Investigation of the Pozzolanic Activity Improvement of Yellow Phosphorus Slag with Thermal Activation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. XRD Analysis
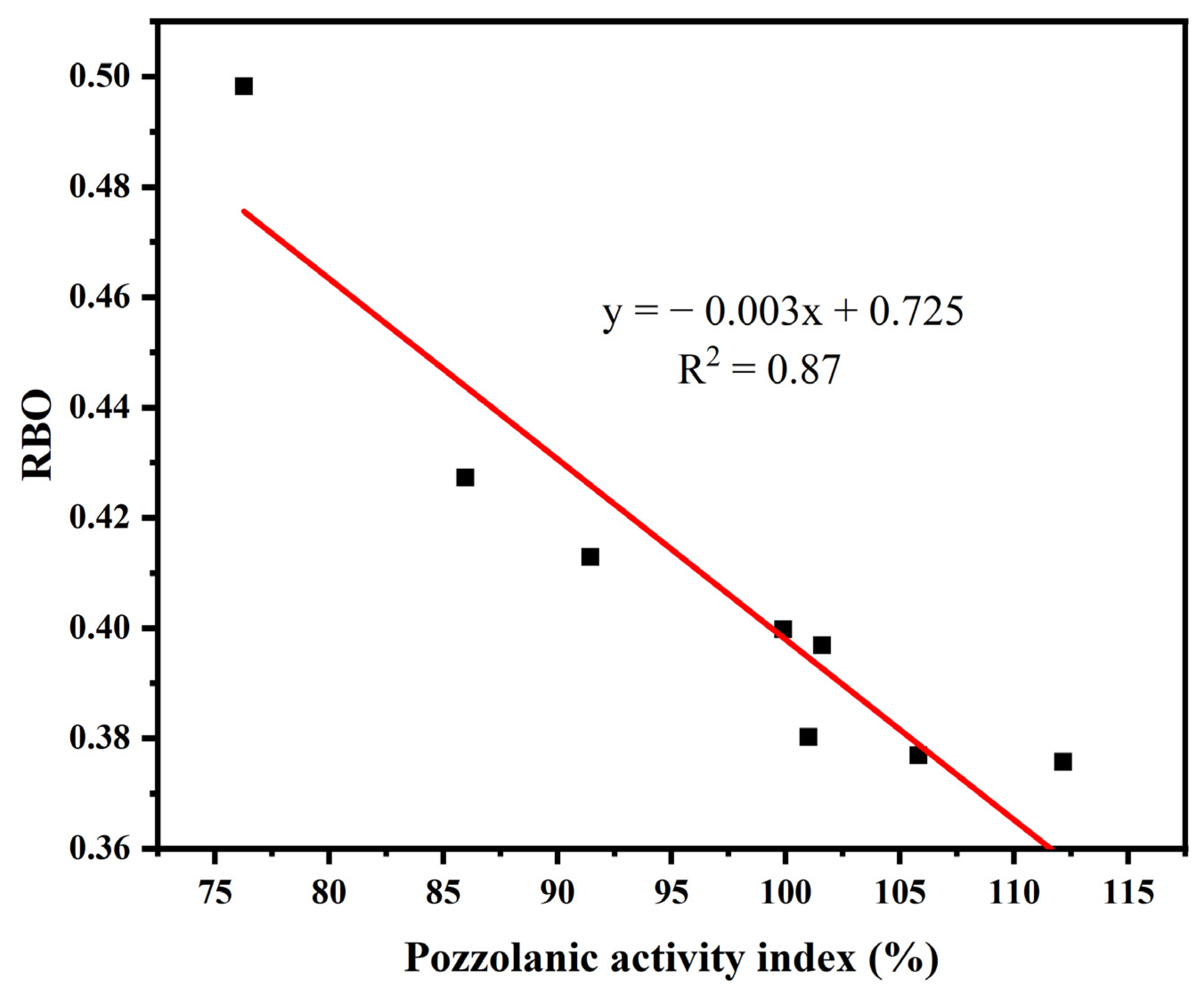
3.2. Pozzolanic Activity Evaluation by Compressive Strength
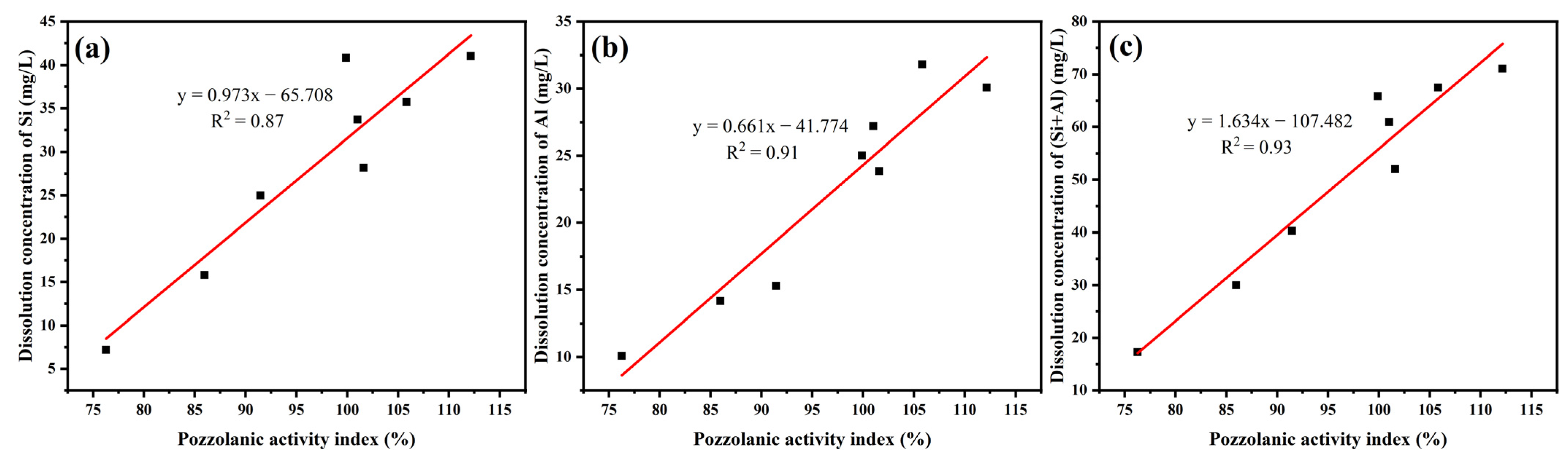
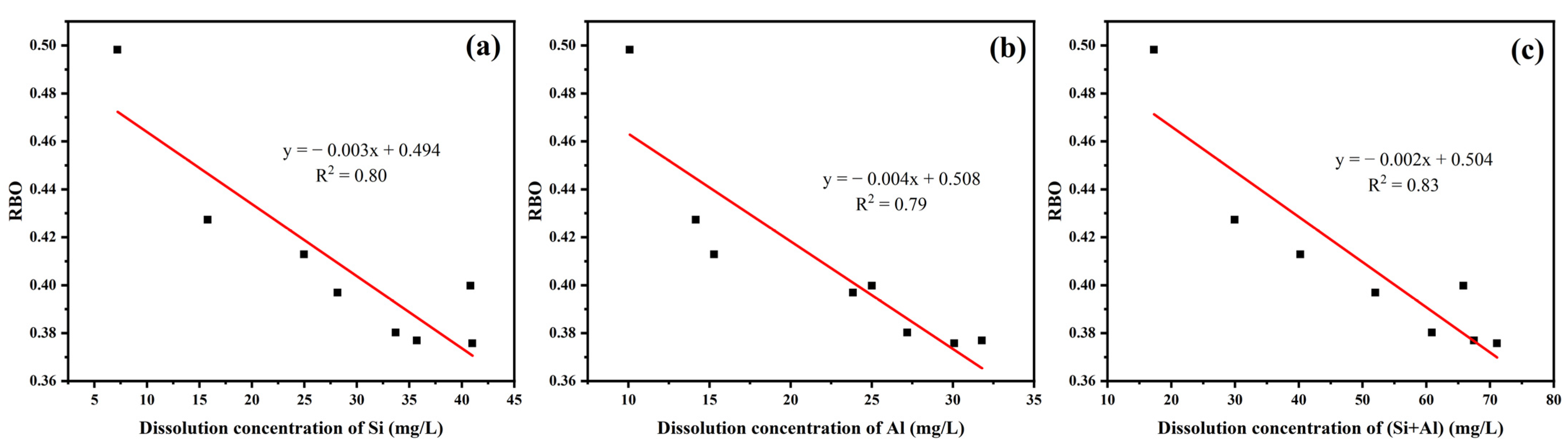
3.3. Pozzolanic Activity Evaluation by Dissolution Concentrations of Si and Al
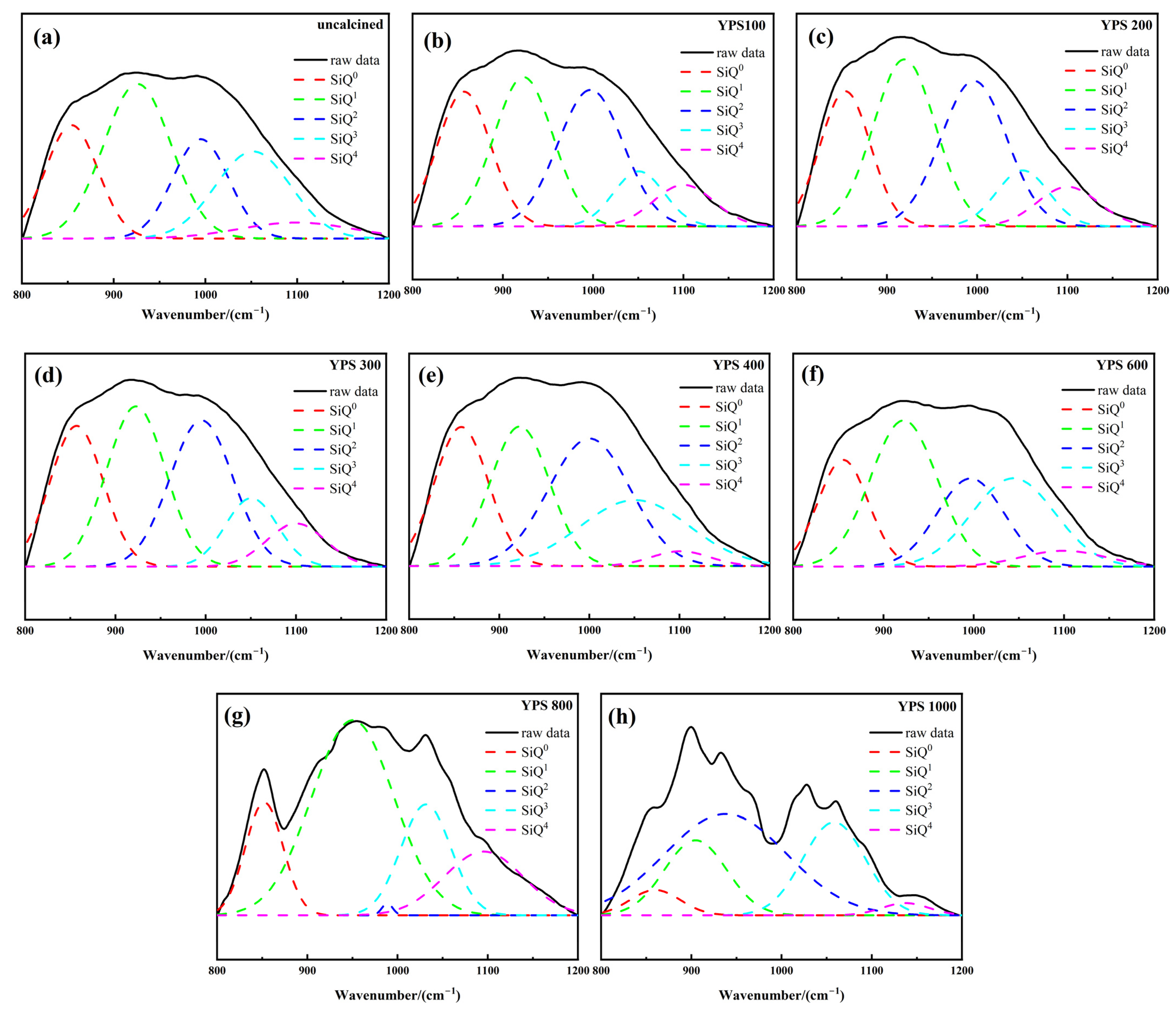
3.4. Pozzolanic Activity Evaluation by FTIR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassankhani-Majd, Z.; Anbia, M. Recovery of Valuable Materials from Phosphorus Slag Using Nitric Acid Leaching Followed by Precipitation Method. Resour. Conserv. Recycl. 2021, 169, 105547. [Google Scholar] [CrossRef]
- Akcil, A.; Karshigina, Z.B.; Bochevskaya, Y.G.; Abisheva, Z.S. Conditions of Nitric Acid Treatment of Phosphorus Slag for Rems Recovery and Production of Precipitated Silicon Dioxide. Complex Use Miner. Resour. 2018, 305, 28–38. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Karshigina, Z.B.; Bochevskaya, Y.G.; Akcil, A.; Sargelova, E.A.; Kvyatkovskaya, M.N.; Silachyov, I.Y. Recovery of Rare Earth Metals as Critical Raw Materials from Phosphorus Slag of Long-Term Storage. Hydrometallurgy 2017, 173, 271–282. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Zhang, Z. Recycling and Comprehensive Utilization of Yellow Phosphorus Slag in Building Materials: A Review. Constr. Build. Mater. 2023, 396, 132384. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, V.C.; Nguyen, T.C.; Tran, D.M.T.; Nguyen, T.T.T.; Vu, Q.T.; Nguyen, D.T.; Thai, H. Treatment of Yellow Phosphorus Slag and Reuse of It as an Absorbent of Chromium (VI) Ions and Methylene Blue. J. Chem. 2020, 2020, 1834829. [Google Scholar] [CrossRef]
- Hassankhani-Majd, Z.; Anbia, M.; Tavoussi-Shirazi, D.S. A Zero-Waste Process for the Extraction of Highly Pure Nanoporous Silica Particles from Phosphorus Slag. JOM 2022, 74, 1002–1011. [Google Scholar] [CrossRef]
- Naseroleslami, R.; Bakhshi, J.; Nemati Chari, M.; Yaghoobi, M.A.; Haji Mahdi, M. Properties of Cement Mortars Containing Phosphorous Slag. J. Mater. Civ. Eng. 2023, 35, 04022384. [Google Scholar] [CrossRef]
- Pavlíková, M.; Rovnaníková, P.; Záleská, M.; Pavlík, Z. Diatomaceous Earth—Lightweight Pozzolanic Admixtures for Repair Mortars—Complex Chemical and Physical Assessment. Materials 2022, 15, 6881. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodloorad, H.; Allahverdi, A. Efflorescence Formation and Control in Alkali-Activated Phosphorus Slag Cement. Int. J. Civ. Eng. 2016, 14, 425–438. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Shao, X.; Mo, K.H.; Ling, T.C. Enhancement of Early Age Cementitious Properties of Yellow Phosphorus Slag via CO2 Aqueous Carbonation. Cem. Concr. Compos. 2022, 133, 104702. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Kani, E. Modeling the Influence of Chemical Composition on Compressive Strength Behavior of Alkali-Activated Phosphorus Slag Cement Using Statistical Design. Can. J. Civ. Eng. 2018, 45, 1073–1083. [Google Scholar] [CrossRef]
- Golewski, G. Evaluation of Morphology and Size of Cracks of the Interfacial Transition Zone (ITZ) in Concrete Containing Fly Ash (FA). J. Hazard. Mater. 2018, 357, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Mahinroosta, M.; Pilehvar, S. A Temperature–Age Model For Prediction of Compressive Strength of Chemically Activated High Phosphorus Slag Content Cement. Int. J. Civ. Eng. 2017, 15, 839–847. [Google Scholar] [CrossRef]
- Shaikezhan, A.; Anuarova, A.D.; Antonovic, V. Cement Slurry from Electro-Phosphoric Slag. Mag. Civ. Eng. 2020, 98, 9806. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A.; Dong, P.; Bassim, N.; Mahinroosta, M. Resistance of Red Clay Brick Waste/Phosphorus Slag-Based Geopolymer Mortar to Acid Solutions of Mild Concentration. J. Build. Eng. 2021, 34, 102066. [Google Scholar] [CrossRef]
- Mahambetova, U.; Nuranbayeva, B.; Estemesov, Z.; Sadykov, P.; Mamyrbayev, O.; Oralbekova, D. Development And Research Of The Influence Of The Composition And Concentration Of Activators On The Strength Of Phosphorus Slag Binders. East.-Eur. J. Enterp. Technol. 2021, 5, 54–61. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Z.; Wang, D. Influence of High-Volume Electric Furnace Nickel Slag and Phosphorous Slag on the Properties of Massive Concrete. J. Therm. Anal. Calorim. 2018, 131, 873–885. [Google Scholar] [CrossRef]
- Yang, R.; Yu, R.; Shui, Z.; Gao, X.; Xiao, X.; Zhang, X.; Wang, Y.; He, Y. Low Carbon Design of an Ultra-High Performance Concrete (UHPC) Incorporating Phosphorous Slag. J. Clean. Prod. 2019, 240, 118157. [Google Scholar] [CrossRef]
- Allahverdi, A.; Bahri Rasht Abadi, M.M. Resistance of Chemically Activated High Phosphorous Slag Content Cement against Frost-Salt Attack. Cold Reg. Sci. Technol. 2014, 98, 18–25. [Google Scholar] [CrossRef]
- Maghsoodloorad, H.; Allahverdi, A. Developing Low-Cost Activators for Alkali-Activated Phosphorus Slag-Based Binders. J. Mater. Civ. Eng. 2017, 29, 04017006. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Xiao, H.; Zhao, J.; Zhang, Y.; Liu, X. Structural Characterization of Phosphorous Slag Regarding Occurrence State of Phosphorus in Dicalcium Silicate. Materials 2022, 15, 7450. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. Strength Development and Acid Resistance of Geopolymer Based on Waste Clay Brick Powder and Phosphorous Slag. Struct. Concr. 2019, 20, 1596–1606. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Najafi Kani, E.; Palomo Sanchez, A.; Fernandez-Jimenez, A. Rheology of Activated Phosphorus Slag with Lime and Alkaline Salts. Cem. Concr. Res. 2018, 113, 121–129. [Google Scholar] [CrossRef]
- Salehi, A.; Najafi Kani, E. Green Cylindrical Mesoporous Adsorbent Based on Alkali-Activated Phosphorous Slag: Synthesis, Dye Removal, and RSM Modeling. Adsorption 2018, 24, 647–666. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Najafi Kani, E. Rheology and Apparent Activation Energy of Alkali Activated Phosphorous Slag. Constr. Build. Mater. 2018, 171, 197–204. [Google Scholar] [CrossRef]
- Maghsoodloorad, H.; Khalili, H.; Allahverdi, A. Alkali-Activated Phosphorous Slag Performance under Different Curing Conditions: Compressive Strength, Hydration Products, and Microstructure. J. Mater. Civ. Eng. 2017, 30, 04017253. [Google Scholar] [CrossRef]
- He, X.; Ye, Q.; Yang, J.; Dai, F.; Su, Y.; Wang, Y.; Bohumír, S. Physico-Chemical Characteristics of Wet-Milled Ultrafine-Granulated Phosphorus Slag as a Supplementary Cementitious Material. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 625–633. [Google Scholar] [CrossRef]
- Hu, J. Comparison between the Effects of Superfine Steel Slag and Superfine Phosphorus Slag on the Long-Term Performances and Durability of Concrete. J. Therm. Anal. Calorim. 2017, 128, 1251–1263. [Google Scholar] [CrossRef]
- Allahverdi, A.; Mahinroosta, M. Mechanical Activation of Chemically Activated High Phosphorous Slag Content Cement. Powder Technol. 2013, 245, 182–188. [Google Scholar] [CrossRef]
- Allahverdi, A.; Akhondi, M.; Mahinroosta, M. Superior Sodium Sulfate Resistance of a Chemically Activated Phosphorus Slag–Based Composite Cement. J. Mater. Civ. Eng. 2017, 29, 04016231. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Yang, J. Hydration Mechanisms of Composite Binders Containing Phosphorus Slag at Different Temperatures. Constr. Build. Mater. 2017, 147, 720–732. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, R.; Hu, W.; Jiang, X.; Zhang, X.; Huang, B. Effect of Granulated Phosphorus Slag on Physical, Mechanical and Microstructural Characteristics of Class F Fly Ash Based Geopolymer. Constr. Build. Mater. 2021, 291, 123287. [Google Scholar] [CrossRef]
- Allahverdi, A.; Pilehvar, S.; Mahinroosta, M. Influence of Curing Conditions on the Mechanical and Physical Properties of Chemically-Activated Phosphorous Slag Cement. Powder Technol. 2016, 288, 132–139. [Google Scholar] [CrossRef]
- Administration, M.; Standardization, A. GB/T 26751-2022; The Chinese Standard Ground Granulated Electric Furnace Phosphorus Slag Powder Used for Cement and Concrete, in Chinese Code GB/T 26751-2022. Chinese Standard Press: Beijing, China, 2022.
- Wang, Y.; Liu, X.; Xie, Z.; Wang, H.; Zhang, W.; Xue, Y. Rapid Evaluation of the Pozzolanic Activity of Bayer Red Mud by a Polymerization Degree Method: Correlations with Alkali Dissolution of (Si + Al) and Strength. Materials 2021, 14, 5546. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, Y.; Zhang, Z.; Liu, X.; Wang, Y. Harmless Treatment of Electrolytic Manganese Residue: Ammonia Nitrogen Recovery, Preparation of Struvite and Nonsintered Bricks. Chem. Eng. J. 2023, 455, 140739. [Google Scholar] [CrossRef]
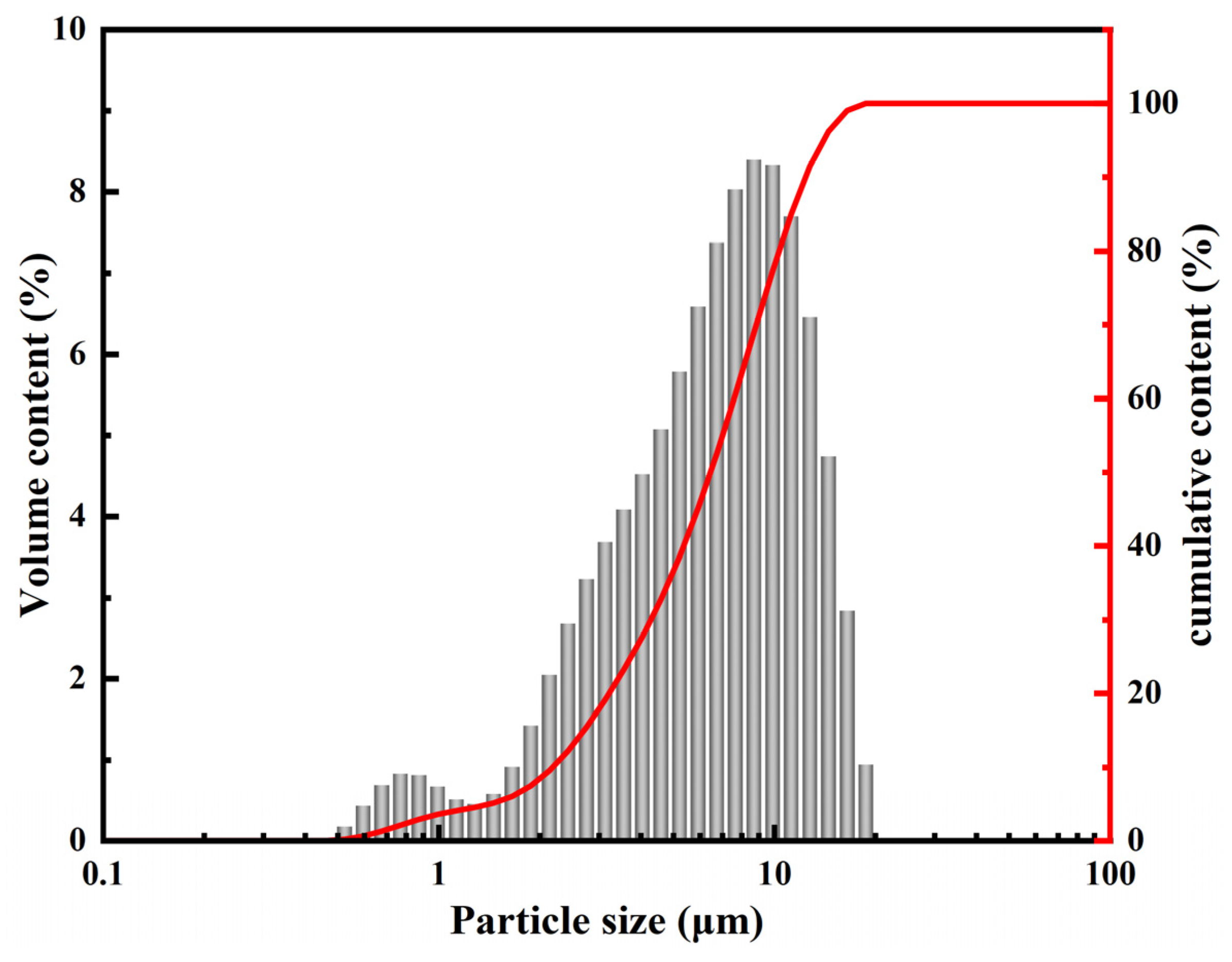


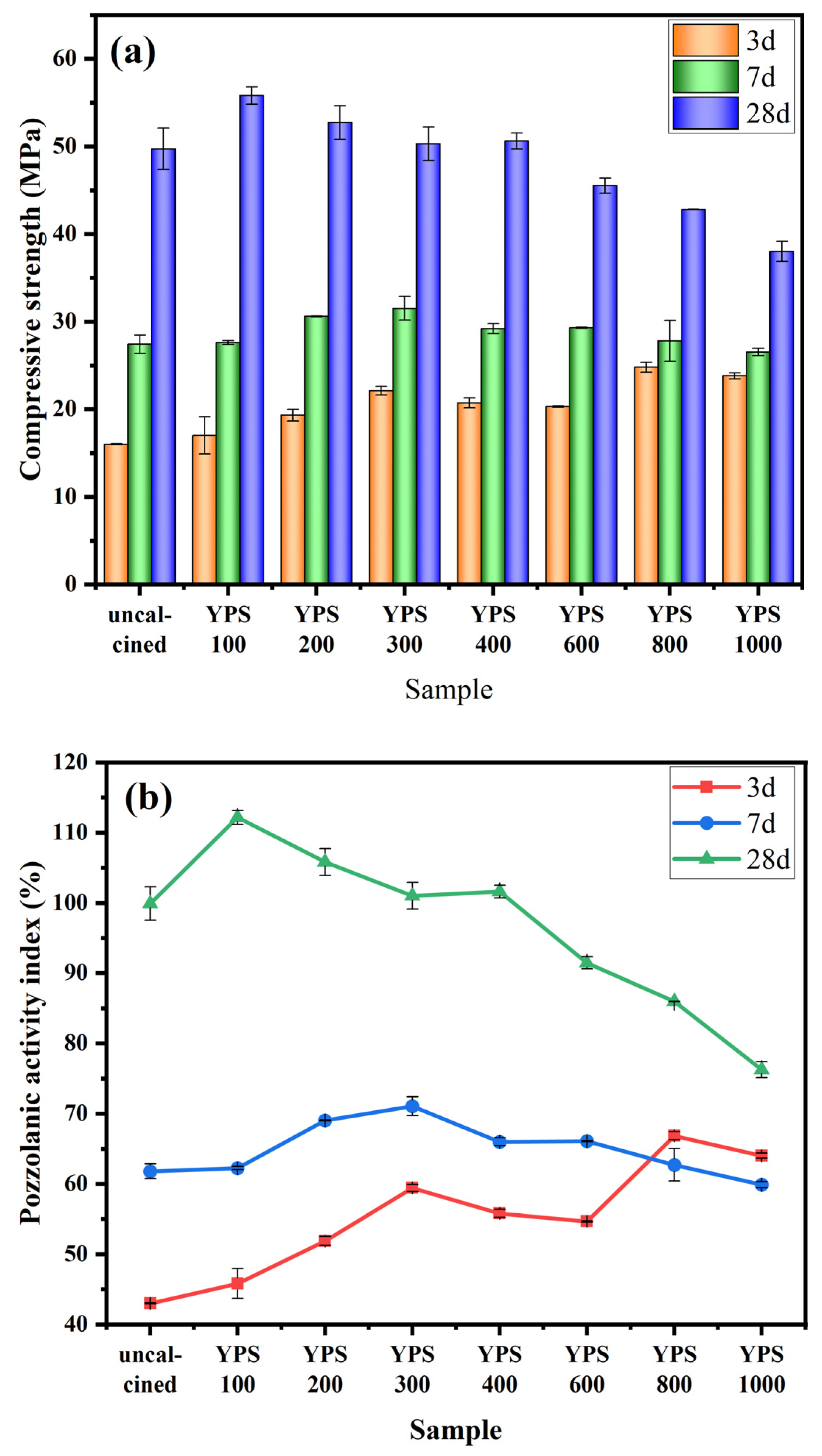
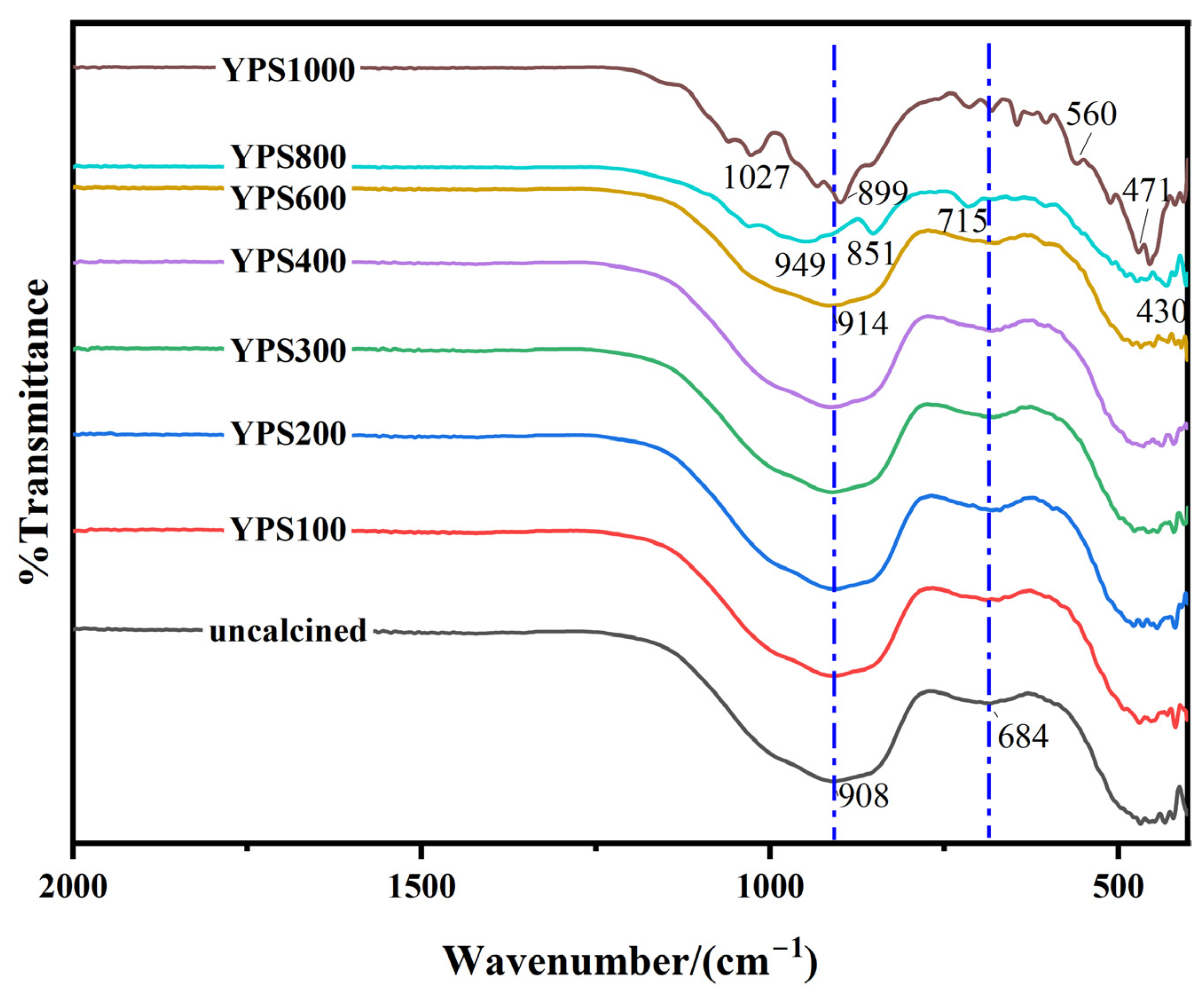
| Oxides | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | SO3 | P2O5 | F |
|---|---|---|---|---|---|---|---|---|---|---|
| YPS | 44.72 | 34.84 | 6.00 | 1.19 | 1.94 | 0.35 | 1.43 | 2.36 | 3.18 | 3.24 |
| Cement | 63.48 | 20.70 | 5.00 | 3.59 | 2.76 | 0.14 | 0.82 | 2.59 | 0.075 | 0.096 |
| Sample | YPS Ash/g | Cement/g | Standard Sand/g | Water/g | w/c Ratio |
|---|---|---|---|---|---|
| YPS sample | 135 | 315 | 1350 | 225 | 0.5 |
| Control sample | 0 | 450 | 1350 | 225 | 0.5 |
| Sample | Dissolution Concentration of Si | Dissolution Concentration of Al | Dissolution Concentration of (Si + Al) |
|---|---|---|---|
| uncalcined | 40.83 | 25.01 | 65.84 |
| YPS100 | 41.01 | 30.08 | 71.09 |
| YPS200 | 35.71 | 31.79 | 67.50 |
| YPS300 | 33.71 | 27.19 | 60.90 |
| YPS400 | 28.17 | 23.84 | 52.01 |
| YPS600 | 24.97 | 15.29 | 40.26 |
| YPS800 | 15.79 | 14.16 | 29.95 |
| YPS1000 | 7.197 | 10.08 | 17.28 |
| Sample | Relative Content/% | RBO | R2 | ||||
|---|---|---|---|---|---|---|---|
| SiQ0 | SiQ1 | SiQ2 | SiQ3 | SiQ4 | |||
| uncalcined | 19.24 | 34.83 | 18.43 | 21.78 | 5.72 | 0.3998 | 0.996 |
| YPS100 | 23.22 | 29.34 | 29.75 | 9.33 | 8.36 | 0.3757 | 0.996 |
| YPS200 | 20.63 | 32.40 | 30.14 | 9.24 | 7.59 | 0.3769 | 0.997 |
| YPS300 | 22.75 | 29.38 | 28.86 | 11.07 | 7.94 | 0.3802 | 0.997 |
| YPS400 | 21.48 | 24.35 | 30.66 | 20.95 | 2.56 | 0.3969 | 0.996 |
| YPS600 | 17.78 | 33.02 | 19.95 | 24.81 | 4.44 | 0.4128 | 0.997 |
| YPS800 | 13.68 | 51.86 | 0.42 | 17.95 | 16.09 | 0.4273 | 0.983 |
| YPS1000 | 5.14 | 19.10 | 49.46 | 23.94 | 2.36 | 0.4982 | 0.963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, X.; Zhang, Z.; Wei, C.; Zeng, Q.; Li, Y.; Ma, S. Investigation of the Pozzolanic Activity Improvement of Yellow Phosphorus Slag with Thermal Activation. Materials 2023, 16, 6047. https://doi.org/10.3390/ma16176047
Liu X, Liu X, Zhang Z, Wei C, Zeng Q, Li Y, Ma S. Investigation of the Pozzolanic Activity Improvement of Yellow Phosphorus Slag with Thermal Activation. Materials. 2023; 16(17):6047. https://doi.org/10.3390/ma16176047
Chicago/Turabian StyleLiu, Xinyue, Xiaoming Liu, Zengqi Zhang, Chao Wei, Qingsen Zeng, Yantian Li, and Shanliang Ma. 2023. "Investigation of the Pozzolanic Activity Improvement of Yellow Phosphorus Slag with Thermal Activation" Materials 16, no. 17: 6047. https://doi.org/10.3390/ma16176047
APA StyleLiu, X., Liu, X., Zhang, Z., Wei, C., Zeng, Q., Li, Y., & Ma, S. (2023). Investigation of the Pozzolanic Activity Improvement of Yellow Phosphorus Slag with Thermal Activation. Materials, 16(17), 6047. https://doi.org/10.3390/ma16176047









