Mesoporous Materials for Metal-Laden Wastewater Treatment
Abstract
1. Introduction
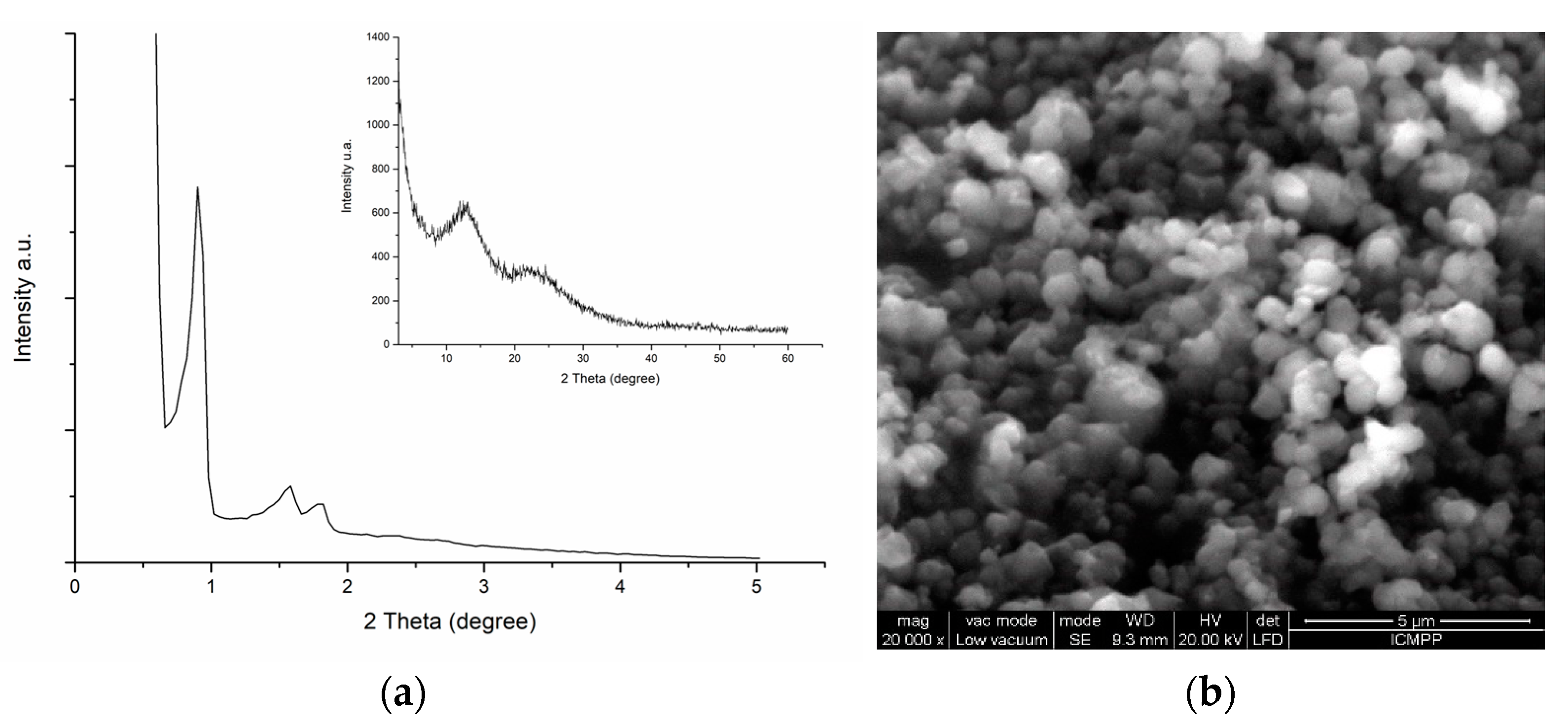
2. Mesoporous Materials Synthesis and Characterization
3. Application of Mesoporous Materials for Metal Removal from Wastewater
4. Mechanisms of Metal Ions’ Removal by Mesoporous Materials
5. Mesoporous Adsorbents Reusability and Cost
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayuo, J.; Rwiza, M.J.; Sillanpää, M.; Mtei, K.M. Removal of Heavy Metals from Binary and Multicomponent Adsorption Systems Using Various Adsorbents–a Systematic Review. RSC Adv. 2023, 13, 13052–13093. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, M.P. Heavy Metal Contamination in Water and Its Possible Sources. Heavy Met. Environ. Impact Assess. Remediat. 2021, 179–189. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. EXS 2012, 101, 133. [Google Scholar] [CrossRef]
- Flores, D.; Almeida, C.M.R.; Gomes, C.R.; Balula, S.S.; Granadeiro, C.M. Tailoring of Mesoporous Silica-Based Materials for Enhanced Water Pollutants Removal. Molecules 2023, 28, 4038. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Rani, L.; Basnet, B.; Kumar, A. Mercury Toxicity. Encycl. Environ. Health 2022, 325–332. [Google Scholar] [CrossRef]
- Royer, A.; Sharman, T. Copper Toxicity; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Khan, A.A.; Mondal, M. Low-Cost Adsorbents, Removal Techniques, and Heavy Metal Removal Efficiency. New Trends Remov. Heavy Met. Ind. Wastewater 2021, 83–103. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, G.; Jadeja, R.N. Physical and Chemical Methods for Heavy Metal Removal. In Pollutants and Water Management: Resources, Strategies and Scarcity; Wiley: Hoboken, NJ, USA, 2021; pp. 377–397. [Google Scholar] [CrossRef]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy Metal Removal Applications Using Adsorptive Membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Sen, T.K.; Gomez, D. Adsorption of Zinc (Zn2+) from Aqueous Solution on Natural Bentonite. Desalination 2011, 267, 286–294. [Google Scholar] [CrossRef]
- Bashir, M.; Tyagi, S.; Annachhatre, A.P. Adsorption of Copper from Aqueous Solution onto Agricultural Adsorbents: Kinetics and Isotherm Studies. Mater. Today Proc. 2020, 28, 1833–1840. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Otero-Gonzalez, L.; Folens, K.; Mees, B.; Hennebel, T.; Du Laing, G. Selective and Enhanced Nickel Adsorption from Sulfate- and Calcium-Rich Solutions Using Chitosan. Sep. Purif. Technol. 2021, 276, 119283. [Google Scholar] [CrossRef]
- Türkmen, D.; Bakhshpour, M.; Akgönüllü, S.; Aşır, S.; Denizli, A. Heavy Metal Ions Removal From Wastewater Using Cryogels: A Review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A Critical Review on Recent Developments in MOF Adsorbents for the Elimination of Toxic Heavy Metals from Aqueous Solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef]
- Jang, E.H.; Pack, S.P.; Kim, I.; Chung, S. A Systematic Study of Hexavalent Chromium Adsorption and Removal from Aqueous Environments Using Chemically Functionalized Amorphous and Mesoporous Silica Nanoparticles. Sci. Rep. 2020, 10, 5558. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ma, L.; Wang, C.-C.; Wang, P.; Gutierrez, L.; Zheng, W. Light-Response Adsorption and Desorption Behaviors of Metal–Organic Frameworks. Environ. Funct. Mater. 2022, 1, 49–66. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Mao, Y. Metal-Organic Framework-Based Materials for Adsorption and Detection of Uranium(VI) from Aqueous Solution. ACS Omega 2022, 7, 14430–14456. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, G. Charge Reversal and Anion Effects during Adsorption of Metal Ions at Clay Surfaces: Mechanistic Aspects and Influence Factors. Chem. Phys. 2020, 529, 110575. [Google Scholar] [CrossRef]
- Rajamathi, M.; Thomas, G.S.; Kamath, P.V. The Many Ways of Making Anionic Clays. J. Chem. Sci. 2001, 113, 671–680. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Carbon Derived Nanomaterials for the Sorption of Heavy Metals from Aqueous Solution: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578. [Google Scholar] [CrossRef]
- Stor, M.; Czelej, K.; Krasiński, A.; Gradoń, L. Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation. Nanomaterials 2023, 13, 1162. [Google Scholar] [CrossRef] [PubMed]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated Carbons for Arsenic Removal from Natural Waters and Wastewaters: A Review. Water 2021, 13, 2982. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Zhou, S.; Gao, X. Adsorption Mechanism of Cr(VI) on Woody-Activated Carbons. Heliyon 2023, 9, e13267. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Yushin, N.; Abdusamadzoda, D.; Grozdov, D.; Humelnicu, I.; Ignat, M.; Humelnicu, D. Removal of Vanadium Ions from Aqueous Solutions Using Different Type of Hydroxyapatites: Adsorption Isotherm, Kinetics and Thermodynamic Studies. Environ. Eng. Manag. J. 2021, 20, 871–881. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Ignat, M.; Humelnicu, I.; Grozdov, D.; Vershinina, T. Removal of Indium Ions from Aqueous Solutions Using Hydroxyapatite and Its Two Modifications. Separations 2023, 10, 401. [Google Scholar] [CrossRef]
- Smržová, D.; Szatmáry, L.; Ecorchard, P.; Machálková, A.; Maříková, M.; Salačová, P.; Straka, M. Carbon and Zeolite-Based Composites for Radionuclide and Heavy Metal Sorption. Heliyon 2022, 8, e12293. [Google Scholar] [CrossRef]
- Paramitha, T.; Wulandari, W.; Rizkiana, J.; Sasongko, D. Performance Evaluation of Coal Fly Ash Based Zeolite A for Heavy Metal Ions Adsorption of Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012095. [Google Scholar] [CrossRef]
- Nagappan, S.; Park, S.S.; Kim, B.K.; Yoo, D.G.; Jo, N.J.; Lee, W.K.; Ha, C.S. Synthesis and Functionalisation of Mesoporous Materials for Transparent Coatings and Organic Dye Adsorption. New J. Chem. 2018, 42, 10254–10262. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, J.; Wu, D.; Li, X.; Luo, Y.; Han, C.; Ma, W.; He, S. Investigating the Heavy Metal Adsorption of Mesoporous Silica Materials Prepared by Microwave Synthesis. Nanoscale Res. Lett. 2017, 12, 323. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A Review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2022, 13, 2. [Google Scholar] [CrossRef]
- Kanatzidis, M.G.; Poeppelmeier, K.R.; Bobev, S.; Guloy, A.M.; Hwu, S.-J.; Lachgar, A.; Latturner, S.E.; Raymond; Schaak, E.; Seo, D.-K.; et al. Report from the Third Workshop on Future Directions of Solid-State Chemistry: The Status of Solid-State Chemistry and Its Impact in the Physical Sciences. Prog. Solid State Chem. 2008, 36, 1–133. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Wang, Z.; Gao, P.; Li, G.K. Synthesis and Application of Mesoporous Materials: Process Status, Technical Problems, and Development Prospects: A Mini-Review. Energy Fuels 2023, 37, 3413–3427. [Google Scholar] [CrossRef]
- Yu, Q.; Hui, J.; Wang, P.; Xu, B.; Zhuang, J.; Wang, X. Hydrothermal Synthesis of Mesoporous Silica Spheres: Effect of the Cooling Process. Nanoscale 2012, 4, 7114–7120. [Google Scholar] [CrossRef] [PubMed]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous Materials for Clean Energy Technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials. Mater. Today Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, H.J.; Xia, Z.N. Advances in Microwave Assisted Synthesis of Ordered Mesoporous Materials. Trans. Nonferrous Met. Soc. China 2009, 19, s656–s664. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous Materials as Multifunctional Tools in Biosciences: Principles and Applications. Mater. Sci. Eng. C 2015, 49, 114–151. [Google Scholar] [CrossRef] [PubMed]
- de Greñu, B.D.; de los Reyes, R.; Costero, A.M.; Amorós, P.; Ros-Lis, J.V. Recent Progress of Microwave-Assisted Synthesis of Silica Materials. Nanomaterials 2020, 10, 1092. [Google Scholar] [CrossRef]
- Chircov, C.; Spoială, A.; Păun, C.; Crăciun, L.; Ficai, D.; Ficai, A.; Andronescu, E.; Turculeƫ, S.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules 2020, 25, 3814. [Google Scholar] [CrossRef]
- Kim, C.; Yoon, S.; Lee, J.H. Facile Large-Scale Synthesis of Mesoporous Silica Nanoparticles at Room Temperature in a Monophasic System with Fine Size Control. Microporous Mesoporous Mater. 2019, 288, 109595. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.; Gautam, D.; Hooda, S. Characterization of Mesoporous Materials. In Advanced Functional Porous Materials; Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 175–204. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874. [Google Scholar] [CrossRef]
- da Silva, F.; das, C.M.; Costa, M.J.d.S.; da Silva, L.K.R.; Batista, A.M.; da Luz, G.E. Functionalization Methods of SBA-15 Mesoporous Molecular Sieve: A Brief Overview. SN Appl. Sci. 2019, 1, 654. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Grozdov, D.; Ignat, M.; Demcak, S.; Humelnicu, I. Sorption of Ce(III) by Silica SBA-15 and Titanosilicate ETS-10 from Aqueous Solution. Water 2021, 13, 3263. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Grozdov, D.; Ignat, M.; Humelnicu, I. Adsorption Capacity of Silica SBA-15 and Titanosilicate ETS-10 toward Indium Ions. Materials 2023, 16, 3201. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.Z. Use of SBA-15 Ordered Nano Mesoporous Silica for Removal of Copper(II) from Aqueous Media: Studies on Equilibrium, Isotherm, Kinetics and Thermodynamics. J. Environ. Chem. Eng. 2019, 7, 103069. [Google Scholar] [CrossRef]
- Nguyen, Q.N.K.; Yen, N.T.; Hau, N.D.; Tran, H.L. Synthesis and Characterization of Mesoporous Silica SBA-15 and ZnO/SBA-15 Photocatalytic Materials from the Ash of Brickyards. J. Chem. 2020, 2020, 8456194. [Google Scholar] [CrossRef]
- Kumar, P.; Mal, N.; Oumi, Y.; Yamana, K.; Sano, T. Mesoporous Materials Prepared Using Coal Fly Ash as the Silicon and Aluminium Source. J. Mater. Chem. 2001, 11, 3285–3290. [Google Scholar] [CrossRef]
- Bae, J.; Kim, S.; Kim, K.S.; Hwang, H.K.; Choi, H. Adsorptive Removal of Arsenic by Mesoporous Iron Oxide in Aquatic Systems. Water 2020, 12, 3147. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe 3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; AbdulWahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive Removal of Different Pollutants Using Metal-Organic Framework Adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Babapour, M.; Hadi Dehghani, M.; Alimohammadi, M.; Moghadam Arjmand, M.; Salari, M.; Rasuli, L.; Mubarak, N.M.; Ahmad Khan, N. Adsorption of Cr(VI) from Aqueous Solution Using Mesoporous Metal-Organic Framework-5 Functionalized with the Amino Acids: Characterization, Optimization, Linear and Nonlinear Kinetic Models. J. Mol. Liq. 2022, 345, 117835. [Google Scholar] [CrossRef]
- Nqombolo, A.; Munonde, T.S.; Makhetha, T.A.; Moutloali, R.M.; Nomngongo, P.N. Cobalt/Zinc Based Metal Organic Frameworks as an Effective Adsorbent for Improved Removal of As(V) and Cr(VI) in a Wide PH Range. J. Mater. Res. Technol. 2021, 12, 1845–1855. [Google Scholar] [CrossRef]
- Karbalaee Hosseini, A.; Tadjarodi, A. Novel Zn Metal–Organic Framework with the Thiazole Sites for Fast and Efficient Removal of Heavy Metal Ions from Water. Sci. Rep. 2023, 13, 11430. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Naidu, G.; Moon, H.; Vigneswaran, S. Selective Copper Extraction by Multi-Modified Mesoporous Silica Material, SBA-15. Sci. Total Environ. 2019, 697, 134070. [Google Scholar] [CrossRef] [PubMed]
- Dudarko; Kobylinska, N.; Mishra, B.; Kessler, V.G.; Tripathi, B.P.; Seisenbaeva, G.A. Facile Strategies for Synthesis of Functionalized Mesoporous Silicas for the Removal of Rare-Earth Elements and Heavy Metals from Aqueous Systems. Microporous Mesoporous Mater. 2021, 315, 110919. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, S.; Abu Khadra, M.R. Phosphate Removal from Solution by Composite of MCM-41 Silica with Rice Husk: Kinetic and Equilibrium Studies. Microporous Mesoporous Mater. 2016, 224, 51–57. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, Y.; Guo, Y.; Miao, S.; Chen, Q.; Chen, Z. Functionalization of SBA-15 Mesoporous Materials with 2-Acetylthiophene for Adsorption of Cr(III) Ions. Microporous Mesoporous Mater. 2020, 292, 109754. [Google Scholar] [CrossRef]
- Awual, M.R. Mesoporous Composite Material for Efficient Lead(II) Detection and Removal from Aqueous Media. J. Environ. Chem. Eng. 2019, 7, 103124. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wen, N.; Wei, D.; Zhang, Y. Highly Effective Removal of Lead and Cadmium Ions from Wastewater by Bifunctional Magnetic Mesoporous Silica. Sep. Purif. Technol. 2021, 265, 118341. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. 2-Mercaptothiazoline Modified Mesoporous Silica for Mercury Removal from Aqueous Media. J. Hazard. Mater. 2006, 134, 245–256. [Google Scholar] [CrossRef]
- Thirupathi, K.; Rajesh, S.; Madhappan, S.; Gnanasekaran, L.; Guganathan, L.; Phan, T.T.V.; Kim, S.C. Selective Removal of Copper (II) Ions from Aqueous Solution Using Pyridyl-Bridged Mesoporous Organosilica Hybrid Adsorbent. Environ. Res. 2023, 224, 115439. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, F.; Kheshti, Z.; Ghasemi, S.; Altaee, A. Enhancement of Cd 2+ Removal from Aqueous Solution by Multifunctional Mesoporous Silica: Equilibrium Isotherms and Kinetics Study. Sep. Purif. Technol. 2019, 224, 199–208. [Google Scholar] [CrossRef]
- Wang, J.; Tong, X.; Chen, Y.; Sun, T.; Liang, L.; Wang, C. Enhanced Removal of Cr(III) in High Salt Organic Wastewater by EDTA Modified Magnetic Mesoporous Silica. Microporous Mesoporous Mater. 2020, 303, 110262. [Google Scholar] [CrossRef]
- Peng, Y.; Luo, L.; Luo, S.; Peng, K.; Zhou, Y.; Mao, Q.; Yang, J.; Yang, Y. Efficient Removal of Antimony(III) in Aqueous Phase by Nano-Fe3O4 Modified High-Iron Red Mud: Study on Its Performance and Mechanism. Water 2021, 13, 809. [Google Scholar] [CrossRef]
- Abdulridha, S.; Jiao, Y.; Xu, S.; Zhang, R.; Garforth, A.A.; Fan, X. Mesoporous Zeolitic Materials (MZMs) Derived From Zeolite Y Using a Microwave Method for Catalysis. Front. Chem. 2020, 8, 482. [Google Scholar] [CrossRef]
- Sabarish, R.; Unnikrishnan, G. Synthesis, Characterization and Evaluations of Micro/Mesoporous ZSM-5 Zeolite Using Starch as Bio Template. SN Appl. Sci. 2019, 1, 989. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Qin, Z.; Mintova, S.; Liu, X. Intra-Crystalline Mesoporous ZSM-5 Zeolite by Grinding Synthesis Method. Microporous Mesoporous Mater. 2020, 306, 110437. [Google Scholar] [CrossRef]
- Liu, D.; Li, G.; Liu, J.; Wei, Y.; Guo, H. Mesoporous Titanium-Silicalite Zeolite Containing Organic Templates as a Bifunctional Catalyst for Cycloaddition of CO2 and Epoxides. ACS Appl. Mater. Interfaces 2018, 10, 22119–22129. [Google Scholar] [CrossRef]
- Li, X.; Su, M.; Chen, Y.; Nisa, M.U.; Zhao, N.; Jiang, X.; Li, Z. The Modification of Titanium in Mesoporous Silica for Co-Based Fischer-Tropsch Catalysts. Front. Chem. Sci. Eng. 2022, 16, 1224–1236. [Google Scholar] [CrossRef]
- Qiao, M.; Guo, P.F.; Zhang, C.Y.; Sun, X.Y.; Chen, M.L.; Wang, J.H. Titanium Dioxide-Functionalized Dendritic Mesoporous Silica Nanoparticles for Highly Selective Isolation of Phosphoproteins. J. Sep. Sci. 2021, 44, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Ederer, J.; Šťastný, M.; Došek, M.; Henych, J.; Janoš, P. Mesoporous Cerium Oxide for Fast Degradation of Aryl Organophosphate Flame Retardant Triphenyl Phosphate. RSC Adv. 2019, 9, 32058–32065. [Google Scholar] [CrossRef] [PubMed]
- Özkan, E.; Hofmann, A.; Votsmeier, M.; Wang, W.; Huang, X.; Kübel, C.; Badaczewski, F.; Turke, K.; Werner, S.; Smarsly, B.M. Comprehensive Characterization of a Mesoporous Cerium Oxide Nanomaterial with High Surface Area and High Thermal Stability. Langmuir 2021, 37, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, P.; Roy, A.; Ullah, H.; Sujatha Devi, P.; Tahir, A.A.; Mallick, T.K.; Sundaram, S. Soft-Template Synthesis of High Surface Area Mesoporous Titanium Dioxide for Dye-Sensitized Solar Cells. Int. J. Energy Res. 2019, 43, 523–534. [Google Scholar] [CrossRef]
- Kuvayskaya, A.; Lotsi, B.; Mohseni, R.; Vasiliev, A. Mesoporous Adsorbents for Perfluorinated Compounds. Microporous Mesoporous Materials 2020, 305, 110374. [Google Scholar] [CrossRef]
- Kouznetsova, T.; Ivanets, A.; Prozorovich, V.; Hosseini-Bandegharaei, A.; Tran, H.N.; Srivastava, V.; Sillanpää, M. Sorption and Mechanism Studies of Cu2+, Sr2+ and Pb2+ Ions on Mesoporous Aluminosilicates/Zeolite Composite Sorbents. Water Sci. Technol. 2020, 82, 984–997. [Google Scholar] [CrossRef]
- Costa, J.A.S.; Costa, V.C.; Pereira-Filho, E.R.; Paranhos, C.M. Removal of Cr(VI) from Wastewater of the Tannery Industry by Functionalized Mesoporous Material. Silicon 2020, 12, 1895–1903. [Google Scholar] [CrossRef]
- Yang, S.; Qian, J.; Kuang, L.; Hua, D. Ion-Imprinted Mesoporous Silica for Selective Removal of Uranium from Highly Acidic and Radioactive Effluent. ACS Appl. Mater. Interfaces 2017, 9, 29337–29344. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Popoola, L.T.; Babatunde, E.O. Adsorption of Cadmium Ion from Aqueous Solutions by Copper-Based Metal Organic Framework: Equilibrium Modeling and Kinetic Studies. Appl. Water Sci. 2019, 9, 106. [Google Scholar] [CrossRef]
- Conte, N.; Díez, E.; Almendras, B.; Gómez, J.M.; Rodríguez, A. Sustainable Recovery of Cobalt from Aqueous Solutions Using an Optimized Mesoporous Carbon. J. Sustain. Metall. 2023, 9, 266–279. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Effective Removal of Hexavalent Chromium from Aqueous Solutions by Adsorption on Mesoporous Carbon Microspheres. J. Colloid Interface Sci. 2016, 462, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Calderon, J.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Mesoporous Activated Carbon as a Green Adsorbent for the Removal of Heavy Metals and Congo Red: Characterization, Adsorption Kinetics, and Isotherm Studies. J. Contam. Hydrol. 2021, 243, 103869. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Y.; Wang, X.; Chen, Z. Rapid Removal of Zinc(II) from Aqueous Solutions Using a Mesoporous Activated Carbon Prepared from Agricultural Waste. Materials 2017, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Lv, X.; Li, Q.; Ren, T.; Song, H.; Yang, Q. Removal of Hexavalent Chromium from Aqueous Solution by Mesoporous α-FeOOH Nanoparticles: Performance and Mechanism. Microporous Mesoporous Mater. 2020, 299, 110101. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Choi, K.; Kim, H.G.; Park, J.A.; Cho, S.H.; Hong, S.W.; Lee, J.H.; Lee, J.H.; Lee, S.; et al. Investigation of the Mechanism of Chromium Removal in (3-Aminopropyl)Trimethoxysilane Functionalized Mesoporous Silica. Sci. Rep. 2018, 8, 12078. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J.; Adegoke, K.A.; Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O. Adsorption Mechanism and Modeling of Radionuclides and Heavy Metals onto ZnO Nanoparticles: A Review. Appl. Water Sci. 2022, 13, 20. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetics and Isotherm Models of Heavy Metals by Various Adsorbents: An Overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Wang, B.; Lan, J.; Bo, C.; Gong, B.; Ou, J. Adsorption of Heavy Metal onto Biomass-Derived Activated Carbon: Review. RSC Adv. 2023, 13, 4275–4302. [Google Scholar] [CrossRef]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of Copper (II) on Mesoporous Silica: The Effect of Nano-Scale Confinement. Geochem. Trans. 2018, 19, 13. [Google Scholar] [CrossRef]
- Liu, S.; Phenrat, T.; Sui, Q.; Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y. Zinc Removal from Water via EDTA-Modified Mesoporous SBA-16 and SBA-15. Toxics 2023, 11, 205. [Google Scholar] [CrossRef]
- Humelnicu, D.; Ignat, M.; Dinu, M.V.; Dragan, E.S. Optimization of Arsenic Removal from Aqueous Solutions Using Amidoxime Resin Hosted by Mesoporous Silica. ACS Omega 2022, 7, 31069–31080. [Google Scholar] [CrossRef]
- Kobylinska, N.G.; Kessler, V.G.; Seisenbaeva, G.A.; Dudarko, O.A. In Situ Functionalized Mesoporous Silicas for Sustainable Remediation Strategies in Removal of Inorganic Pollutants from Contaminated Environmental Water. ACS Omega 2022, 7, 23576–23590. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, H.; Rohani, S.; Batool, S.; Yusuff, A.S. An Overview of the Use of Water-Stable Metal-Organic Frameworks in the Removal of Cadmium Ion. J. Environ. Chem. Eng. 2023, 11, 109131. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. [Google Scholar] [CrossRef]
- Mei, D.; Liu, L.; Yan, B. Adsorption of Uranium (VI) by Metal-Organic Frameworks and Covalent-Organic Frameworks from Water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hafez, I.; Tajvidi, M.; Amirbahman, A. Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials 2021, 11, 2818. [Google Scholar] [CrossRef]
- Du, L.; Gao, P.; Liu, Y.; Minami, T.; Yu, C. Removal of Cr(VI) from Aqueous Solution by Polypyrrole/Hollow Mesoporous Silica Particles. Nanomaterials 2020, 10, 686. [Google Scholar] [CrossRef]
- Humelnicu, D.; Zinicovscaia, I.; Humelnicu, I.; Ignat, M.; Yushin, N.; Grozdov, D. Study on the SBA-15 Silica and ETS-10 Titanosilicate as Efficient Adsorbents for Cu(II) Removal from Aqueous Solution. Water 2022, 14, 857. [Google Scholar] [CrossRef]
- Río, D.A.d.H.-D.; Aguilera-Alvarado, A.F.; Cano-Aguilera, I.; Martínez-Rosales, M.; Holmes, S. Synthesis and Characterization of Mesoporous Aluminosilicates for Copper Removal from Aqueous Medium. Mater. Sci. Appl. 2012, 3, 485–491. [Google Scholar] [CrossRef][Green Version]
- Khalifa, M.E.; Abdelrahman, E.A.; Hassanien, M.M.; Ibrahim, W.A. Application of Mesoporous Silica Nanoparticles Modified with Dibenzoylmethane as a Novel Composite for Efficient Removal of Cd(II), Hg(II), and Cu(II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 1234, 30, 2182–2196. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Wen, X.; Wu, W. Efficient Removal of Cadmium Using Facile Functionalized of Mesoporous Silica via a Biomimetic Coating. RSC Adv. 2016, 6, 18340–18347. [Google Scholar] [CrossRef]
- Thakur, A.K.; Nisola, G.M.; Limjuco, L.A.; Parohinog, K.J.; Torrejos, R.E.C.; Shahi, V.K.; Chung, W.J. Polyethylenimine-Modified Mesoporous Silica Adsorbent for Simultaneous Removal of Cd(II) and Ni(II) from Aqueous Solution. J. Ind. Eng. Chem. 2017, 49, 133–144. [Google Scholar] [CrossRef]
- Rabie, A.M.; Abd El-Salam, H.M.; Betiha, M.A.; El-Maghrabi, H.H.; Aman, D. Mercury Removal from Aqueous Solution via Functionalized Mesoporous Silica Nanoparticles with the Amine Compound. Egypt. J. Pet. 2019, 28, 289–296. [Google Scholar] [CrossRef]
- Da’na, E.; Sayari, A. Adsorption of Copper on Amine-Functionalized SBA-15 Prepared by Co-Condensation: Equilibrium Properties. Chem. Eng. J. 2011, 166, 445–453. [Google Scholar] [CrossRef]
- Khezami, L.; Modwi, A.; Taha, K.K.; Bououdina, M.; Hamadi, B.; Assadi, N.; Tran, H.N.; Khezami, L.; Modwi, A.; Taha, K.K.; et al. Mesoporous Zr-G-C3N4 Sorbent as an Exceptional Cu (II) Ion Adsorbent in Aquatic Solution: Equilibrium, Kinetics, and Mechanisms Study. Water 2023, 15, 1202. [Google Scholar] [CrossRef]
- Othman, A.; Vargo, P.; Andreescu, S. Recyclable Adsorbents Based on Ceria Nanostructures on Mesoporous Silica Beads for the Removal and Recovery of Phosphate from Eutrophic Waters. ACS Appl. Nano Mater. 2019, 2, 7008–7018. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of Adsorbent Preparation and Usage in Wastewater Treatment: A Review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Pham, T.H.; Lee, B.K.; Kim, J. Improved Adsorption Properties of a Nano Zeolite Adsorbent toward Toxic Nitrophenols. Process Saf. Environ. Prot. 2016, 104, 314–322. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Nasr, M. Regression Model, Artificial Intelligence, and Cost Estimation for Phosphate Adsorption Using Encapsulated Nanoscale Zero-Valent Iron. Sep. Sci. Technol. 2018, 54, 13–26. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Vergel, K.; Popova, N.; Artemiev, G.; Safonov, A. Metal Removal from Nickel-Containing Effluents Using Mineral–Organic Hybrid Adsorbent. Materials 2020, 13, 4462. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, C.; Rao, G.P. Comparisons of Sorbent Cost for the Removal of Ni2+ from Aqueous Solution by Carbon Nanotubes and Granular Activated Carbon. J. Hazard. Mater. 2008, 151, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Okolie, J.A.; Niu, C.; Dalai, A.K. Techno–Economic Analysis of Activated Carbon Production from Spent Coffee Grounds: Comparative Evaluation of Different Production Routes. Energy Convers. Manag. X 2022, 14, 100218. [Google Scholar] [CrossRef]

| Material | Technique Applied for Synthesis | Surface Area, m2/g | Pore Size, nm | Reference |
|---|---|---|---|---|
| Mesostructured zeolitic materials (MZMs) | Conventional hydrothermal treatment | 210–814 | 2.2–10 | [69] |
| Micro/mesoporous ZSM-5 zeolite | Hydrothermal crystallization route, using starch mesotemplate | - | 5–15 | [70] |
| Mesoporous ZSM-5 zeolite | Facile grinding synthesis method | 77 | 3–25 | [71] |
| Mesoporous TS-1 zeolite (MTS-1) | Hydrothermal method using polydiallyldimethylammonium chloride (PDDA) as mesopore template | 61.79 | 5–20 | [72] |
| SBA-15 mesoporous silica with incorporated titanium | One-pot hydrothermal crystallization method | 472 | 6.2 | [73] |
| Titanium dioxide-functionalized dendritic mesoporous silica | Post-grafting method | 666.66. | 22.2 | [74] |
| Mesoporous cerium oxide | Calcination of basic cerous carbonate (as a precursor) obtained by precipitation from an aqueous solution | 6–102 | 3–4 | [75] |
| Mesoporous cerium oxide | Thermal hydrolysis method | 190–205 | 27 | [76] |
| Mesoporous titanium dioxide | Soft-template method with titanium isopropoxide as a titanium source | 90 | 19.2 | [77] |
| Bridged silsesquioxanes | Sol–gel condensation of bis [3-(trimethoxysilyl)propyl]amine and N- methyl-3,30-bis(trimethoxysilyl)dipropylamine in acidic media in the presence of surfactants. | 18.7–189.4 | 10.2 | [78] |
| Mesoporous aluminosilicate/zeolite composite | Template co-precipitation method | 320 | 7 | [79] |
| PABA-MCM-41 mesoporous material | Hydrothermal/co-condensation method | 556 | 4.2 | [80] |
| Ion-imprinted mesoporous silica | Co-condensation method with uranyl as the template ion and diethylphosphatoethyltriethoxysilane as the functional ligands | 224–681 | 1.06–9.55 | [81] |
| Cu-MOF | Solvothermal method | 1057 | 20 | [82] |
| Mesoporous carbon | Replica method | 439–924 | 9–13 | [83] |
| Mesoporous carbon microspheres | Spray-drying method | 1061 | 9.5 | [84] |
| Mesoporous activated carbon | Self-activation method | 843.3 | 3.55 | [85] |
| Mesoporous activated carbon | Chemical activation | 688.2 | 3.2 | [86] |
| Mesoporous α-FeOOH nanoparticles | Freeze-drying technique | 46 | 11 | [87] |
| (3-aminopropyl)trimethoxysilane functionalized mesoporous silica | Post-synthesis grafting method | 857.88 | 2.7 | [88] |
| Amino-functionalized mesoporous silica nanoparticles | Base-catalysed hydrolysis and condensation | 517.4 | 8.94 | [17] |
| NZVI-SH-HMS | Gel–sol and wet impregnation methods | 312.84 | 2.56 | [63] |
| HRM@nFe3O4 | Co-precipitation method | 171.63 | 22.76 | [68] |
| Sorbent | Metal | pH | q, mg/g | Isotherm Model | Surface Area, m2/g | Reference |
|---|---|---|---|---|---|---|
| Mesoporous iron oxide | As(III) | 5–9 | 136.89 | Freundlich | 269 | [52] |
| As(V) | 5–9 | 31.82 | Langmuir | |||
| Iron oxide nanoparticles immobilized on cellulose nanofibril aerogels | As(III) | 7 | 48 | Langmuir | 165 | [101] |
| As(V) | 7 | 91 | ||||
| ZIF-67/ZIF-8 | As(V) | 6.5 | 71.4 | Langmuir | 950 | [56] |
| Cr(VI) | 6.5 | 69.4 | ||||
| MOF-5 | Cr(VI) | 2.0 | 78.12 | Langmuir | 500.8 | [55] |
| Mesoporous α-FeOOH nanoparticles | Cr(VI) | 3 | 16.58 | Langmuir | 46 | [87] |
| (3-aminopropyl)trimethoxysilane functionalized mesoporous silica | Cr(VI) | 3 | 89.4 | Temkin | 857.88 | [88] |
| Amino-functionalized mesoporous silica nanoparticles | Cr(VI) | 2.0 | 42.2 | Langmuir | 517.4 | [17] |
| Mesoporous carbon microspheres | Cr(VI) | 3.0 | 156.3 | Langmuir | 1061 | [84] |
| Polypyrrole/hollow mesoporous silica particle | Cr(VI) | 322 | Langmuir | 325 | [102] | |
| Multi-modified SBA-15 (Mn-SBA-15-NH2) | Cu | 5.0 | 2.01 mmol/g | Langmuir | 310 | [58] |
| SBA-15 Silica | Cu | 5.0 | 52.71 | Langmuir | 802.493 | [103] |
| ETS-10 titanosilicate | Cu | 6.0 | 172.53 | Langmuir | 31.473 | [103] |
| Mesoporous aluminosilicates | Cu | 4.0 | 16 | Langmuir | 243 | [104] |
| Mesoporous activated carbon | Cu | 6.0 | 12 | Langmuir | 843.3 | [85] |
| Mesoporous silica nanoparticles modified by dibenzoylmethane | Cu | 6 | 31.76 | Langmuir | - | [105] |
| Mesoporous carbon | Co | 4.0–6.0 | 5.85 | Langmuir | 439–924 | [83] |
| NZVI-SH-HMS | Cd | 4.5 | 330.0 | Langmuir | 312.84 | [63] |
| Mesoporous material (DMOS) | Cd | 6.0 | 107 | Langmuir | 431 | [106] |
| Mesoporous silica nanoparticles modified by dibenzoylmethane | Cd | 6.0 | 35.37 | Langmuir | - | [105] |
| PEI/MCM-41 * | Cd | 6.0 | 156.0 | Langmuir/Freundlich | 440 | [107] |
| Mesoporous silica nanoparticles modified by dibenzoylmethane | Hg | 6.0 | 25.17 | Langmuir | - | [105] |
| DA-KIT–6 | Hg | 10 | 50 | Langmuir | 185 | [108] |
| SBA-15 Silica | In | 6.0 | 2036 | Langmuir | 802.493 | [48] |
| Mesoporous activated carbon | Pb | 6.0 | 12.7 | Langmuir | 843.3 | [85] |
| Mesoporous composite material | Pb | 6.0 | 196.35 | Langmuir | 527 | [62] |
| NZVI-SH-HMS | Pb | 5.5 | 487.8 | Langmuir | 312.84 | [63] |
| Mesoporous activated carbon | Zn | 5.2 | 100.76 | Langmuir | 688.2 | [86] |
| PEI/MCM-41 * | Ni | 6.0 | 139.7 | Langmuir/Freundlich | 440 | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grozdov, D.; Zinicovscaia, I. Mesoporous Materials for Metal-Laden Wastewater Treatment. Materials 2023, 16, 5864. https://doi.org/10.3390/ma16175864
Grozdov D, Zinicovscaia I. Mesoporous Materials for Metal-Laden Wastewater Treatment. Materials. 2023; 16(17):5864. https://doi.org/10.3390/ma16175864
Chicago/Turabian StyleGrozdov, Dmitrii, and Inga Zinicovscaia. 2023. "Mesoporous Materials for Metal-Laden Wastewater Treatment" Materials 16, no. 17: 5864. https://doi.org/10.3390/ma16175864
APA StyleGrozdov, D., & Zinicovscaia, I. (2023). Mesoporous Materials for Metal-Laden Wastewater Treatment. Materials, 16(17), 5864. https://doi.org/10.3390/ma16175864







