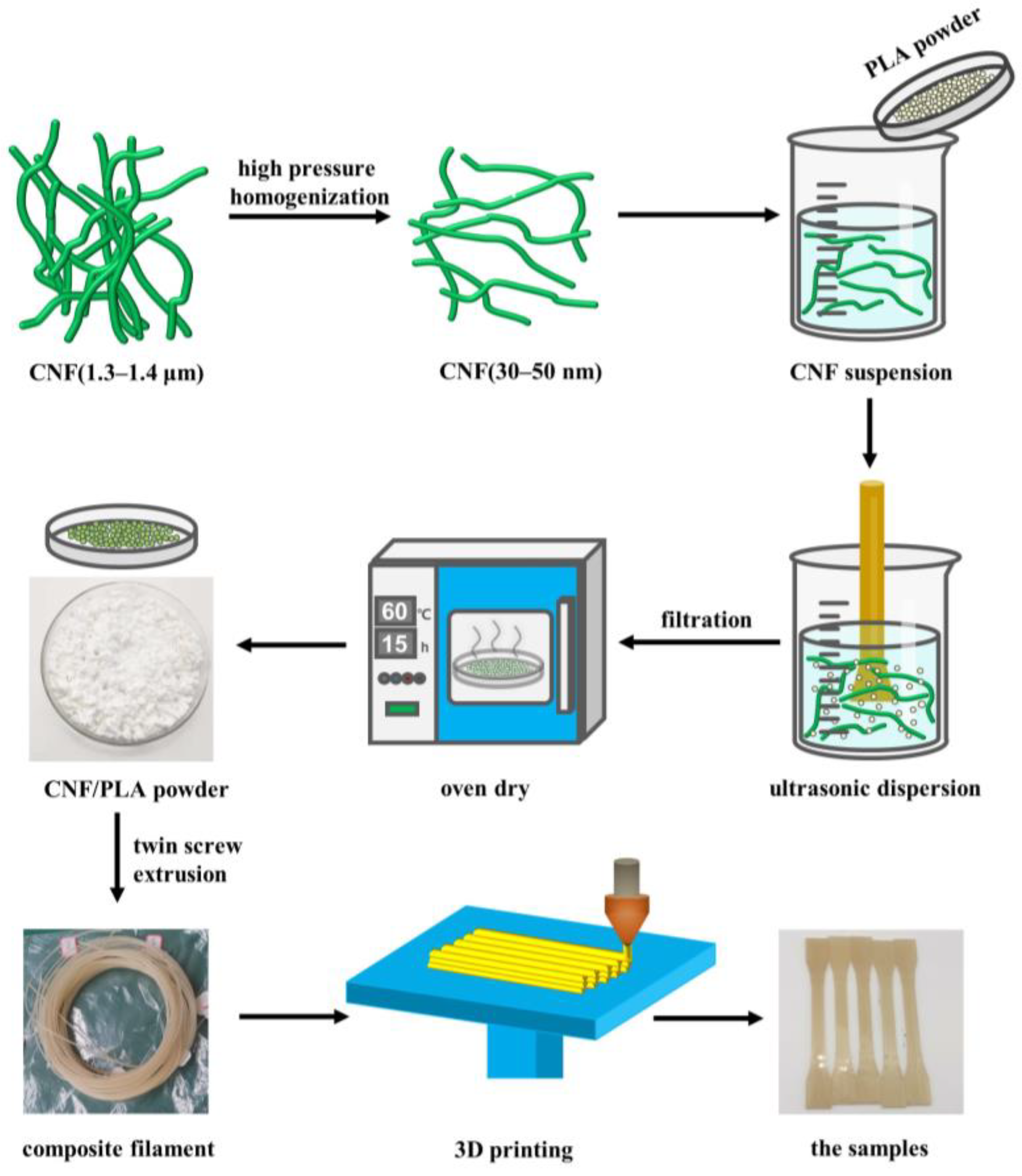
2.2. Preparation of CNF/PLA Composites
2.3. In Vitro Degradation
2.4. Characterization
3. Results and Discussion
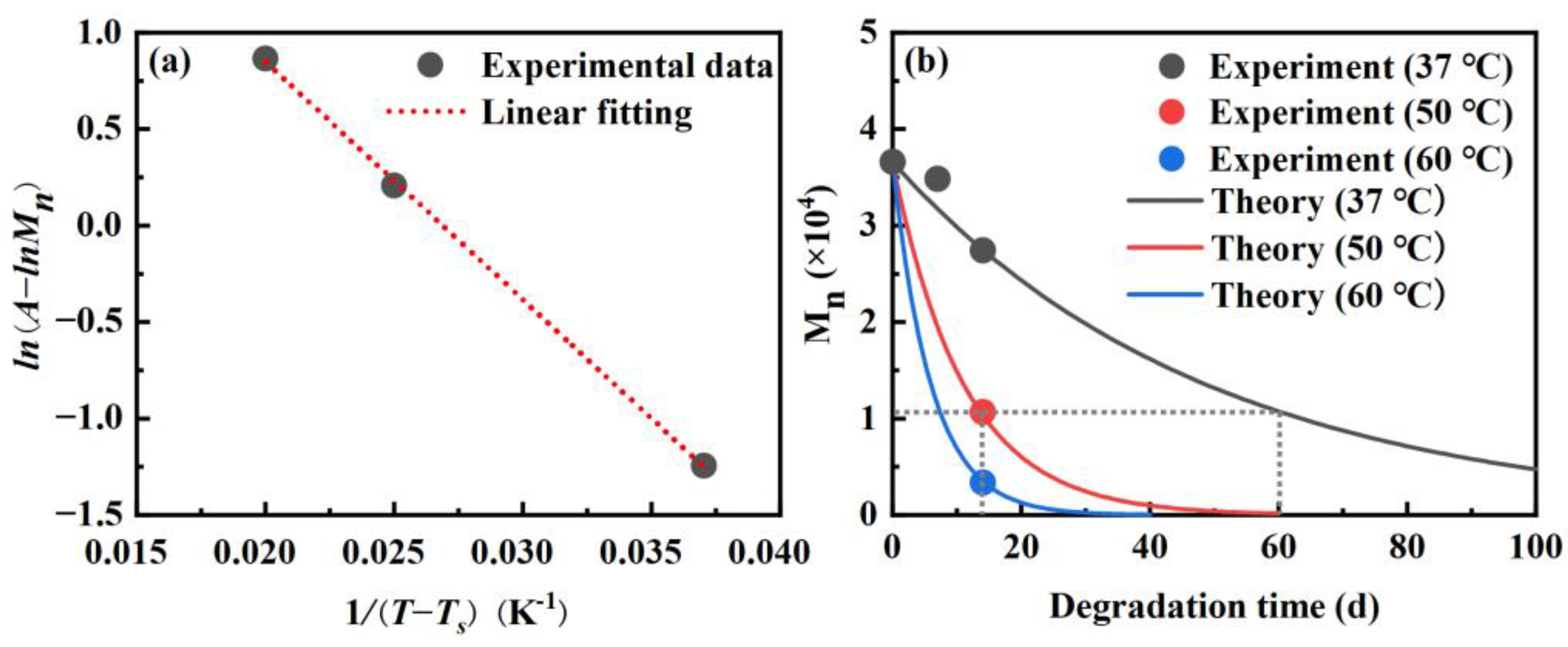
3.1. Molecular Weight Analysis
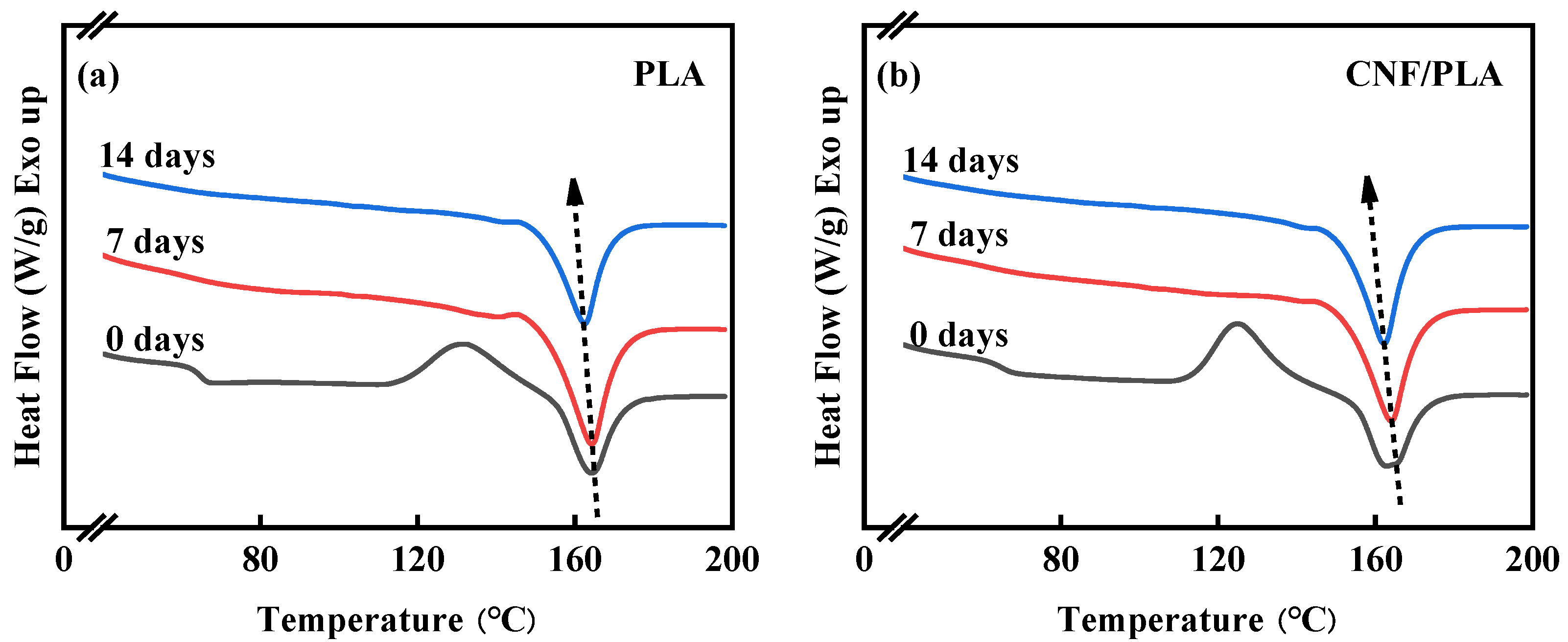
3.2. Thermal Properties Analysis
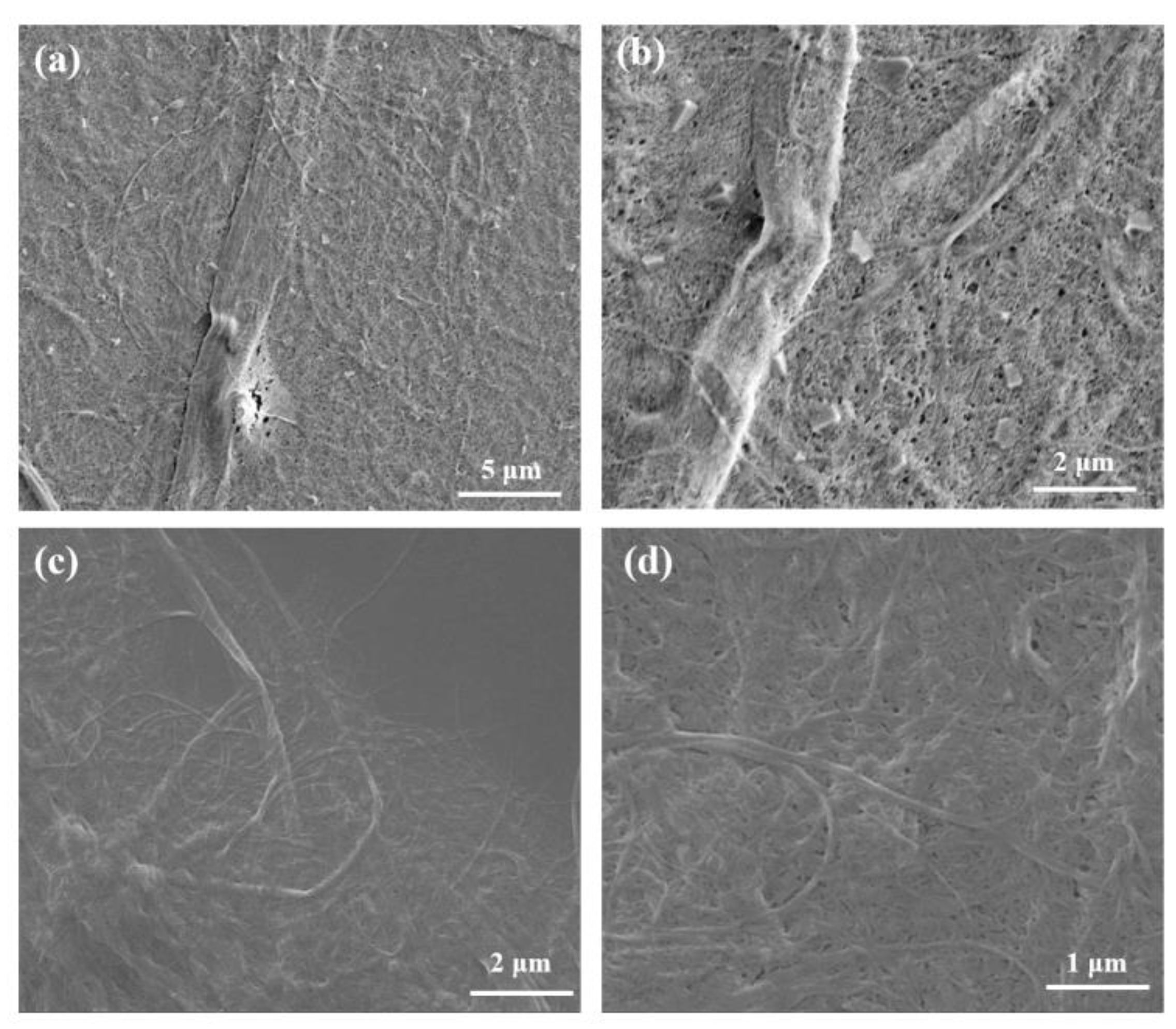
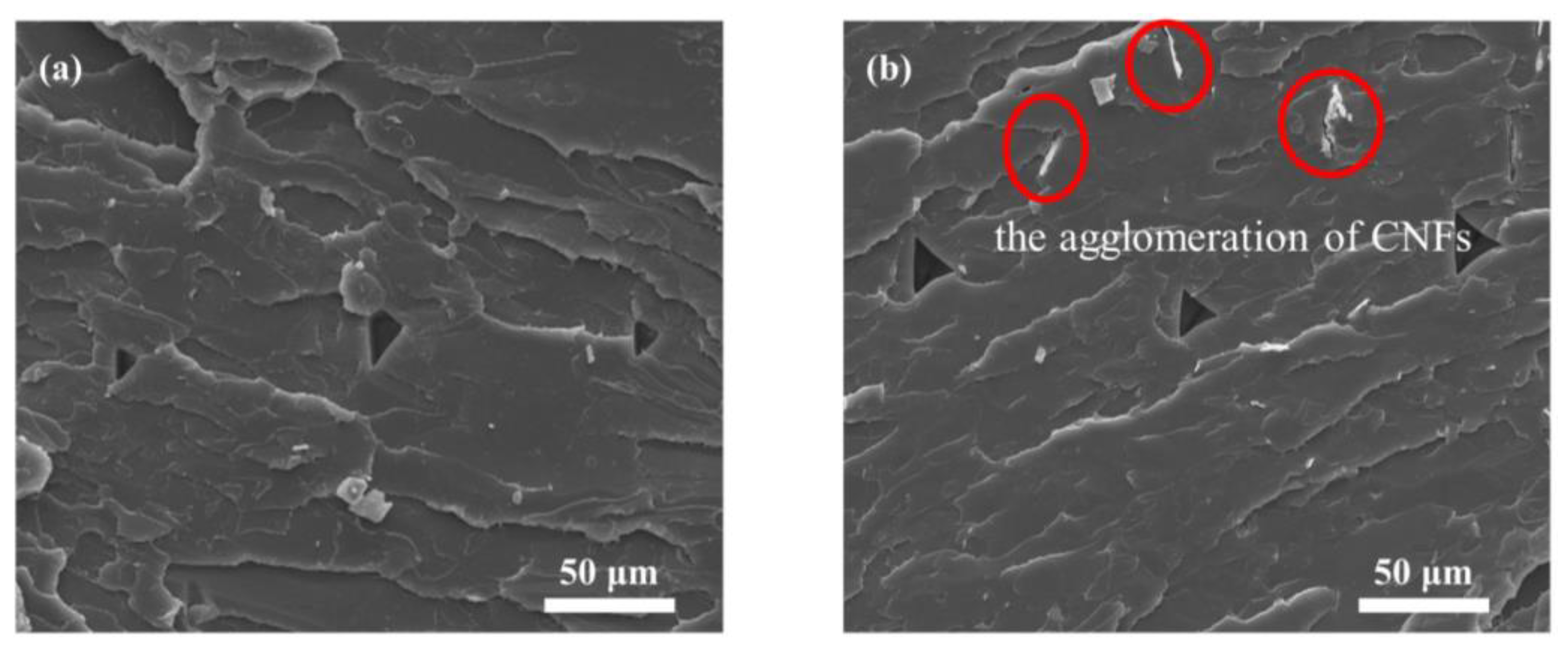
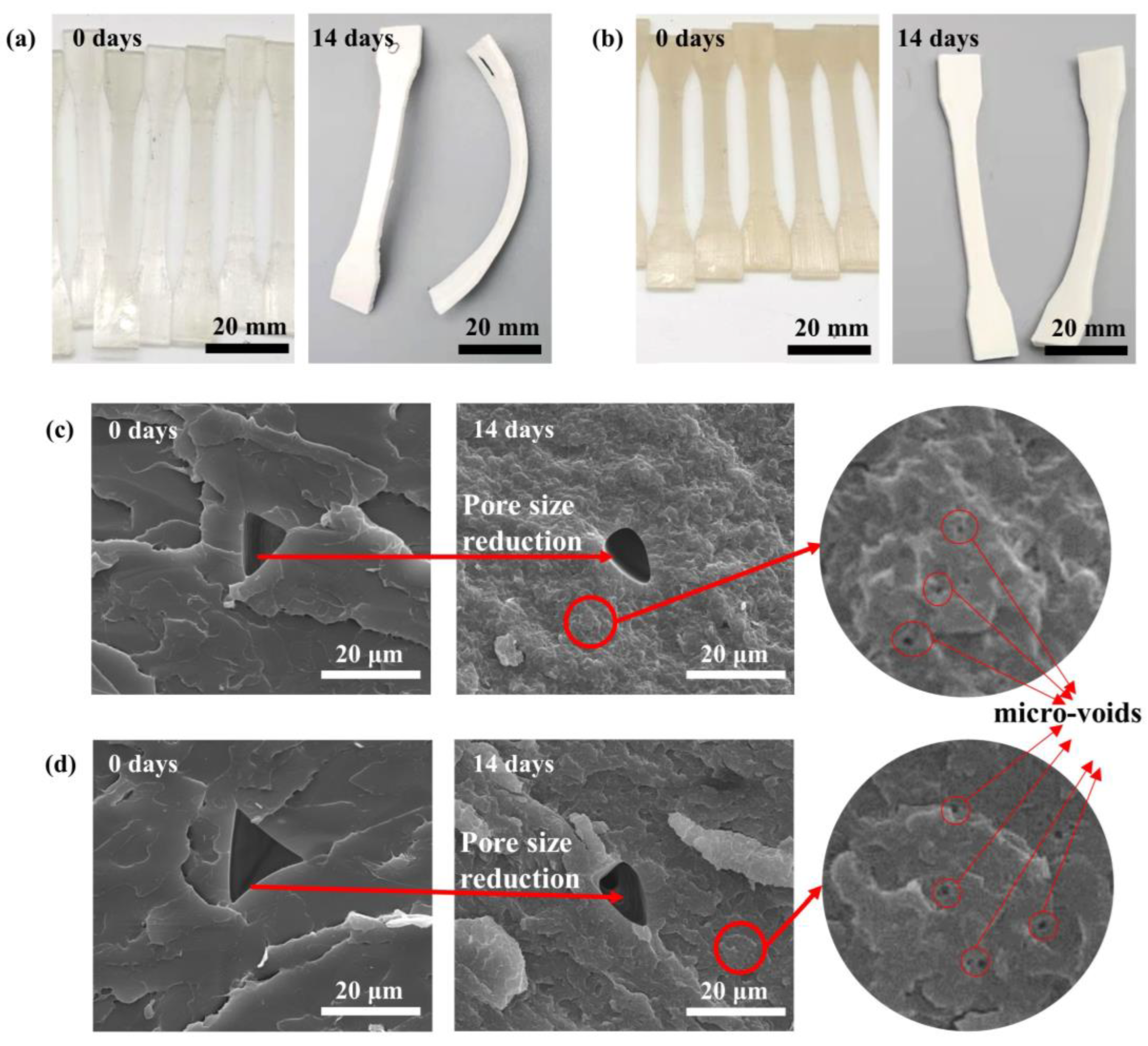
3.3. Morphological Analysis
3.4. Deformation Analysis
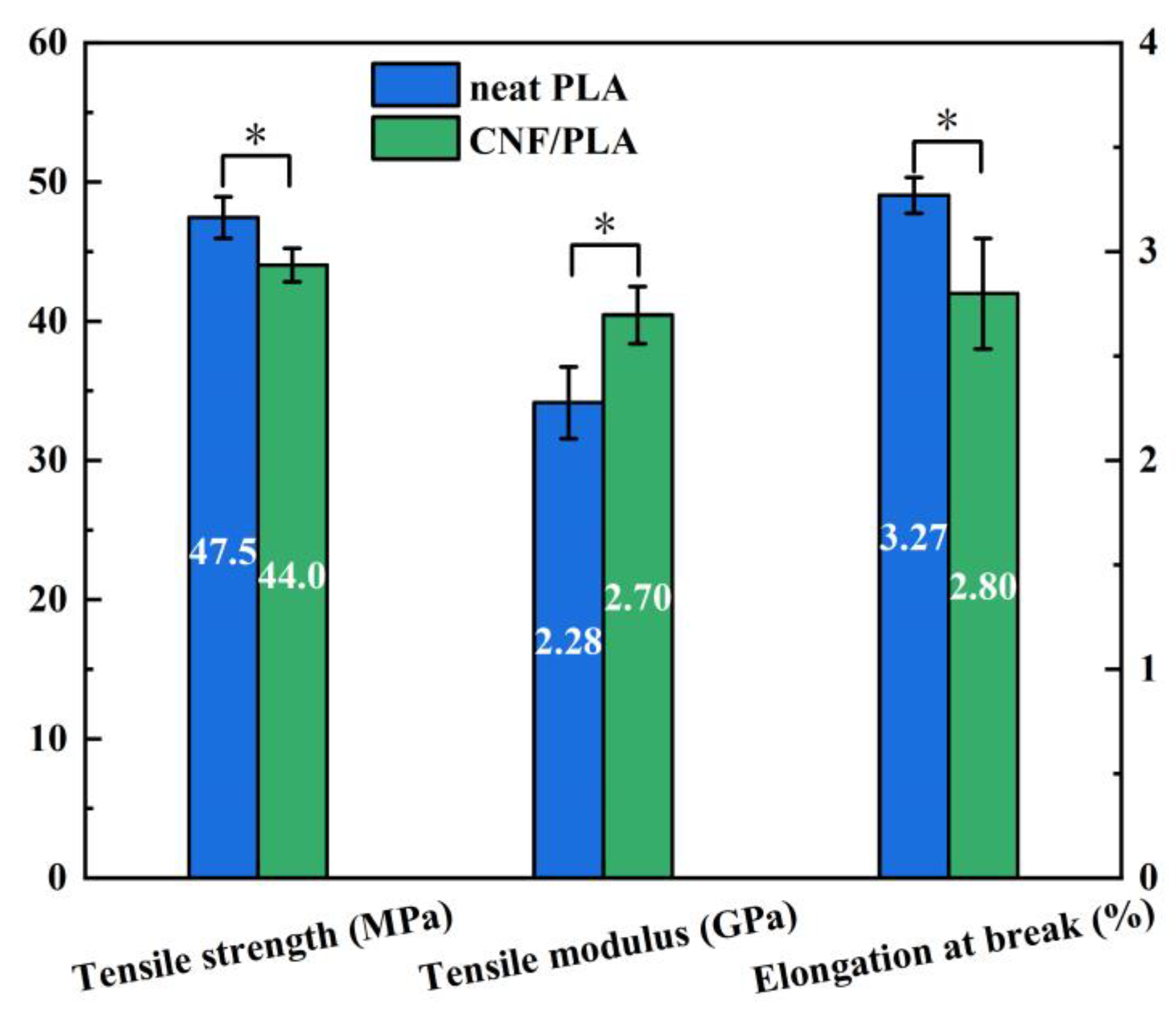
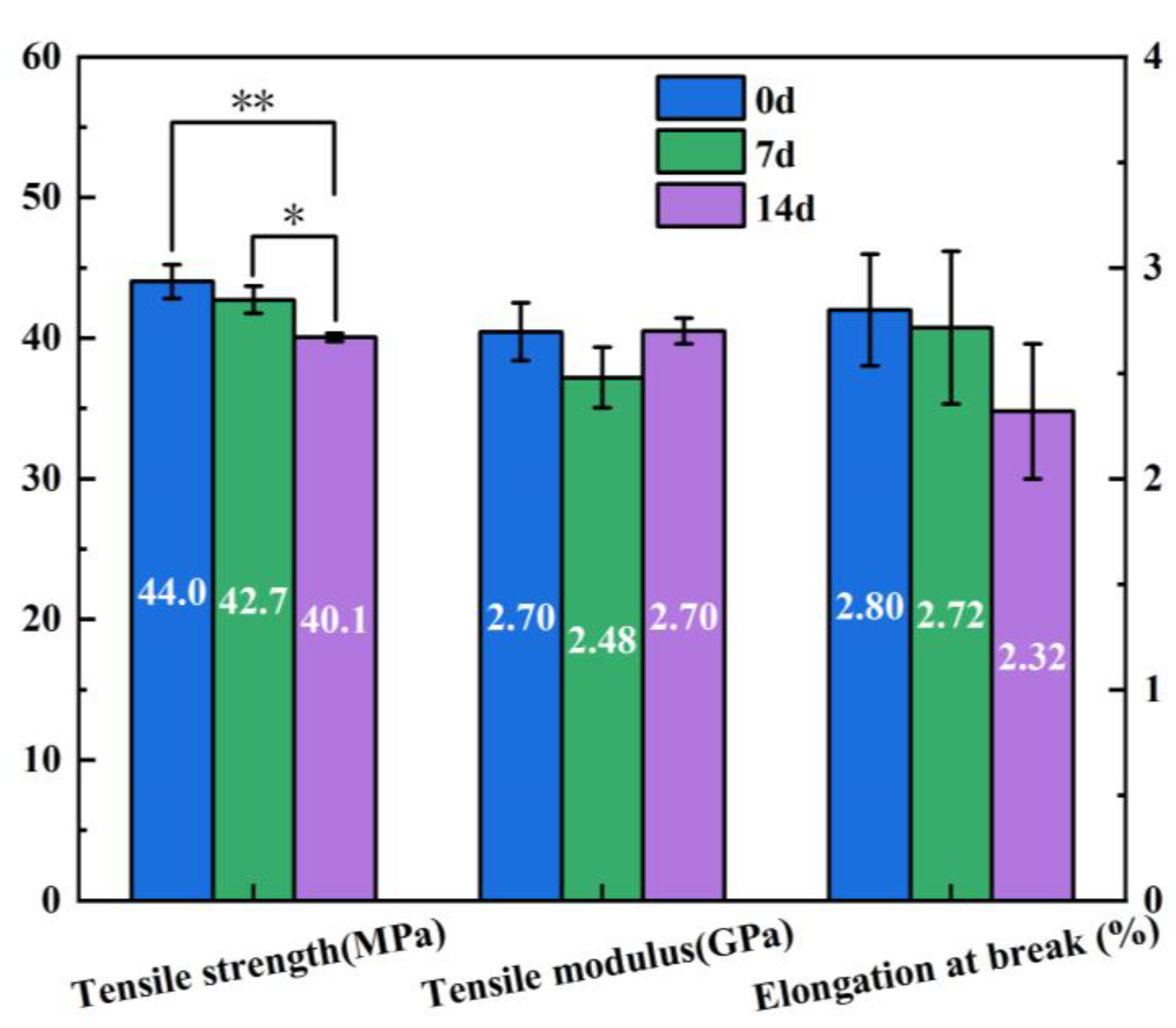
3.5. Mechanical Performance Analysis
4. Conclusions
- (1)
- The addition of CNF increased the tensile modulus of the composites by 18.4% compared to neat PLA because of the high modulus of elasticity exhibited by CNF, while the strength of the composites was 7.4% lower due to poor interfacial bonding between CNF and PLA matrix. Over a degradation period of 14 days at 37 °C, the tensile strength of the CNF/PLA composites exhibited a decreased of 8.9%. This indicates a considerable susceptibility to the degradation conditions.
- (2)
- The presence of CNF accelerated the degradation of the PLA. When subjected to accelerated degradation at 50 °C, the decreases of Mn and Mw of PLA matrix in the CNF/PLA composites were 7.96% and 4.91% higher than that of neat PLA.
- (3)
- The mapping relationship between accelerated degradation and normal degradation implies that the degradation rate that occurs during 60 days at 37 °C is equivalent to that experienced during 14 days at 50 °C by examining the alteration in Mn.
- (4)
- The degraded samples exhibited improved crystallinity and crystallization capacity, while they appeared as a non-transparent milky white color with considerable amount of micro-voids generated inside the samples, and the addition of CNF caused a more pronounced change in the sample morphology.
- (5)
- The degradation process caused a notable C-shape deformation in the 3D-printed parts due to residual stress generated during the 3D printing process. It is suggested to employ an appropriate post-treatment method to alleviate the residual stress in these 3D-printed parts prior to their application.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Shalawi, F.D.; Hanim, M.A.; Ariffin, M.K.A.; Kim, C.L.S.; Brabazon, D.; Calin, R.; Al-Osaimi, M.O. Biodegradable synthetic polymer in orthopaedic application: A review. Mater. Today Proc. 2023, 74, 540–546. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. An overview of biodegradable poly (lactic acid) production from fermentative lactic acid for biomedical and bioplastic applications. Biomass Convers. Biorefinery 2022, 1–20. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation Behavior In Vitro of Carbon Nanotubes (CNTs)/Poly(Lactic Acid) (PLA) Composite Suture. Polymers 2019, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, S.; Hashmi, F.A.; Imran, I. Recent progress in development and applications of biomaterials. Mater. Today Proc. 2022, 62, 385–391. [Google Scholar] [CrossRef]
- Hu, X.; Lin, Z.; He, J.; Zhou, M.; Yang, S.; Wang, Y.; Li, K. Recent progress in 3D printing degradable polylactic acid-based bone repair scaffold for the application of cancellous bone defect. MedComm–Biomater. Appl. 2022, 1, e14. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Fully Bioresorbable Vascular Scaffolds: Lessons Learned and Future Directions. Nat. Rev. Cardiol. 2019, 16, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Toncheva, A.; Mincheva, R.; Kancheva, M.; Manolova, N.; Rashkov, I.; Dubois, P.; Markova, N. Antibacterial PLA/PEG Electrospun Fibers: Comparative Study between Grafting and Blending PEG. Eur. Polym. J. 2016, 75, 223–233. [Google Scholar] [CrossRef]
- Nedaipour, F.; Bagheri, H.; Mohammadi, S. “Polylactic Acid-Polyethylene Glycol-Hydroxyapatite Composite” an Efficient Composition for Interference Screws. Nanocomposites 2020, 6, 99–110. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.A.; Majumdar, A.; Butola, B.S. Tailoring the Mechanical and Thermal Properties of Polylactic Acid-Based Bionanocomposite Films Using Halloysite Nanotubes and Polyethylene Glycol by Solvent Casting Process. J. Mater. Sci. 2019, 54, 8971–8983. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Yu, L.; Min, A.; Yang, S.; Shuai, C. Accelerated Degradation of Poly(L-Lactide) Bone Scaffold: Crystallinity and Hydrophilicity. Mater. Chem. Phys. 2021, 266, 124545. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A New Approach in Compatibilization of the Poly(Lactic Acid)/Thermoplastic Starch (PLA/TPS) Blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Graphene Modifies the Biodegradation of Poly(Lactic Acid)-Thermoplastic Cassava Starch Reactive Blend Films. Polym. Degrad. Stabil. 2019, 164, 187–197. [Google Scholar] [CrossRef]
- Momeni, S.; Rezvani Ghomi, E.; Shakiba, M.; Shafiei-Navid, S.; Abdouss, M.; Bigham, A.; Khosravi, F.; Ahmadi, Z.; Faraji, M.; Abdouss, H.; et al. The Effect of Poly (Ethylene Glycol) Emulation on the Degradation of PLA/Starch Composites. Polymers 2021, 13, 1019. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, Y.; Tian, J. Study on the Biodegradability of Modified Starch/Polylactic Acid (PLA) Composite Materials. RSC Adv. 2020, 10, 26298–26307. [Google Scholar] [CrossRef]
- Watai, J.S.; Calvao, P.S.; Rigolin, T.R.; do Prado Bettini, S.H.; Souza, A.M.C. Retardation Effect of Nanohydroxyapatite on the Hydrolytic Degradation of Poly (Lactic Acid). Polym. Eng. Sci. 2020, 60, 2152–2162. [Google Scholar] [CrossRef]
- Naik, A.; Shepherd, D.V.; Shepherd, J.H.; Best, S.M.; Cameron, R.E. The Effect of the Type of HA on the Degradation of PLGA/HA Composites. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.-T. Nanocellulose, a Versatile Platform: From the Delivery of Active Molecules to Tissue Engineering Applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef]
- Iwatake, A.; Nogi, M.; Yano, H. Cellulose Nanofiber-Reinforced Polylactic Acid. Compos. Sci. Technol. 2008, 68, 2103–2106. [Google Scholar] [CrossRef]
- Abudula, T.; Saeed, U.; Memic, A.; Gauthaman, K.; Hussain, M.A.; Al-Turaif, H. Electrospun Cellulose Nano Fibril Reinforced PLA/PBS Composite Scaffold for Vascular Tissue Engineering. J. Polym. Res. 2019, 26, 110. [Google Scholar] [CrossRef]
- Benitez, A.J.; Walther, A. Cellulose Nanofibril Nanopapers and Bioinspired Nanocomposites: A Review to Understand the Mechanical Property Space. J. Mater. Chem. A 2017, 5, 16003–16024. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Klar, V.; Ajdary, R.; Norberg, N.; Ago, M.; Cunha, A.G.; Rojas, O.J. Absorbent Filaments from Cellulose Nanofibril Hydrogels through Continuous Coaxial Wet Spinning. ACS Appl. Mater. Interfaces 2018, 10, 27287–27296. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cheng, L.; Yuan, C.; Wu, Z.; Yuan, G.; Hou, M.; Chen, J.Y.; Luo, C.; Li, W. Preparation, Characterization and Evaluation of Cellulose Nanocrystal/Poly(Lactic Acid) in Situ Nanocomposite Scaffolds for Tissue Engineering. Int. J. Biol. Macromol. 2019, 134, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, C.; Yue, Y.; Guo, W.; Wu, Y.; Wu, Q. Mechanical Properties and in Vitro Degradation of Electrospun Bio-Nanocomposite Mats from PLA and Cellulose Nanocrystals. Carbohydr. Polym. 2012, 90, 301–308. [Google Scholar] [CrossRef]
- Shuai, C.; Yuan, X.; Yang, W.; Peng, S.; He, C.; Feng, P.; Qi, F.; Wang, G. Cellulose Nanocrystals as Biobased Nucleation Agents in Poly-L-Lactide Scaffold: Crystallization Behavior and Mechanical Properties. Polym. Test 2020, 85, 106458. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun Bio-Nanocomposite Scaffolds for Bone Tissue Engineering by Cellulose Nanocrystals Reinforcing Maleic Anhydride Grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef]
- Bitinis, N.; Fortunati, E.; Verdejo, R.; Bras, J.; Maria Kenny, J.; Torre, L.; Angel Lopez-Manchado, M. Poly(Lactic Acid)/Natural Rubber/Cellulose Nanocrystal Bionanocomposites. Part II: Properties Evaluation. Carbohydr. Polym. 2013, 96, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Pan, M.; Xu, Z.; Luo, W.; Yang, Y.; Teng, T.; Lin, J.; Huang, H. In Vitro and in Vivo Properties Study of a Novel 3D-Printed Absorbable Pancreaticojejunostomy Device Made by Melting Blended Poly(p-Dioxanone)/Poly(Lactic Acid). Mater. Des. 2021, 210, 110088. [Google Scholar] [CrossRef]
- Alam, F.; Varadarajan, K.M.; Kumar, S. 3D Printed Polylactic Acid Nanocomposite Scaffolds for Tissue Engineering Applications. Polym. Test 2020, 81, 106203. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, K.; Li, H.; Zhang, Q.; Tu, D. Rapid residual stress prediction and feedback control during fused deposition modeling of PLA. Int. J. Adv. Manuf. Technol. 2022, 118, 3229–3240. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Božić, Ž.; Zolfagharian, A.; Reinicke, T. Failure analysis of 3D-printed PLA components: Impact of manufacturing defects and thermal ageing. Eng. Fail. Anal. 2022, 136, 106214. [Google Scholar] [CrossRef]
- Ueda, T.; Ishigami, A.; Thumsorn, S.; Kurose, T.; Kobayashi, Y.; Ito, H. Structural, rheological, and mechanical properties of polyvinyl alcohol composites reinforced with cellulose nanofiber treated by ultrahigh-pressure homogenizer. Mater. Today Commun. 2022, 33, 104316. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, L.; Sun, J.; Liu, J. Cellulose nanofibrils filled poly (lactic acid) biocomposite filament for FDM 3D printing. Molecules 2020, 25, 2319. [Google Scholar] [CrossRef] [PubMed]
- ISO 527-2:2012; Plastics-Determination of Tensile Properties-Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Geneva, Switzerland, 2012.
- Ambone, T.; Torris, A.; Shanmuganathan, K. Enhancing the mechanical properties of 3D printed polylactic acid using nanocellulose. Polym. Eng. Sci. 2020, 60, 1842–1855. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and Morphology of Poly(l-Lactide) 4. Effects of Structural Parameters on Long-Term Hydrolysis of Poly(l-Lactide) in Phosphate-Buffered Solution. Polym. Degrad. Stab. 2000, 67, 179–189. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic Degradation of Polylactic Acid (PLA) and Its Composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F. Hydrolysis of Polylactic Acid (PLA) and Polycaprolactone (PCL) in Aqueous Acetonitrile Solutions: Autocatalysis. J. Environ. Polym. Degrad. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Lyu, S.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and Time-Temperature Equivalence of Polymer Degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef]
- ISO 15814:1999; Implants for surgery-Copolymers and blends based on polylactide-In vitro degradation testing. International Organization for Standardization: Geneva, Switzerland, 1999.
- Balcerowiak, W. The degree of crystallinity of polymer systems as determined by DSC. Polimery 1998, 43, 373–378. [Google Scholar] [CrossRef]
- Kalita, N.K.; Bhasney, S.M.; Kalamdhad, A.; Katiyar, V. Biodegradable Kinetics and Behavior of Bio-Based Polyblends under Simulated Aerobic Composting Conditions. J. Environ. Manag. 2020, 261, 110211. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Kinetics of Hydrolytic Degradation of PLA. J. Polym. Environ. 2013, 21, 313–318. [Google Scholar] [CrossRef]
- Dimarzio, E.A.; Gibbs, J.H. Molecular Interpretation of Glass Temperature Depression by Plasticizers. J. Polym. Sci. A Gen. Pap. 1963, 1, 1417–1428. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, J.; Zhu, Q.; Liu, J. Structure and properties of polylactic acid biocomposite films reinforced with cellulose nanofibrils. Molecules 2020, 25, 3306. [Google Scholar] [CrossRef] [PubMed]
- Barrera, D.A.; Zylstra, E.; Lansbury, P.T.; Langer, R. Copolymerization and degradation of poly (lactic acid-co-lysine). Macromolecules 1995, 28, 425–432. [Google Scholar] [CrossRef]
- Polak-Krasna, K.; Abaei, A.R.; Shirazi, R.N.; Parle, E.; Carroll, O.; Ronan, W.; Vaughan, T.J. Physical and Mechanical Degradation Behaviour of Semi-Crystalline PLLA for Bioresorbable Stent Applications. J. Mech. Behav. Biomed. Mater. 2021, 118, 104409. [Google Scholar] [CrossRef]
- Sun, X.C.; Mazur, M.; Cheng, C. A review of void reduction strategies in material extrusion-based additive manufacturing. Addit. Manuf. 2023, 67, 103463. [Google Scholar] [CrossRef]
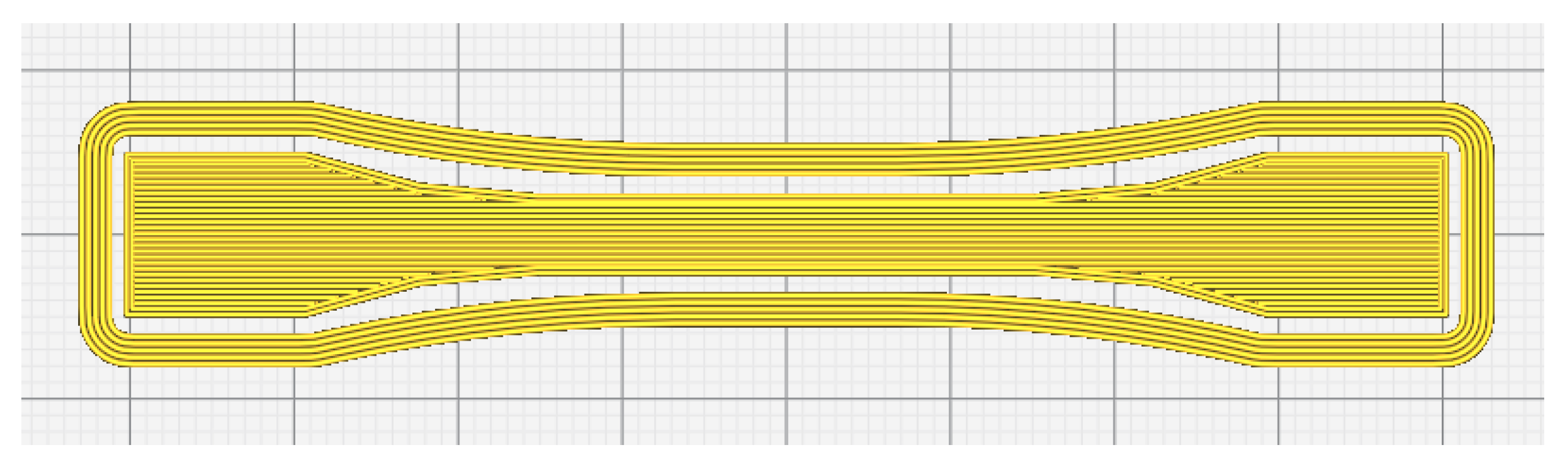


| Parameter | Value |
|---|---|
| Nozzle diameter (mm) | 0.4 |
| Printing temperature (°C) | 190 |
| Bed temperature (°C) | 60 |
| Printing speed (mm/s) | 50 |
| Filling density | 100% |
| Layer thickness (mm) | 0.1 |
| Printing flow | 110% |
| Samples | Time (d) | |||
|---|---|---|---|---|
| neat PLA | 0 | 3.7 × 104 | 6.7 × 104 | 1.82 |
| neat PLA | 14 | 1.3 × 104 | 2.6 × 104 | 2.06 |
| CNF/PLA | 14 | 1.1 × 104 | 2.4 × 104 | 2.24 |
| Samples | Time (d) | (℃) | (℃) | (℃) | (J/g) | (J/g) | (%) |
|---|---|---|---|---|---|---|---|
| neat PLA | 0 | 64.75 | 131.69 | 164.27 | 22.00 | 15.83 | 6.63 |
| neat PLA | 7 | -- | -- | 164.00 | 22.36 | 0 | 24.01 |
| neat PLA | 14 | -- | -- | 162.27 | 22.04 | 0 | 23.67 |
| CNF/PLA | 0 | 64.81 | 127.41 | 165.31 | 24.90 | 21.45 | 3.71 |
| CNF/PLA | 7 | -- | -- | 164.26 | 23.63 | 0 | 25.29 |
| CNF/PLA | 14 | -- | -- | 162.43 | 22.40 | 0 | 24.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cao, B.; Jiang, N. The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites. Materials 2023, 16, 6197. https://doi.org/10.3390/ma16186197
Zhang Z, Cao B, Jiang N. The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites. Materials. 2023; 16(18):6197. https://doi.org/10.3390/ma16186197
Chicago/Turabian StyleZhang, Zhongsen, Bingyan Cao, and Ning Jiang. 2023. "The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites" Materials 16, no. 18: 6197. https://doi.org/10.3390/ma16186197
APA StyleZhang, Z., Cao, B., & Jiang, N. (2023). The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites. Materials, 16(18), 6197. https://doi.org/10.3390/ma16186197








