Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment
Abstract
:1. Introduction
2. Experimental Procedures
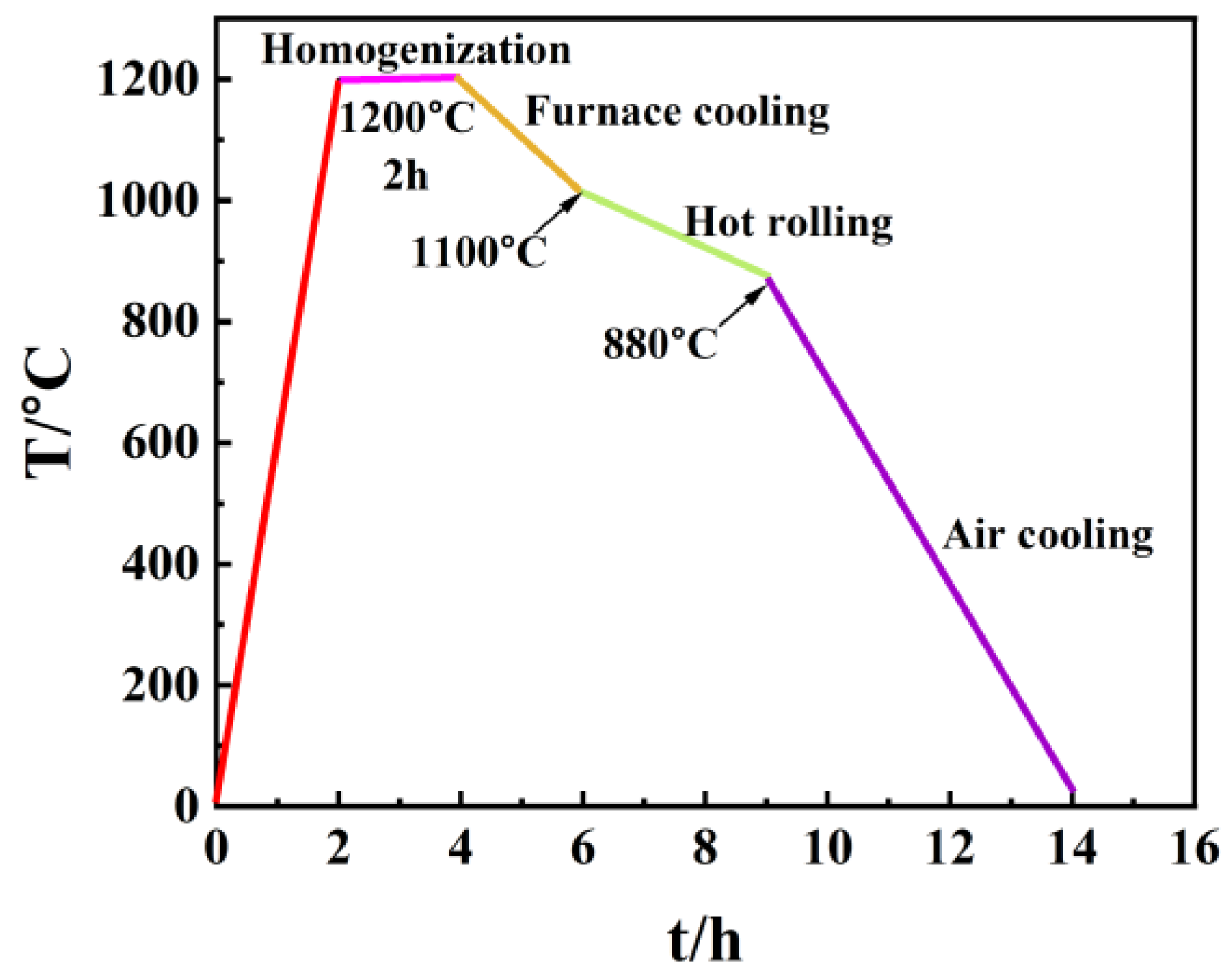
2.1. Materials and Solution
2.2. Microstructural Analysis
2.3. Mechanical Property Test
2.4. Electrochemical Measurements
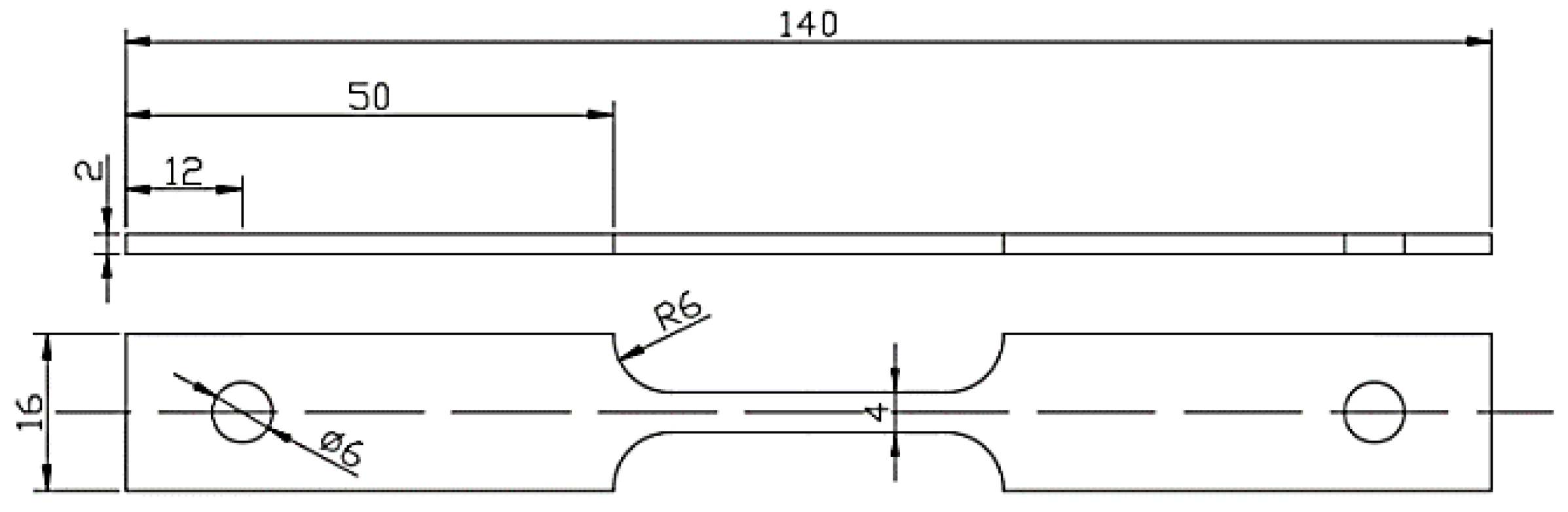
2.5. SSRT Tests
3. Results
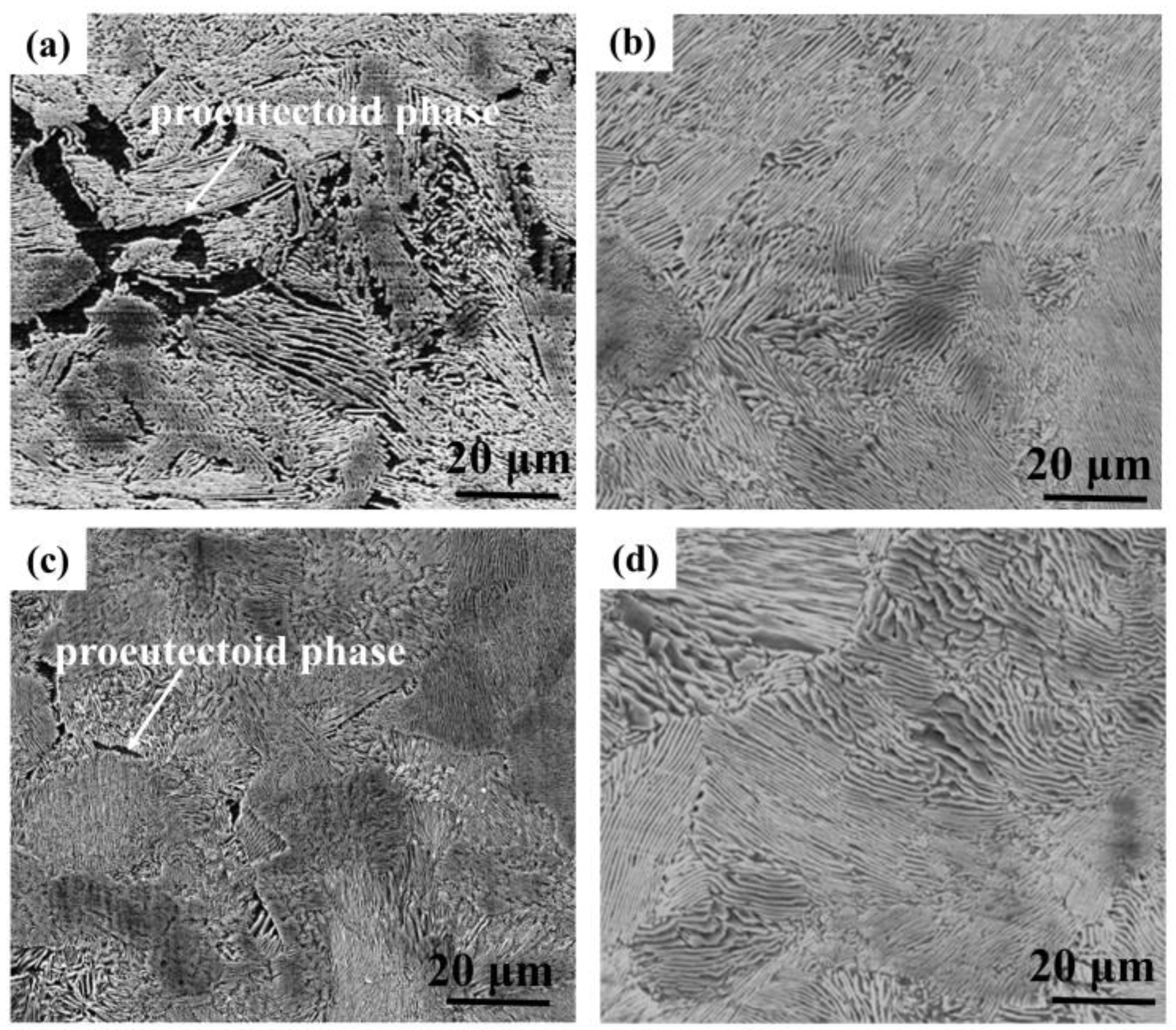
3.1. Microstructural Characterization
3.2. Mechanical Properties
3.3. Electrochemical Properties
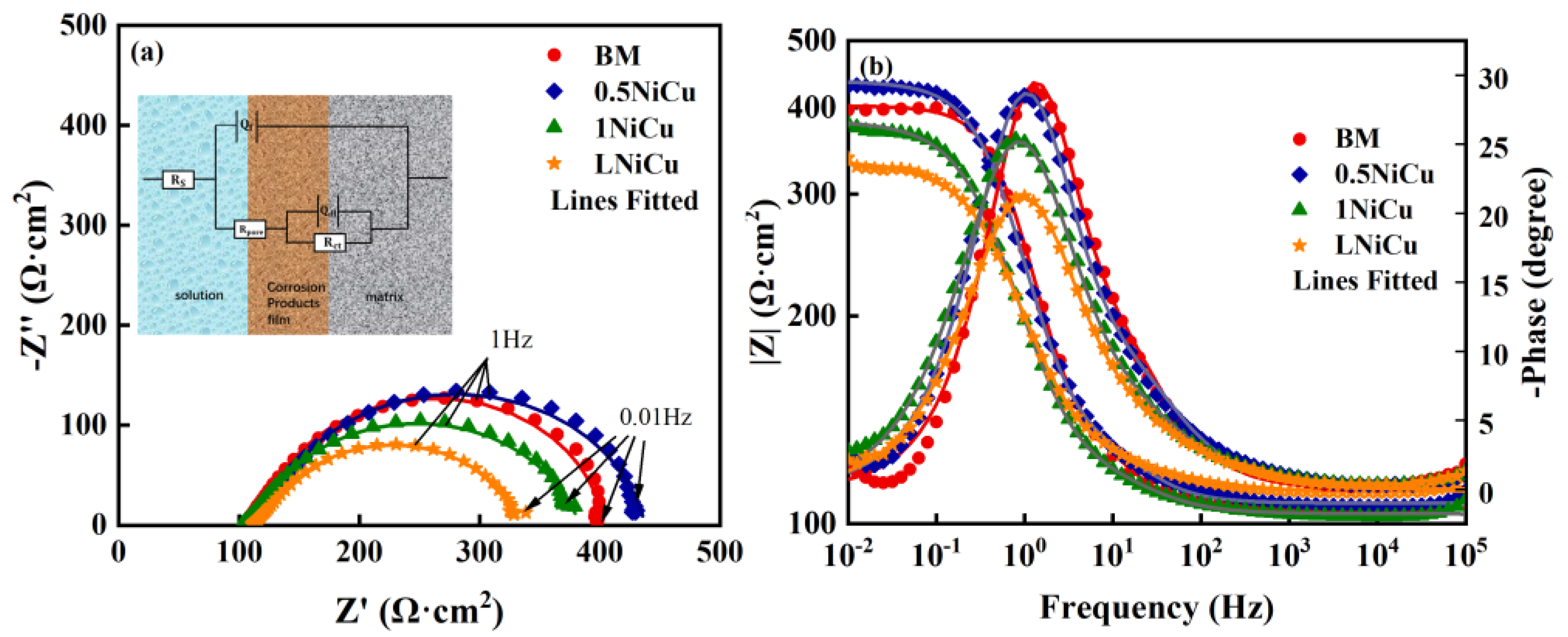
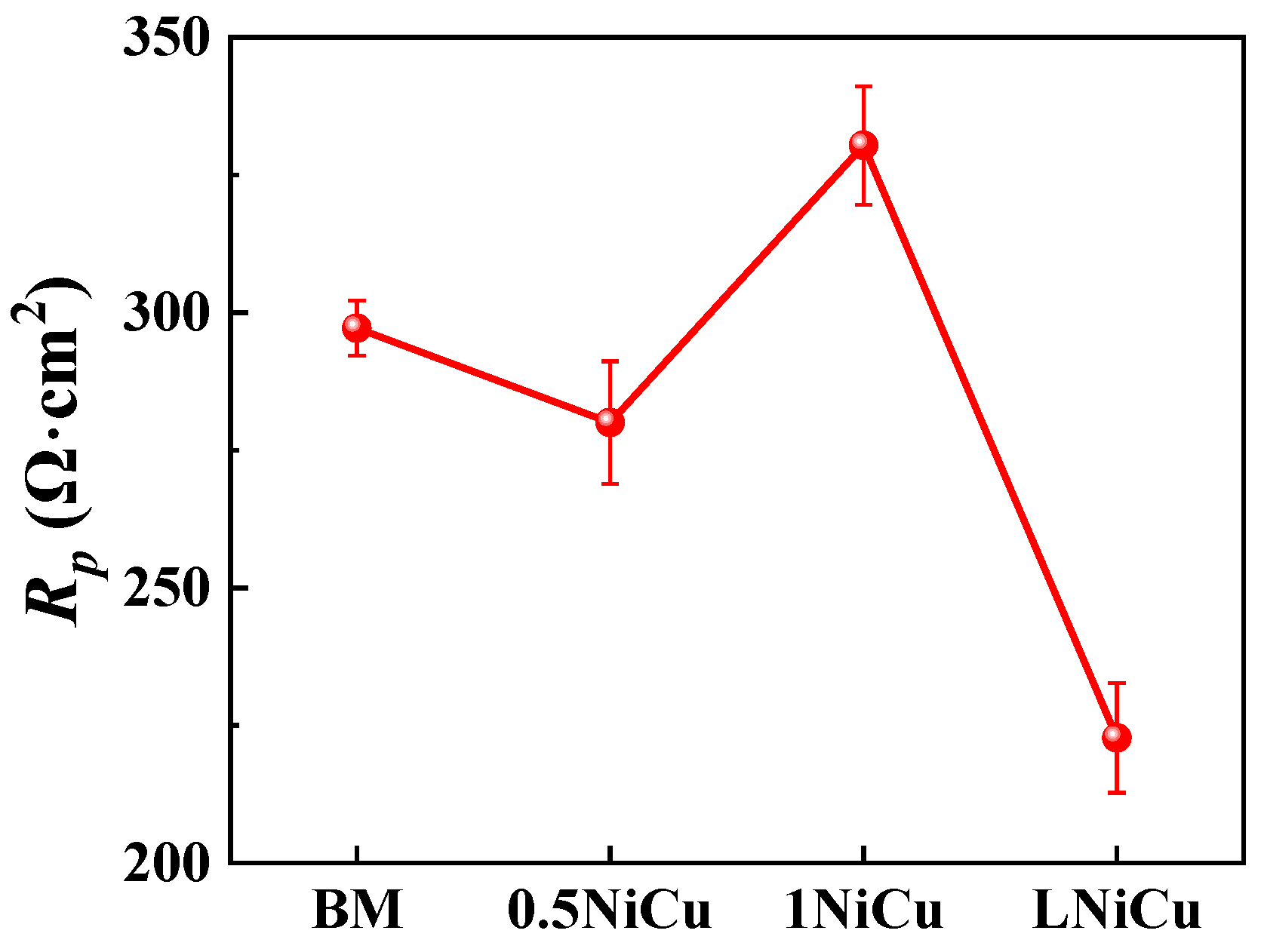
3.3.1. EIS Analysis
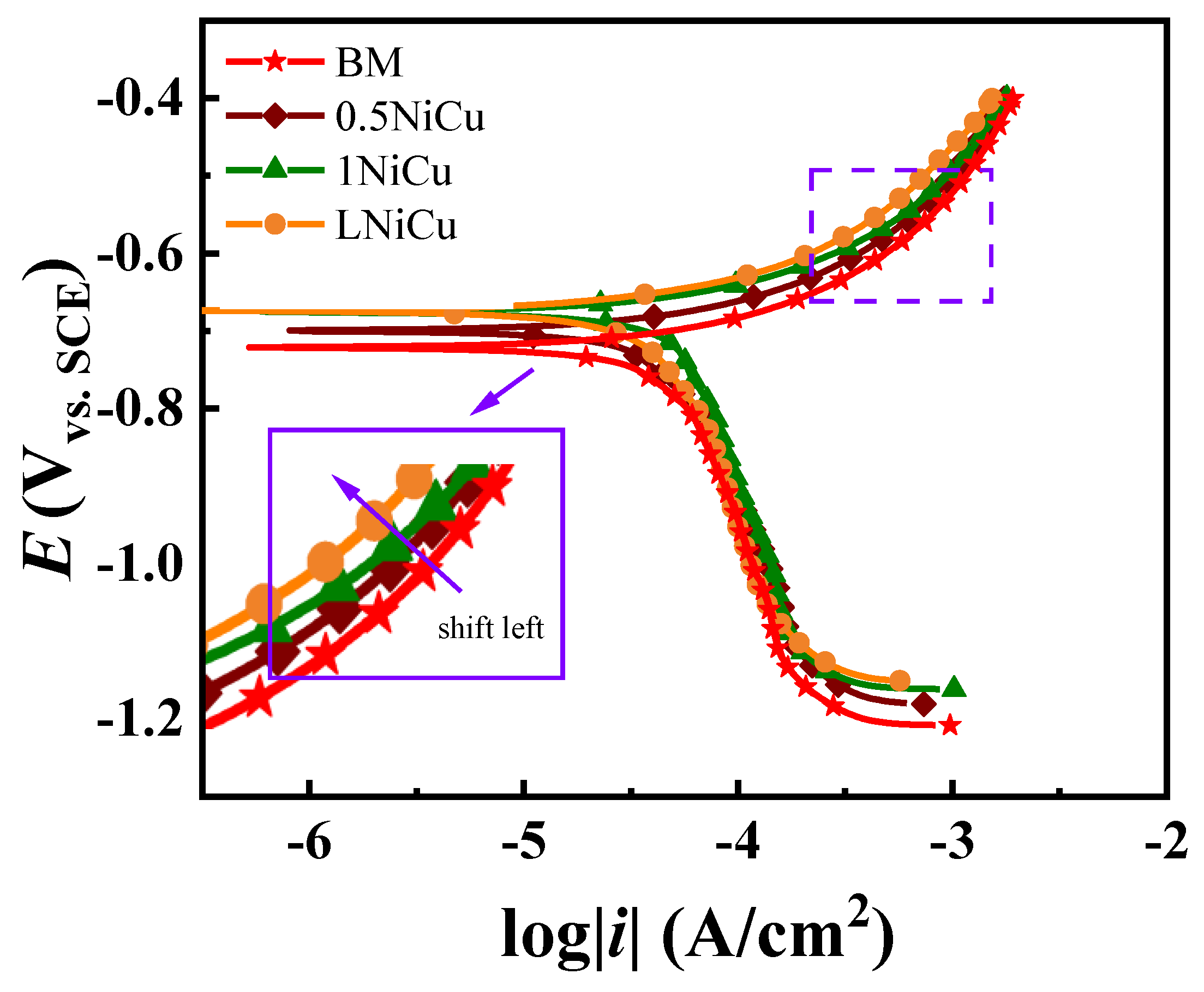
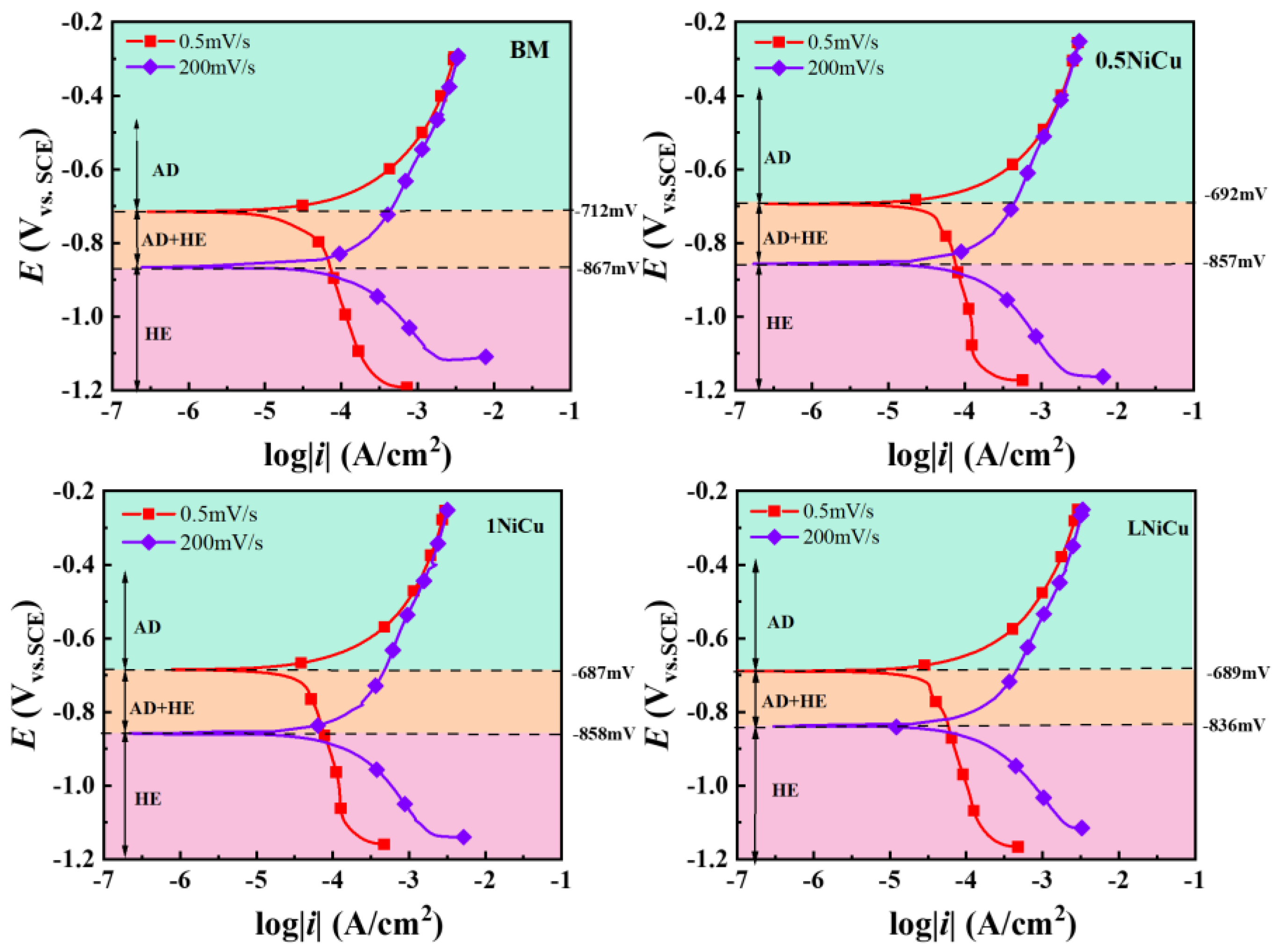
3.3.2. Polarization Curve Analysis
3.4. SSRT Results
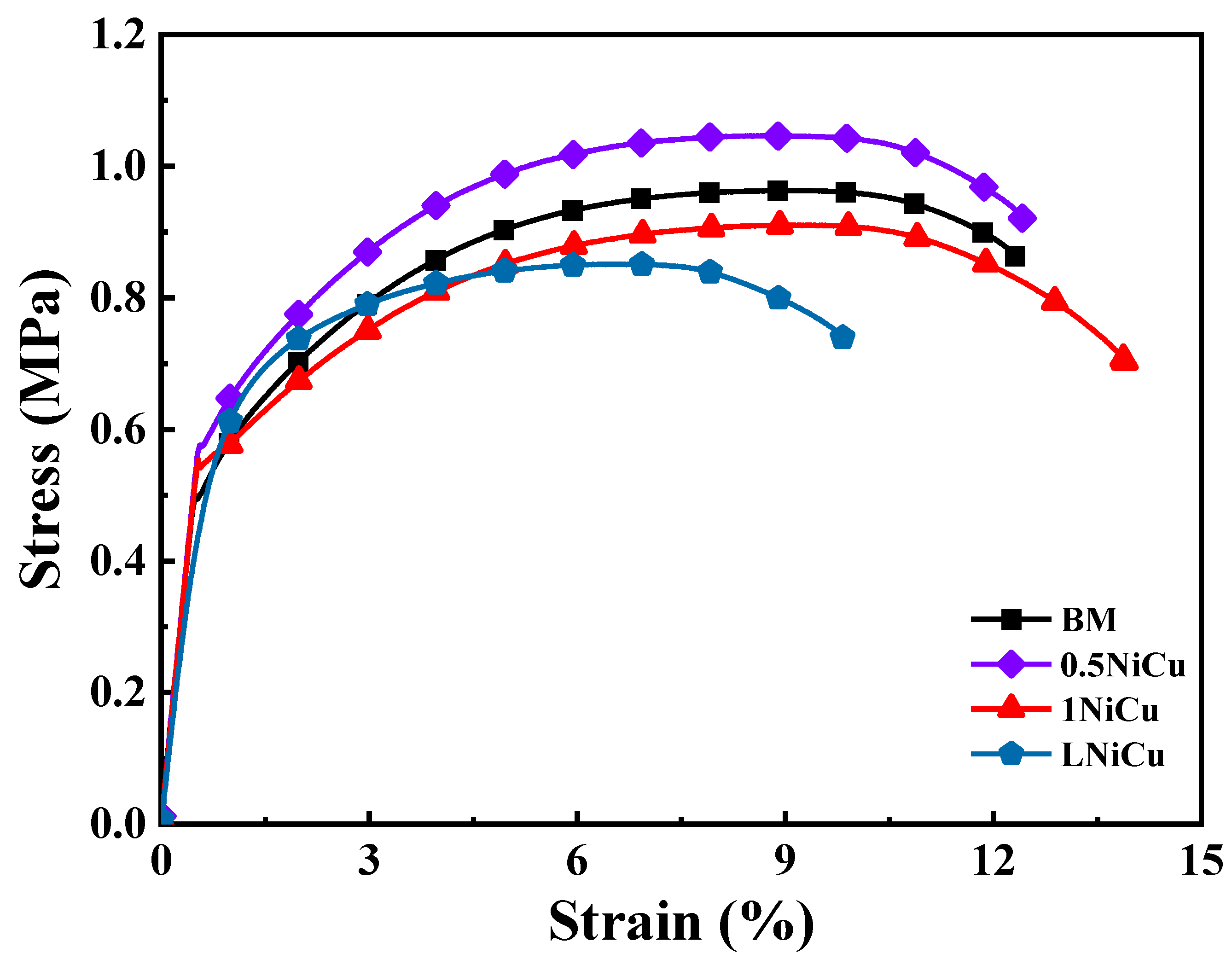
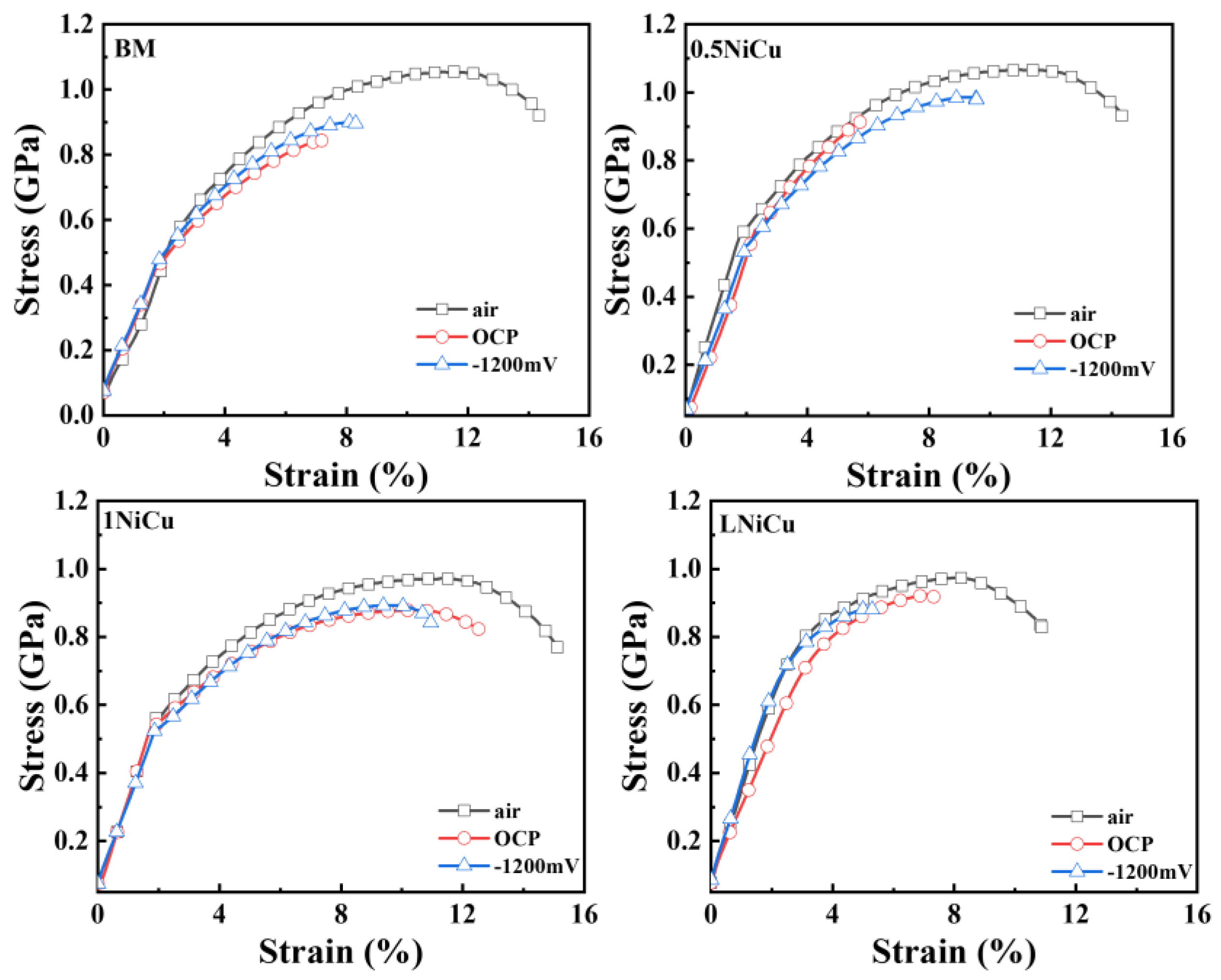
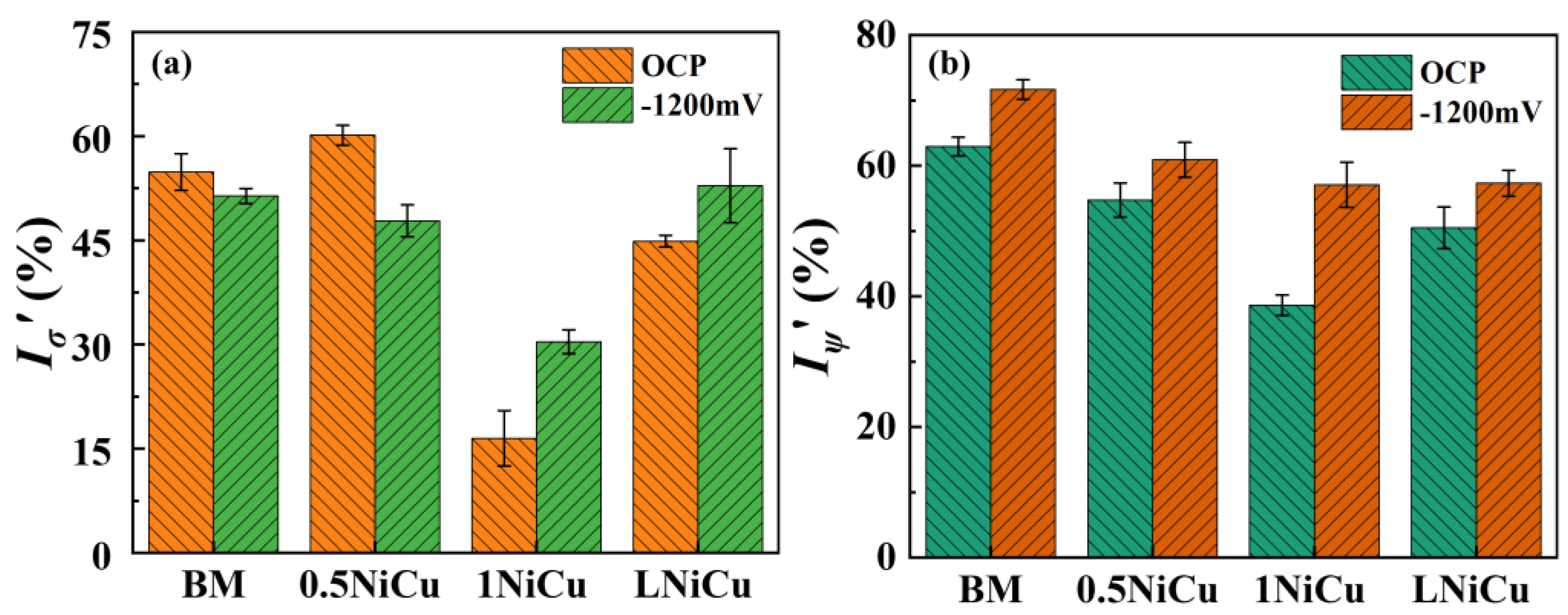
3.4.1. Stress–Strain Curves and SCC Susceptibility
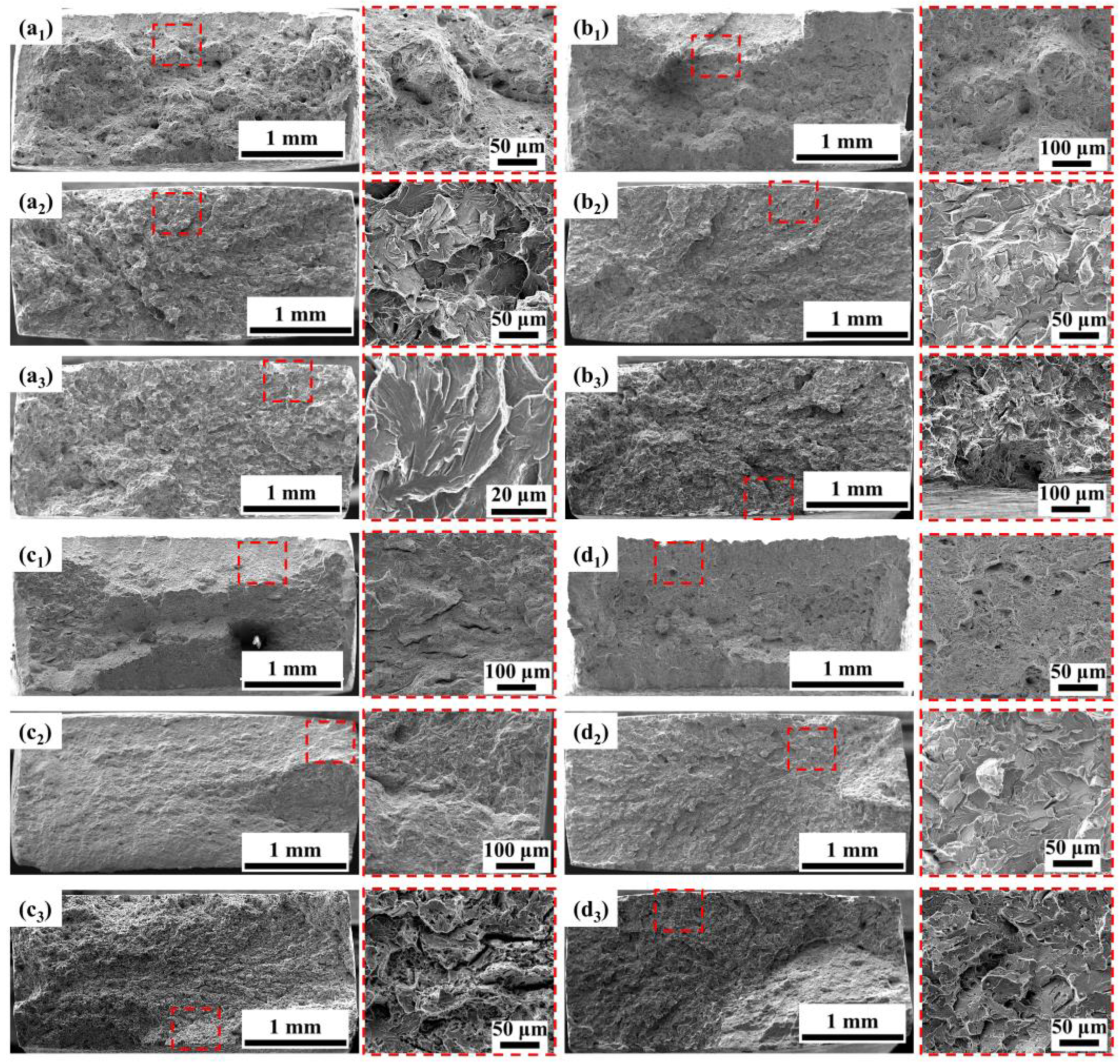
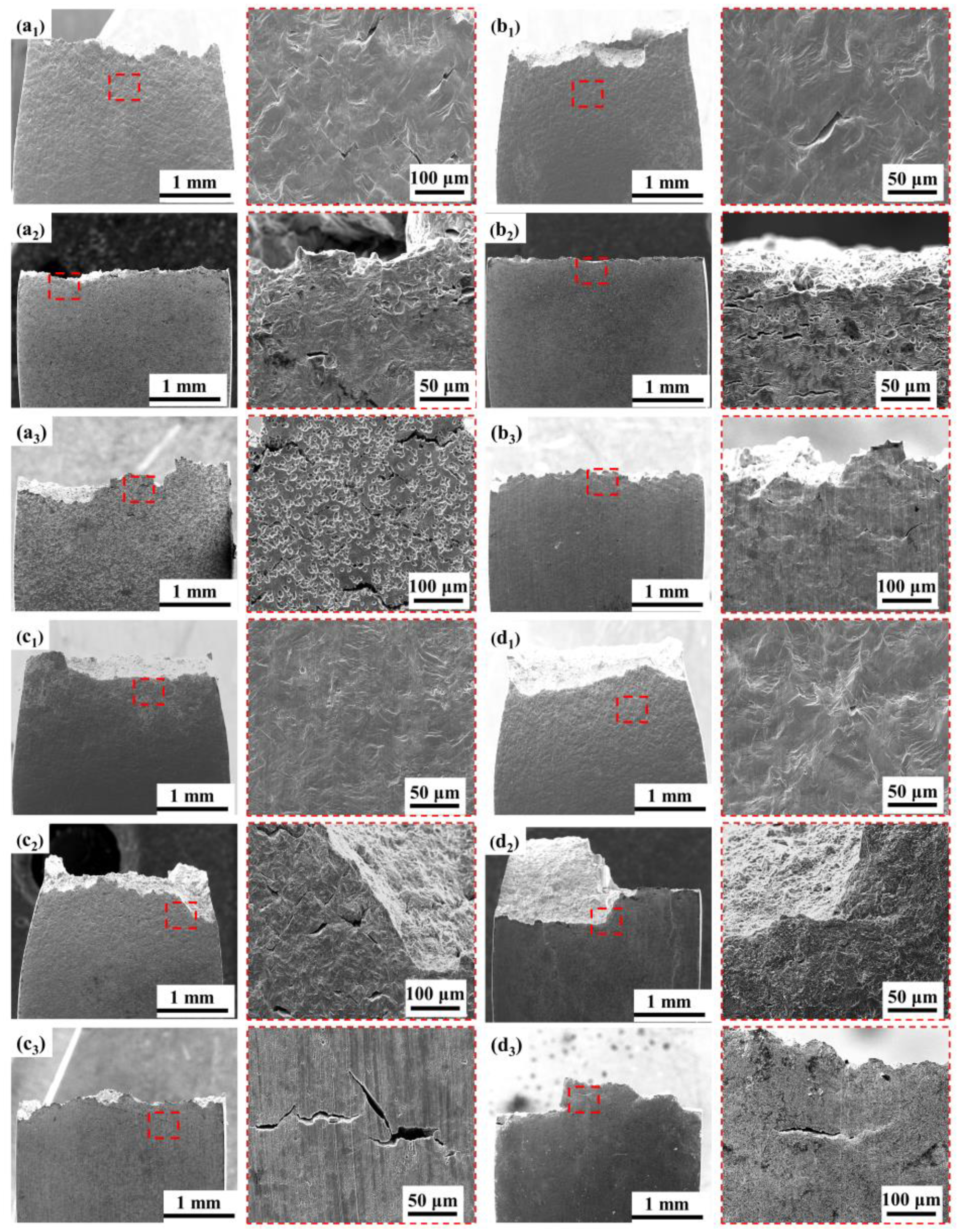
3.4.2. Fracture Analysis
4. Discussion
4.1. Effect of Alloy Elements on the Electrochemical Mechanism
4.2. Effect of Alloy Elements on the SCC Mechanism
5. Conclusions
- (1)
- The four kinds of anchor steels have a similar microstructure, which is mainly composed of pearlite.
- (2)
- The combined addition Ni, Cu, and Sb can improve the mechanical properties of the anchor steel, while reducing C content decreases tensile strength as a result of the loss of the solution-strengthening effect.
- (3)
- The addition of Sb, Cu, Ni, and reducing the content of C enhances the resistance to corrosion and SCC by mitigating AD, while adding Nb improves SCC by inhibiting HE. The combined action of 1% Ni, 0.5% Cu, 0.05% Nb, 0.1% Sb, and 0.5% C presents the highest SCC resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Share Corrosion Data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, X.; Ren, A. Investigations of Rock Anchor Corrosion and Its Influence Factors by Exhumations in Four Typical Field Sites. Eng. Fail. Anal. 2019, 101, 357–382. [Google Scholar] [CrossRef]
- Ananda Rao, M.; Sekhar Babu, R.; Pavan Kumar, M.V. Stress Corrosion Cracking Failure of a SS 316L High Pressure Heater Tube. Eng. Fail. Anal. 2018, 90, 14–22. [Google Scholar] [CrossRef]
- Bestautte, J.; Kalácska, S.; Béchet, D.; Obadia, Z.; Christien, F. Investigation of Quasi-Cleavage in a Hydrogen Charged Maraging Stainless Steel. Corros. Sci. 2023, 218, 111163. [Google Scholar] [CrossRef]
- Das, T.; Brahimi, S.V.; Song, J.; Yue, S. A Fast Fracture Approach to Assess Hydrogen Embrittlement (HE) Susceptibility and Mechanism(s) of High Strength Martensitic Steels. Corros. Sci. 2021, 190, 109701. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Yang, X.; Xu, X.; Cheng, X.; Li, X. Behavior and Evaluation of Stress Corrosion Cracking of Typical Anchor Bolt Steel in Simulated Crevice Environment. J. Mater. Res. Technol. 2023, 25, 7430–7443. [Google Scholar] [CrossRef]
- Matos, T.S.; Portella, K.F.; Henke, S.L.; Bragança, M.O.G.P.; Galvão, M.P.; Dias, B.G.; Lagoeiro, L.; Almeida, L.M. Analysis of Anchor Rod Failure in a Guyed Transmission Tower: Influence of Microstructures and Corrosion Mechanisms. Eng. Fail. Anal. 2021, 121, 105166. [Google Scholar] [CrossRef]
- Cano, H.; Díaz, I.; De La Fuente, D.; Chico, B.; Morcillo, M. Effect of Cu, Cr and Ni Alloying Elements on Mechanical Properties and Atmospheric Corrosion Resistance of Weathering Steels in Marine Atmospheres of Different Aggressivities. Mater. Corros. 2018, 69, 8–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Xu, Y.; Liu, Z. Effects of Cr, Ni and Cu on the Corrosion Behavior of Low Carbon Microalloying Steel in a Cl− Containing Environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Cano, H.; Neff, D.; Morcillo, M.; Dillmann, P.; Diaz, I.; De La Fuente, D. Characterization of Corrosion Products Formed on Ni 2.4 wt%–Cu 0.5 wt%–Cr 0.5 wt% Weathering Steel Exposed in Marine Atmospheres. Corros. Sci. 2014, 87, 438–451. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.; Wang, Q.; Li, X. Improving the Resistance of High-Strength Steel to SCC in a SO2-Polluted Marine Atmosphere through Nb and Sb Microalloying. Corros. Sci. 2020, 170, 108693. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, W.; Zhao, X.; Zhang, Y.; Zhao, X. The Potential Significance of Microalloying with Nb in Enhancing the Resistance to Hydrogen-Induced Delayed Fracture of 1300-MPa-Grade High-Strength Bolt Steel. Eng. Fail. Anal. 2022, 135, 106144. [Google Scholar] [CrossRef]
- Shi, R.; Ma, Y.; Wang, Z.; Gao, L.; Yang, X.-S.; Qiao, L.; Pang, X. Atomic-Scale Investigation of Deep Hydrogen Trapping in NbC/α-Fe Semi-Coherent Interfaces. Acta Mater. 2020, 200, 686–698. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, R.; Li, W.; Chen, Y.-H.; Chou, T.-H.; Wang, X.; Liu, Y.; Zhu, Y.; Huang, J.C. Effect of Interstitial Carbon and Nitrogen on Corrosion of FeCoCrNi Multi-Principal Element Alloys Made by Selective Laser Melting. J. Mater. Sci. Technol. 2023, 148, 52–63. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic materials—Tensile testing—Part 1: Method of test at room temperature. Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- Parkins, R.N. Predictive Approaches to Stress Corrosion Cracking Failure. Corros. Sci. 1980, 20, 147–166. [Google Scholar] [CrossRef]
- Ding, Z.; Liang, B.; Xu, Z.; Tian, R.; Dong, L.; Zhao, J. Formation and Morphology of Grain Boundary Proeutectoid Pearlite in 100Mn13 Steel during Low Temperature Aging Treatment. Mater. Today Commun. 2022, 33, 104887. [Google Scholar] [CrossRef]
- Jung, M.; Cho, W.; Park, J.; Jung, J.-G.; Lee, Y.-K. Variation of Carbon Concentration in Proeutectoid Ferrite during Austenitization in Hypoeutectoid Steel. Mater. Charact. 2014, 94, 161–169. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, E.; Zhang, C.; Kong, L.; Yang, H.; Zhang, X.; Wang, Y. Tuning Mechanical Behavior and Deformation Mechanisms in High-Manganese Steels via Carbon Content Modification. Mater. Sci. Eng. A 2023, 881, 145401. [Google Scholar] [CrossRef]
- Qiao, C.; Wang, M.; Hao, L.; Jiang, X.; Liu, X.; Thee, C.; An, X. In-Situ EIS Study on the Initial Corrosion Evolution Behavior of SAC305 Solder Alloy Covered with NaCl Solution. J. Alloys Compd. 2021, 852, 156953. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Sun, B.; Pan, Y.; Yang, J.; Guo, J.; Zhao, B.; Liu, X.; Liu, Z.; Li, X. Microstructure Evolution and SSCC Behavior of Strain-Strengthened 304 SS Pre-Strained at Room Temperature and Cryogenic Temperature. Corros. Sci. 2023, 210, 110855. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Dong, B.; Yang, W.; Chen, L.; Sun, Y.; Li, H.; Zhang, B. Clarifying the Effect of Cu Element on the Corrosion Properties of Ni-Mo Low Alloy Steel in Marine Environment. Corros. Sci. 2023, 216, 111107. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Dong, B.; Mao, R.; Sun, Y.; Chen, L. Corrosion of Cu-Doped Ni–Mo Low-Alloy Steel in a Severe Marine Environment. J. Phys. Chem. Solids 2022, 163, 110584. [Google Scholar] [CrossRef]
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Wang, X.; Cui, H. Corrosion Evolution and Stress Corrosion Cracking Behavior of a Low Carbon Bainite Steel in the Marine Environments: Effect of the Marine Zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.Z.; Du, C.W.; Li, J.K.; Li, X.G. Effect of Hydrogen-Induced Plasticity on the Stress Corrosion Cracking of X70 Pipeline Steel in Simulated Soil Environments. Mater. Sci. Eng. A 2016, 658, 348–354. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Z.; Zhang, Y.; Li, X.; Du, C. Effect of AC Current Density on the Stress Corrosion Cracking Behavior and Mechanism of E690 High-Strength Steel in Simulated Seawater. J. Mater. Eng. Perform. 2019, 28, 6931–6941. [Google Scholar] [CrossRef]
- Seol, J.-B.; Jung, J.E.; Jang, Y.W.; Park, C.G. Influence of Carbon Content on the Microstructure, Martensitic Transformation and Mechanical Properties in Austenite/ε-Martensite Dual-Phase Fe–Mn–C Steels. Acta Mater. 2013, 61, 558–578. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Wang, X.; Gong, K.; Du, J.; Huang, J.C.; Hu, M. Stress Corrosion Cracking Behavior of X80 Steel under the Combined Effects of Sulfide and Cathodic Potential. J. Mater. Res. Technol. 2023, 24, 3925–3936. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, B.; Liu, Z.; Wu, W.; Li, X. Hydrogen Effects on Passivation and SCC of 2205 DSS in Acidified Simulated Seawater. Corros. Sci. 2022, 208, 110640. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y. Long-Term Corrosion and Stress Corrosion Cracking of X65 Steel in H2O-Saturated Supercritical CO2 with SO2 and O2 Impurities. Constr. Build. Mater. 2023, 362, 129746. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu Alloy as a Biodegradable Material for Potential Vascular Stents Application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Gu, T.; Teng, W.; Liu, A.; Deng, Z.; Ling, L.; Zhang, W. Transformation of Nanoscale Zero-Valent Iron with Antimony: Effects of the Sb Spatial Configuration. Chem. Eng. J. 2021, 416, 129073. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, X.; Zhao, J.; Fan, Y.; Li, X. A Study of Rust Layer of Low Alloy Structural Steel Containing 0.1% Sb in Atmospheric Environment of the Yellow Sea in China. Corros. Sci. 2021, 188, 109549. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Song, J.; Zhang, X.; Li, Z.; Luo, H.; Yu, W.; Dong, C.; Xiao, K. Research on Corrosion Behavior of Truck Body Steel in Chlorine-Containing Sulfuric Acid Environment. J. Mater. Res. Technol. 2022, 21, 1878–1889. [Google Scholar] [CrossRef]
- Le, D.P.; Ji, W.S.; Kim, J.G.; Jeong, K.J.; Lee, S.H. Effect of Antimony on the Corrosion Behavior of Low-Alloy Steel for Flue Gas Desulfurization System. Corros. Sci. 2008, 50, 1195–1204. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, L.; Zhang, L.; Wang, Q.; Wang, T. Structure of the Rust Layer of Weathering Steel in A High Chloride Environment: A Detailed Characterization via HRTEM, STEM-EDS, and FIB-SEM. Corros. Sci. 2020, 177, 108997. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, X.; Dong, W.; Wang, Q.; Zhang, F.; Gu, X. Layer-by-Layer Investigation of the Multilayer Corrosion Products for Different Ni Content Weathering Steel via a Novel Pull-off Testing. Corros. Sci. 2022, 195, 109988. [Google Scholar] [CrossRef]
- Maetz, J.-Y.; Douillard, T.; Cazottes, S.; Verdu, C.; Kléber, X. M23C6 Carbides and Cr2N Nitrides in Aged Duplex Stainless Steel: A SEM, TEM and FIB Tomography Investigation. Micron 2016, 84, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, H.; Liang, W. Hydrogen Embrittlement Fracture Mechanism of 430 Ferritic Stainless Steel: The Significant Role of Carbides and Dislocations. Mater. Sci. Eng. A 2022, 829, 142043. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Li, X.; Du, C. Characteristics of Hydrogen Embrittlement in High-PH Stress Corrosion Cracking of X100 Pipeline Steel in Carbonate/Bicarbonate Solution. Constr. Build. Mater. 2020, 263, 120124. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Lu, L.; Huang, Y.Z.; Du, C.W.; Li, X.G. Mechanistic Aspect of Non-Steady Electrochemical Characteristic During Stress Corrosion Cracking of an X70 Pipeline Steel in Simulated Underground Water. Corrosion 2014, 70, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Q.; Liu, J.; Huang, F.; Huang, Y.; Li, X. Understanding the Effect of Niobium on Hydrogen-Induced Blistering in Pipeline Steel: A Combined Experimental and Theoretical Study. Corros. Sci. 2019, 159, 108142. [Google Scholar] [CrossRef]
- Vercruysse, F.; Claeys, L.; Depover, T.; Verleysen, P.; Petrov, R.H.; Verbeken, K. The Effect of Nb on the Hydrogen Embrittlement Susceptibility of Q&P Steel under Static and Dynamic Loading. Mater. Sci. Eng. A 2022, 852, 143652. [Google Scholar] [CrossRef]
- Samanta, S.; Gangavarapu, S.; Jayabalan, B.; Makineni, S.K.; Dutta, M.; Singh, S.B. Atomic-Scale Investigation of H-Trapping by Fine NbC Precipitates in a Low C Ferritic Steel. Scr. Mater. 2023, 234, 115537. [Google Scholar] [CrossRef]
| C | Si | Mn | Cr | Ni | Cu | Nb | Sb | P | S | |
|---|---|---|---|---|---|---|---|---|---|---|
| BM | 0.71 | 0.21 | 0.80 | 0.20 | 0 | 0 | 0 | 0 | 0.018 | 0.002 |
| 0.5NiCu | 0.71 | 0.21 | 0.80 | 0.20 | 0.49 | 0.51 | 0.046 | 0.11 | 0.021 | 0.016 |
| 1NiCu | 0.53 | 0.21 | 0.80 | 0.20 | 0.98 | 0.51 | 0.046 | 0.11 | 0.022 | 0.012 |
| LNiCu | 0.34 | 0.21 | 0.80 | 0.20 | 0.98 | 0.51 | 0.046 | 0.11 | 0.015 | 0.014 |
| NaCl | KNO3 | Na2SO4 | NaHCO3 | NaHSO3 |
|---|---|---|---|---|
| 0.25 | 0.1 | 0.50 | 1.00 | 1.00 |
| Steels | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation Rate (%) | Reduction in Area (%) |
|---|---|---|---|---|
| BM | 962 ± 85 | 525 ± 57 | 9.14 ± 1.5 | 31.97 ± 2.9 |
| 0.5NiCu | 1046 ± 99 | 604 ± 78 | 8.65 ± 1.2 | 32.73 ± 3.8 |
| 1NiCu | 907 ± 83 | 557 ± 55 | 9.30 ± 2.0 | 45.65 ± 5.5 |
| LNiCu | 856 ± 89 | 551 ± 65 | 7.60 ± 1.5 | 37.01 ± 3.5 |
| Steel | Rs/Ω·cm2 | Qf × 10−4/Ω−1cm−2sn | nf | Rpore/Ω·cm2 | Qdl × 10−4/Ω−1cm−2sn | ndl | Rct/Ω·cm2 | χ2 × 10−4 |
|---|---|---|---|---|---|---|---|---|
| BM | 105.5 | 3.99 | 0.93 | 57.37 | 5.94 | 0.95 | 239.8 | 3.64 |
| 0.5NiCu | 103.4 | 7.55 | 0.82 | 32.23 | 10.43 | 0.83 | 247.8 | 1.53 |
| 1NiCu | 107.1 | 4.77 | 0.86 | 49.25 | 7.37 | 0.90 | 281.1 | 2.05 |
| LNiCu | 111.7 | 6.93 | 0.82 | 27.22 | 11.9 | 0.82 | 195.5 | 1.12 |
| Steels | AD | AD + HE | HE |
|---|---|---|---|
| BM | >−0.712 V | −0.712~−0.867 V | <−0.867 V |
| 0.5NiCu | >−0.692 V | −0.857~−0.692 V | <−0.857 V |
| 1NiCu | >−0.687 V | −0.858~−0.687 V | <−0.858 V |
| LNiCu | >−0.689 V | −0.836~−0.688 V | <−0.836 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; An, N.; Wang, X.; Li, Y.; Liu, Z.; Jin, A.; Yang, R.; Pan, Y.; Li, X. Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment. Materials 2023, 16, 5965. https://doi.org/10.3390/ma16175965
Du H, An N, Wang X, Li Y, Liu Z, Jin A, Yang R, Pan Y, Li X. Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment. Materials. 2023; 16(17):5965. https://doi.org/10.3390/ma16175965
Chicago/Turabian StyleDu, Hailong, Na An, Xiyan Wang, Yongliang Li, Zhiyong Liu, Aibing Jin, Renshu Yang, Yue Pan, and Xiaogang Li. 2023. "Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment" Materials 16, no. 17: 5965. https://doi.org/10.3390/ma16175965
APA StyleDu, H., An, N., Wang, X., Li, Y., Liu, Z., Jin, A., Yang, R., Pan, Y., & Li, X. (2023). Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment. Materials, 16(17), 5965. https://doi.org/10.3390/ma16175965






