Bisphosphonates and Dental Implants: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategies
- (“dental implant” OR “oral implant”) AND (bisphosphonate OR diphosphonate)
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Quality Assessment
2.6. Definitions
2.7. Data Extraction
2.8. Analyses
3. Results
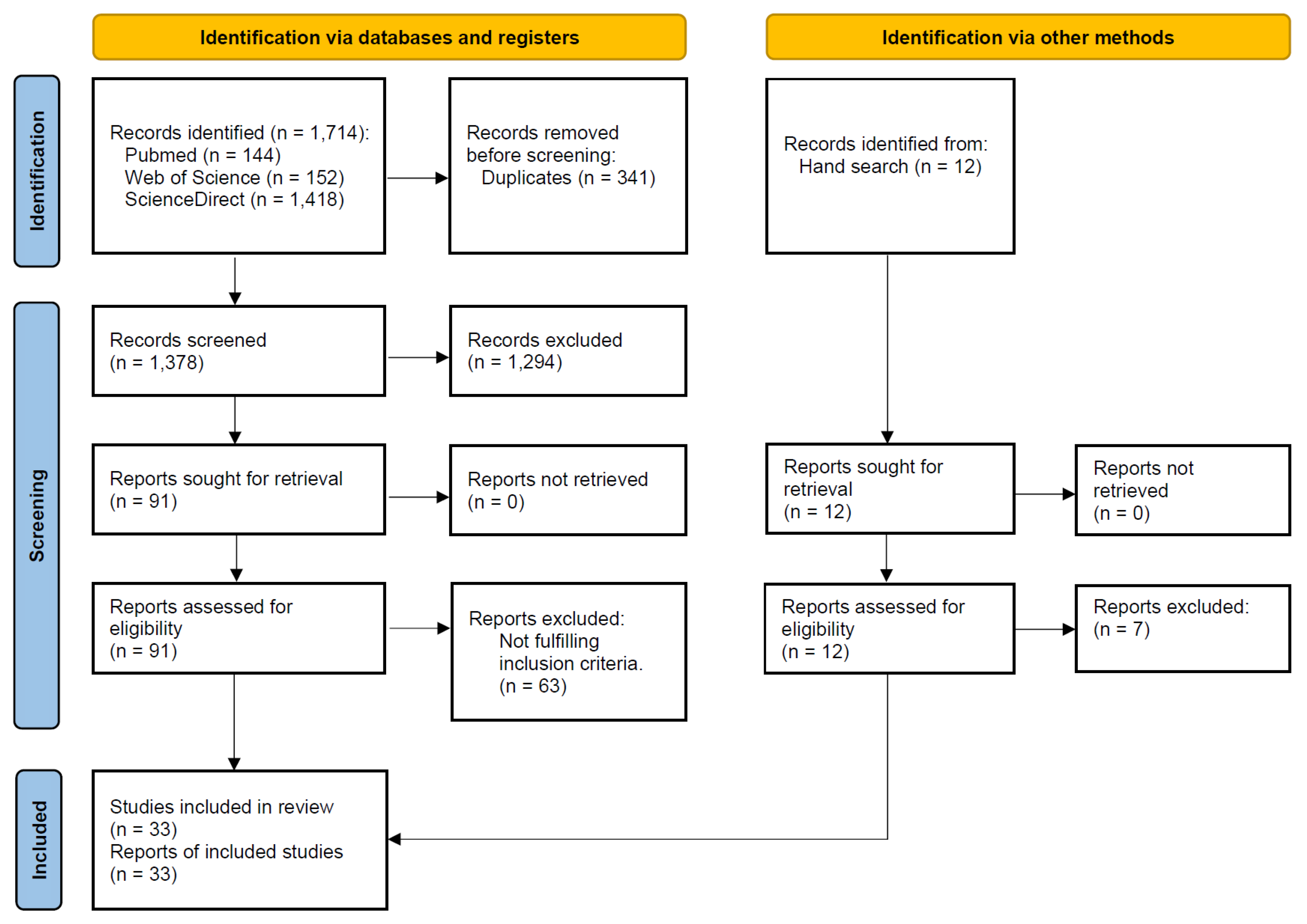
3.1. Literature Search
3.2. Description of the Studies
3.3. Quality Assessment
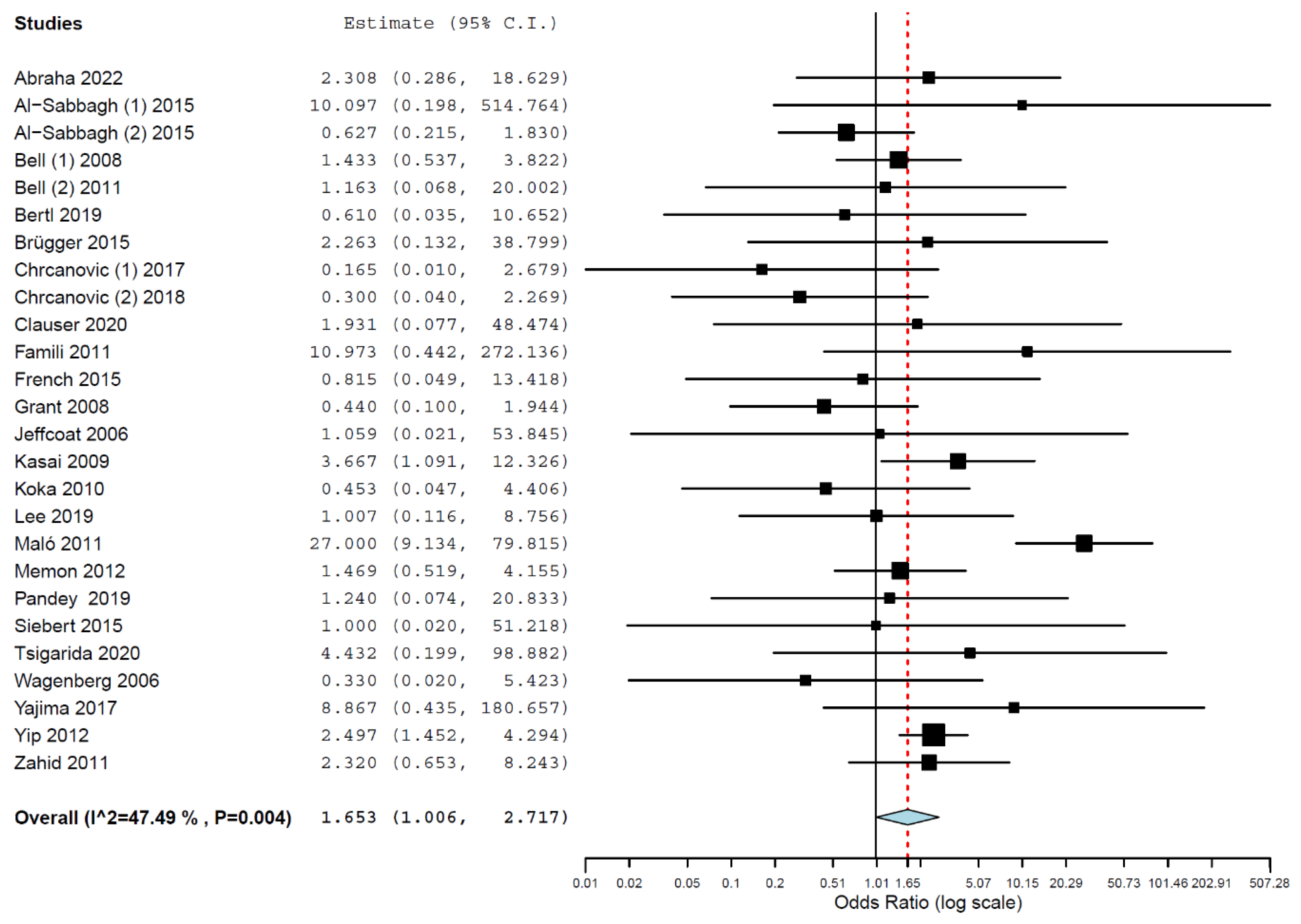
3.4. Meta-Analyses
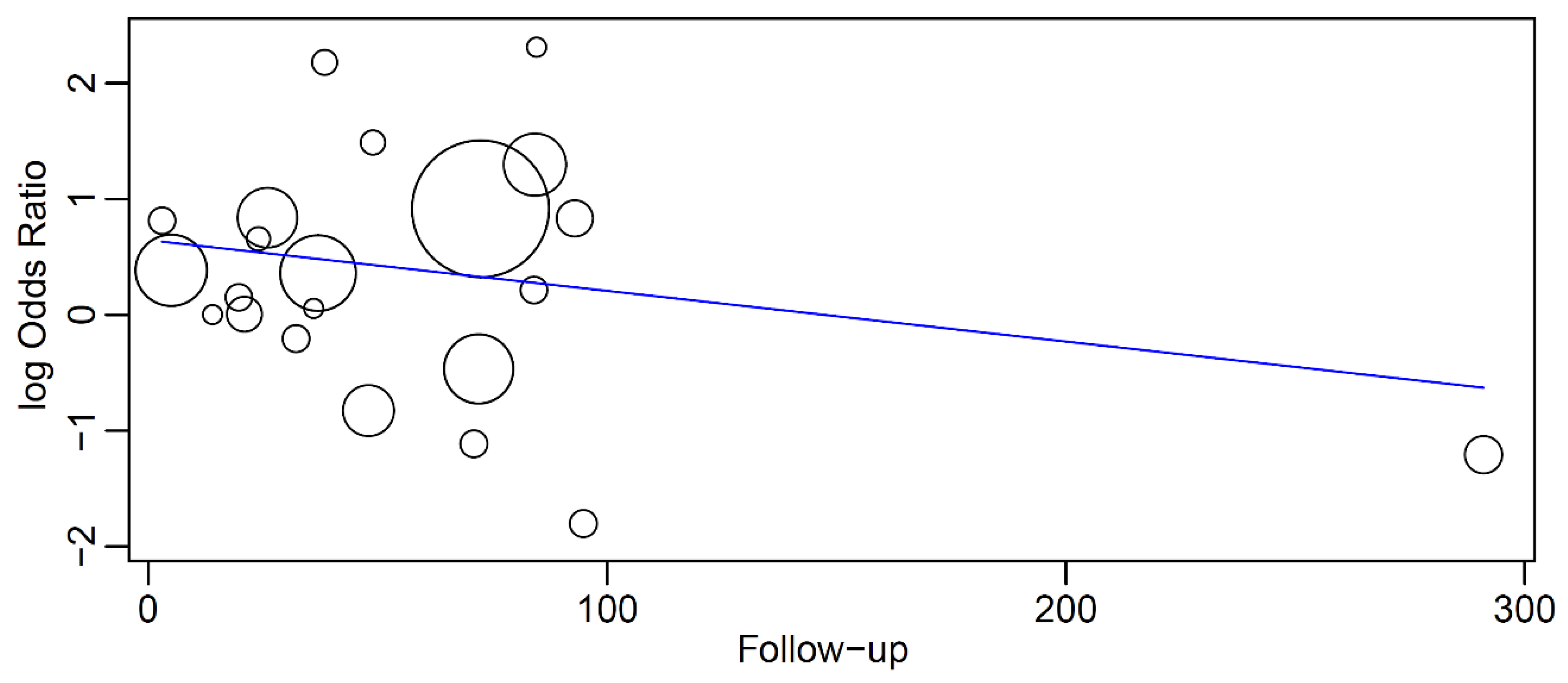
3.5. Meta-Regression
- Intercept = 0.645 (0.049, 1.240), standard error 0.304, p = 0.034
- Follow-up = −0.004 (−0.012, 0.003), standard error 0.004, p = 0.259
3.6. Estimated Survival
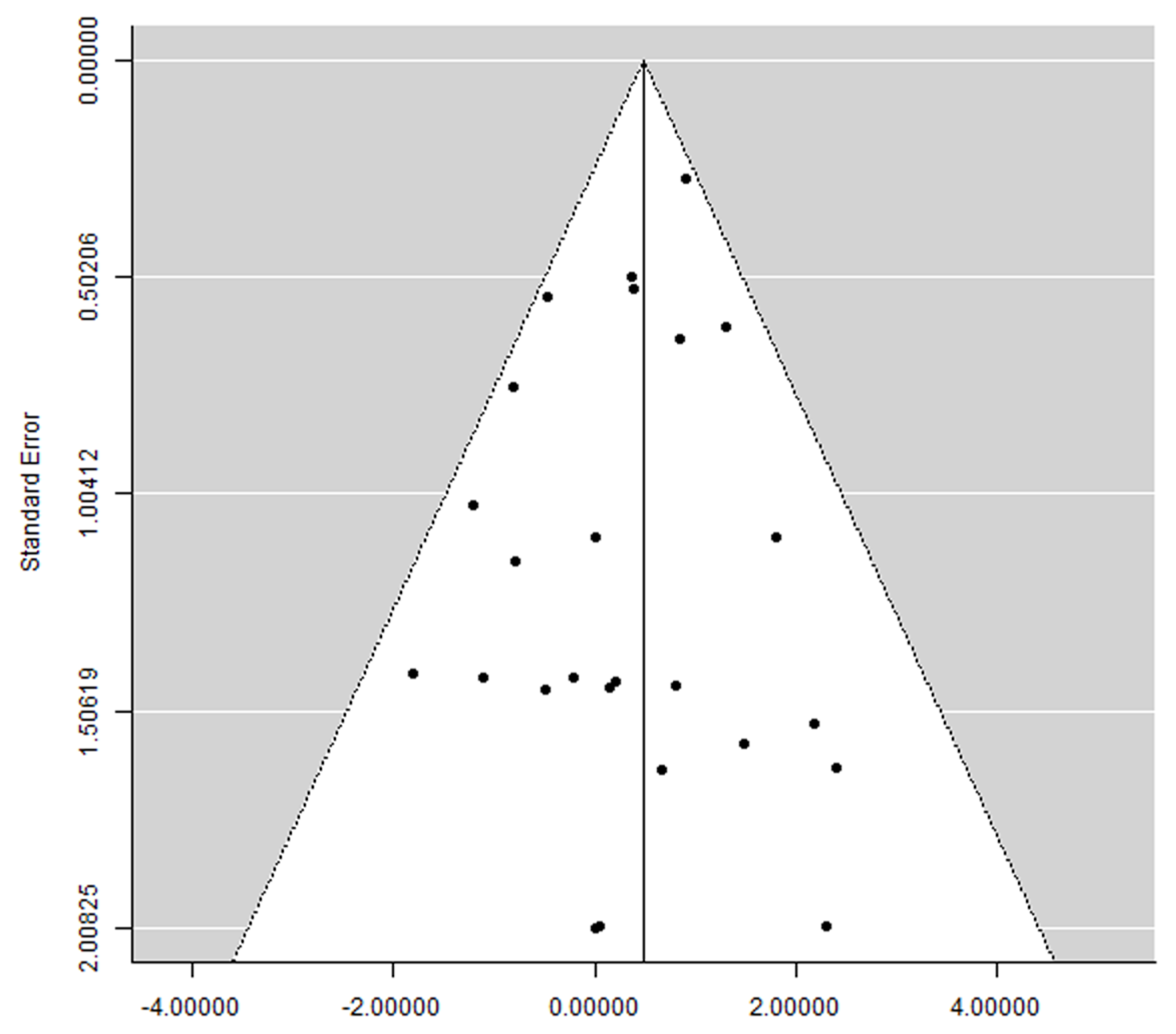
3.7. Publication Bias
4. Discussion
Limitations of the Present Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coughlan, T.; Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. 2014, 14, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Sun, S.; Cherian, P.; Roshandel, S.; Neighbors, J.D.; Hu, E.; Dunford, J.E.; Sedghizadeh, P.P.; McKenna, C.E.; Srinivasan, V.; et al. Bisphosphonates: The role of chemistry in understanding their biological actions and structure-activity relationships, and new directions for their therapeutic use. Bone 2022, 156, 116289. [Google Scholar] [CrossRef]
- Ganesan, K.; Goyal, A.; Roane, D. Bisphosphonate. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, P.D. Bisphosphonate-associated adverse events. Hormones 2009, 8, 96–110. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Landesberg, R.; Woo, V.; Cremers, S.; Cozin, M.; Marolt, D.; Vunjak-Novakovic, G.; Kousteni, S.; Raghavan, S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 2011, 1218, 62–79. [Google Scholar] [CrossRef]
- Ficarra, G.; Beninati, F.; Rubino, I.; Vannucchi, A.; Longo, G.; Tonelli, P.; Pini Prato, G. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J. Clin. Periodontol. 2005, 32, 1123–1128. [Google Scholar] [CrossRef]
- Ripamonti, C.I.; Maniezzo, M.; Campa, T.; Fagnoni, E.; Brunelli, C.; Saibene, G.; Bareggi, C.; Ascani, L.; Cislaghi, E. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann. Oncol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Hokugo, A.; Christensen, R.; Chung, E.M.; Sung, E.C.; Felsenfeld, A.L.; Sayre, J.W.; Garrett, N.; Adams, J.S.; Nishimura, I. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J. Bone Miner. Res. 2010, 25, 1337–1349. [Google Scholar] [CrossRef]
- Bi, Y.; Gao, Y.; Ehirchiou, D.; Cao, C.; Kikuiri, T.; Le, A.; Shi, S.; Zhang, L. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am. J. Pathol. 2010, 177, 280–290. [Google Scholar] [CrossRef]
- Bamias, A.; Kastritis, E.; Bamia, C.; Moulopoulos, L.A.; Melakopoulos, I.; Bozas, G.; Koutsoukou, V.; Gika, D.; Anagnostopoulos, A.; Papadimitriou, C.; et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J. Clin. Oncol. 2005, 23, 8580–8587. [Google Scholar] [CrossRef]
- Sonis, S.T.; Watkins, B.A.; Lyng, G.D.; Lerman, M.A.; Anderson, K.C. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009, 45, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws; American Association of Oral and Maxillofacial Surgeons. Position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar] [CrossRef]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- NIH. Quality Assessment Tool for Case Series Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 January 2020).
- Tonetti, M.S.; Schmid, J. Pathogenesis of implant failures. Periodontol. 2000 1994, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors influencing the fracture of dental implants. Clin. Implant Dent. Relat. Res. 2018, 20, 58–67. [Google Scholar] [CrossRef]
- Gatti, D.; Adami, S. New bisphosphonates in the treatment of bone diseases. Drugs Aging 1999, 15, 285–296. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontol. 2000 2017, 73, 41–50. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D. Principles of and procedures for systematic reviews. In Systematic Reviews in Health Care: Meta-Analysis in Context; Egger, M., Smith, G.D., Altman, D.G., Eds.; BMJ Books: London, UK, 2003; pp. 23–42. [Google Scholar]
- Hawthan, M.; Larsson, C.; Chrcanovic, B.R. Survival of fixed prosthetic restorations on vital and nonvital teeth: A systematic review. J. Prosthodont. 2023. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef]
- Abraha, S.M.; Geng, Y.M.; Naujokat, H.; Terheyden, H. Modified Le Fort I interpositional grafting of the severe atrophied maxilla—A retrospective study of 106 patients over 10 years. Clin. Oral Implants Res. 2022, 33, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabbagh, M.; Robinson, F.G.; Romanos, G.; Thomas, M.V. Osteoporosis and bisphosphonate-related osteonecrosis in a dental school implant patient population. Implant Dent. 2015, 24, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabbagh, M.; Thomas, M.V.; Bhavsar, I.; De Leeuw, R. Effect of Bisphosphonate and Age on Implant Failure as Determined by Patient-Reported Outcomes. J. Oral Implantol. 2015, 41, e287–e291. [Google Scholar] [CrossRef]
- Bell, B.M.; Bell, R.E. Oral bisphosphonates and dental implants: A retrospective study. J. Oral Maxillofac. Surg. 2008, 66, 1022–1024. [Google Scholar] [CrossRef]
- Bell, C.L.; Diehl, D.; Bell, B.M.; Bell, R.E. The immediate placement of dental implants into extraction sites with periapical lesions: A retrospective chart review. J. Oral Maxillofac. Surg. 2011, 69, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Ebner, M.; Knibbe, M.; Pandis, N.; Kuchler, U.; Ulm, C.; Stavropoulos, A. How old is old for implant therapy in terms of early implant losses? J. Clin. Periodontol. 2019, 46, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Brügger, O.E.; Bornstein, M.M.; Kuchler, U.; Janner, S.F.; Chappuis, V.; Buser, D. Implant therapy in a surgical specialty clinic: An analysis of patients, indications, surgical procedures, risk factors, and early failures. Int. J. Oral Maxillofac. Implants 2015, 30, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Intake of Proton Pump Inhibitors Is Associated with an Increased Risk of Dental Implant Failure. Int. J. Oral Maxillofac. Implants 2017, 32, 1097–1102. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin. Implant Dent. Relat. Res. 2018, 20, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Clauser, C.; Sforza, N.M.; Menini, I.; Kalemaj, Z.; Buti, J. Immediate Postextraction Single-Tooth Implants and Provisional Crowns in the Esthetic Area: 2-year Results of a Cohort Prospective Multicenter Study- Patient-Centered Outcomes. Int. J. Oral Maxillofac. Implants 2020, 35, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Demarosi, F.; Carrassi, A.; Leghissa, G.C. Dental implant treatment in oral bisphosphonates patients using a drug holiday protocol: A prospective study. J. Osseointegr. 2010, 2, 73–78. [Google Scholar] [CrossRef]
- Famili, P.; Quigley, S.; Mosher, T. Survival of dental implants among post-menopausal female dental school patients taking oral bisphosphonates: A retrospective study. Compend. Contin. Educ. Dent. 2011, 32, E106–E109. [Google Scholar]
- French, D.; Larjava, H.; Ofec, R. Retrospective cohort study of 4591 Straumann implants in private practice setting, with up to 10-year follow-up. Part 1: Multivariate survival analysis. Clin. Oral Implants Res. 2015, 26, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Fugazzotto, P.A.; Lightfoot, W.S.; Jaffin, R.; Kumar, A. Implant placement with or without simultaneous tooth extraction in patients taking oral bisphosphonates: Postoperative healing, early follow-up, and the incidence of complications in two private practices. J. Periodontol. 2007, 78, 1664–1669. [Google Scholar] [CrossRef]
- Grant, B.T.; Amenedo, C.; Freeman, K.; Kraut, R.A. Outcomes of placing dental implants in patients taking oral bisphosphonates: A review of 115 cases. J. Oral Maxillofac. Surg. 2008, 66, 223–230. [Google Scholar] [CrossRef]
- Jeffcoat, M.K. Safety of oral bisphosphonates: Controlled studies on alveolar bone. Int. J. Oral Maxillofac. Implants 2006, 21, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Pogrel, M.A.; Hossaini, M. The prognosis for dental implants placed in patients taking oral bisphosphonates. J. Calif. Dent. Assoc. 2009, 37, 39–42. [Google Scholar] [CrossRef]
- Koka, S.; Babu, N.M.; Norell, A. Survival of dental implants in post-menopausal bisphosphonate users. J. Prosthodont. Res. 2010, 54, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Cha, J.K.; Sanz-Martin, I.; Sanz, M.; Jung, U.W. A retrospective case series evaluating the outcome of implants with low primary stability. Clin. Oral Implants Res. 2019, 30, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Leonida, A.; Vescovi, P.; Baldoni, M.; Rossi, G.; Lauritano, D. Immediate loading in mandible full-arch: Pilot study in patients with osteoporosis in bisphosphonate therapy. J. Oral Implantol. 2012, 38, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Moss, S.M.; Molina, G.J. A longitudinal study of the survival of All-on-4 implants in the mandible with up to 10 years of follow-up. J. Am. Dent. Assoc. 2011, 142, 310–320. [Google Scholar] [CrossRef]
- Memon, S.; Weltman, R.L.; Katancik, J.A. Oral bisphosphonates: Early endosseous dental implant success and crestal bone changes. A retrospective study. Int. J. Oral Maxillofac. Implants 2012, 27, 1216–1222. [Google Scholar] [PubMed]
- Mozzati, M.; Arata, V.; Giacomello, M.; Del Fabbro, M.; Gallesio, G.; Mortellaro, C.; Bergamasco, L. Failure risk estimates after dental implants placement associated with plasma rich in growth factor-Endoret in osteoporotic women under bisphosphonate therapy. J. Craniofac. Surg. 2015, 26, 749–755. [Google Scholar] [CrossRef]
- Pandey, A.; Verma, S.; Malhan, S.; Mittal, N.; Chaudhary, A.; Gera, T. Evaluation of Effect of Bisphosphonates on Dental Implant Therapy in Postmenopausal Women Using Cone Beam Computed Tomography. J. Contemp. Dent. Pract. 2019, 20, 51–55. [Google Scholar]
- Shabestari, G.O.; Shayesteh, Y.S.; Khojasteh, A.; Alikhasi, M.; Moslemi, N.; Aminian, A.; Masaeli, R.; Eslami, B.; Treister, N.S. Implant placement in patients with oral bisphosphonate therapy: A case series. Clin. Implant Dent. Relat. Res. 2010, 12, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.; Jurkovic, R.; Statelova, D.; Strecha, J. Immediate Implant Placement in a Patient With Osteoporosis Undergoing Bisphosphonate Therapy: 1-Year Preliminary Prospective Study. J. Oral Implantol. 2015, 41, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Canullo, L.; Xhanari, E.; Meloni, S.M. Dental implants treatment outcomes in patient under active therapy with alendronate: 3-year follow-up results of a multicenter prospective observational study. Clin. Oral Implants Res. 2016, 27, 943–949. [Google Scholar] [CrossRef]
- Tsigarida, A.; Chochlidakis, K.; Fraser, D.; Lampraki, E.; Einarsdottir, E.R.; Barmak, A.B.; Papaspyridakos, P.; Ercoli, C. Peri-Implant Diseases and Biologic Complications at Implant-Supported Fixed Dental Prostheses in Partially Edentulous Patients. J. Prosthodont. 2020, 29, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wagenberg, B.; Froum, S.J. A retrospective study of 1925 consecutively placed immediate implants from 1988 to 2004. Int. J. Oral Maxillofac. Implants 2006, 21, 71–80. [Google Scholar]
- Wagenberg, B.D.; Froum, S.J.; Eckert, S.E. Long-term bone stability assessment around 1187 immediately placed implants with 1- to 22-year follow-up. Int. J. Oral Maxillofac. Implants 2013, 28, 605–612. [Google Scholar] [CrossRef]
- Yajima, N.; Munakata, M.; Fuchigami, K.; Sanda, M.; Kasugai, S. Influence of Bisphosphonates on Implant Failure Rates and Characteristics of Postmenopausal Woman Mandibular Jawbone. J. Oral Implantol. 2017, 43, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.K.; Borrell, L.N.; Cho, S.C.; Francisco, H.; Tarnow, D.P. Association between oral bisphosphonate use and dental implant failure among middle-aged women. J. Clin. Periodontol. 2012, 39, 408–414. [Google Scholar] [CrossRef]
- Zahid, T.M.; Wang, B.Y.; Cohen, R.E. Influence of bisphosphonates on alveolar bone loss around osseointegrated implants. J. Oral Implantol. 2011, 37, 335–346. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Bisphosphonates and dental implants: A meta-analysis. Quintessence Int. 2016, 47, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef]
- Fournier, P.; Boissier, S.; Filleur, S.; Guglielmi, J.; Cabon, F.; Colombel, M.; Clezardin, P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002, 62, 6538–6544. [Google Scholar]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Bone Quality and Quantity and Dental Implant Failure: A Systematic Review and Meta-analysis. Int. J. Prosthodont. 2017, 30, 219–237. [Google Scholar] [CrossRef]
- Perazella, M.A.; Markowitz, G.S. Bisphosphonate nephrotoxicity. Kidney Int. 2008, 74, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.E.; Vargo-Gogola, T.; Roeder, R.K. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Adv. Drug. Deliv. Rev. 2016, 99, 12–27. [Google Scholar] [CrossRef]
- Marx, R.E. A decade of bisphosphonate bone complications: What it has taught us about bone physiology. Int. J. Oral Maxillofac. Implants 2014, 29, e247–e258. [Google Scholar] [CrossRef]
- Compain, H.; Berquet, A.; Loison-Robert, L.S.; Ahossi, V.; Zwetyenga, N. Duration of treatment with bisphosphonates at the time of osteonecrosis of the jaw onset in patients with rheumatoid arthritis. Review. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.L.; Schousboe, J.T.; Gold, D.T. Oral bisphosphonate compliance and persistence: A matter of choice? Osteoporos. Int. 2011, 22, 21–26. [Google Scholar] [CrossRef]
- Conte, P.; Guarneri, V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004, 9 (Suppl. 4), 28–37. [Google Scholar] [CrossRef]
- Cremers, S.C.; Pillai, G.; Papapoulos, S.E. Pharmacokinetics/pharmacodynamics of bisphosphonates: Use for optimisation of intermittent therapy for osteoporosis. Clin. Pharmacokinet. 2005, 44, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Analysis of risk factors for cluster behavior of dental implant failures. Clin. Implant Dent. Relat. Res. 2017, 19, 632–642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, N.; Fadhul, F.; Chrcanovic, B.R. Bisphosphonates and Dental Implants: A Systematic Review and Meta-Analysis. Materials 2023, 16, 6078. https://doi.org/10.3390/ma16186078
Sulaiman N, Fadhul F, Chrcanovic BR. Bisphosphonates and Dental Implants: A Systematic Review and Meta-Analysis. Materials. 2023; 16(18):6078. https://doi.org/10.3390/ma16186078
Chicago/Turabian StyleSulaiman, Nabaa, Fadi Fadhul, and Bruno Ramos Chrcanovic. 2023. "Bisphosphonates and Dental Implants: A Systematic Review and Meta-Analysis" Materials 16, no. 18: 6078. https://doi.org/10.3390/ma16186078
APA StyleSulaiman, N., Fadhul, F., & Chrcanovic, B. R. (2023). Bisphosphonates and Dental Implants: A Systematic Review and Meta-Analysis. Materials, 16(18), 6078. https://doi.org/10.3390/ma16186078






